Abstract
AIM: To evaluate the utility of diffusion-weighted imaging (DWI) in screening and differential diagnosis of benign and malignant focal hepatic lesions.
METHODS: Magnetic resonance imaging (MRI) examinations were performed using the Signa Excite Xl Twin Speed 1.5T system (GE Healthcare, Milwaukee, WI, USA). Seventy patients who had undergone MRI of the liver [29 hepatocellular carcinomas (HCC), four cholangiocarcinomas, 34 metastatic liver cancers, 10 hemangiomas, and eight cysts] between April 2004 and August 2008 were retrospectively evaluated. Visualization of lesions, relative contrast ratio (RCR), and apparent diffusion coefficient (ADC) were compared between benign and malignant lesions on DWI. Superparamagnetic iron oxide (SPIO) was administered to 59 patients, and RCR was compared pre- and post-administration.
RESULTS: DWI showed higher contrast between malignant lesions (especially in multiple small metastatic cancers) and surrounding liver parenchyma than did contrast-enhanced computed tomography. ADCs (mean ± SD × 10-3 mm2/s) were significantly lower (P < 0.05) in malignant lesions (HCC: 1.31 ± 0.28 and liver metastasis: 1.11 ± 0.22) and were significantly higher in benign lesions (hemangioma: 1.84 ± 0.37 and cyst: 2.61 ± 0.45) than in the surrounding hepatic tissues. RCR between malignant lesions and surrounding hepatic tissues significantly improved after SPIO administration, but RCRs in benign lesions were not improved.
CONCLUSION: DWI is a simple and sensitive method for screening focal hepatic lesions and is useful for differential diagnosis.
Keywords: Hepatic tumor, Liver imaging, Magnetic resonance imaging, Diffusion-weighted imaging, Apparent diffusion coefficient
INTRODUCTION
Diffusion is the thermally induced motion of water molecules, which is also referred to as Brownian motion[1]. Diffusion-weighted imaging (DWI) is a new magnetic resonance imaging (MRI) technique that provides imaging of diffusion in biological tissues.
DWI has been reported to be useful in evaluating the early stages of brain ischemia[2-4]. Recent technical developments have reduced the image distortion associated with this technique and have increased the signal-to-noise ratio, thus making DWI of the body feasible[5,6]. Screening, accurate detection, and characterization of focal hepatic lesions are important for planning treatment of malignant hepatic lesions. The differential diagnosis of malignant and benign focal hepatic lesions remains a diagnostic challenge; however, to improve the diagnosis of such lesions, new methods for existing modalities, such as MRI, computed tomography (CT), angiography, and ultrasonography are being developed.
Recently, some studies have reported that the apparent diffusion coefficient (ADC), which is one of calculated parameters of DWI, might be useful for differential diagnosis of benign and malignant lesions in the liver[7]. Superparamagnetic iron oxide (SPIO)-enhanced MRI was reported to be as useful as CT during arterioportography and CT during hepatic arteriography for diagnosing metastatic liver tumors[8,9]. SPIO improves the contrast-to-noise ratio (CNR) between focal hepatic lesion and the surrounding liver parenchyma on T2-weighted imaging[9]. SPIO has also been reported to further improve the performance of DWI of the liver because SPIO reduces the signal in normal liver parenchyma[10].
The purpose of the present study was to retrospectively evaluate the utility of DWI in screening, accurate detection, and differential diagnosis of benign and malignant focal hepatic lesions.
MATERIALS AND METHODS
Patients
We retrospectively evaluated 70 patients (52 men and 18 women; age, 39-86 years; mean age, 65.3 years) with 85 lesions [29 hepatocellular carcinomas (HCC), four cholangiocarcinomas, 34 metastatic liver cancers, 10 hemangiomas, and eight cysts] who had been examined between April 2004 and August 2008. The locations of each focal hepatic lesion were: posterior segment in 32, anterior segment in 33, median segment in 15, and lateral segment in five patients. These lesions measured 1.0 to 10.0 cm (mean 3.1 cm) along their long axes on CT images.
The criteria for selecting patients to evaluate in the present study were as follows: (1) the diagnoses of HCC were pathology confirmed in 25 lesions and other lesions were confirmed by measurement of the serum α-fetal protein (AFP) level, clinical data, ultrasonography, angiography and CT or MRI or both; (2) hepatic metastasis of primary lesions was pathologically confirmed; (3) the diagnosis of cholangiocarcinomas was pathologically confirmed in three lesions and the other lesion was confirmed by clinical data, ultrasonography, CT and MRI; (4) the diagnosis of cavernous hemangioma and hepatic cysts was confirmed by clinical data, ultrasonography, CT or MRI or both, and follow-up observation.
Imaging protocol
MR examinations were performed using the Signa Excite Xl Twin Speed 1.5T system (GE Healthcare, Milwaukee, WI, USA) with a four-channel torso-array coil. Diffusion weighted single-shot echo-planar imaging was performed in individual patients using the following parameters: repetition time/echo time (TR/TE) = 6000/73.1 ms, 7-8 mm thickness, water selective excitation for fat suppression, matrix size = 128 × 128, field of view = 36 cm × 36 cm, number of excitations = 6.0, slice thickness/gap = 8 mm/0 mm, 20 axial slices, scan time = 2 min 24 s, b value = 0 and 1000 s/mm2, under free breathing. A parallel imaging technique, array spatial sensitivity encoding technique (ASSET) was used. Motion-probing gradient pulses were placed along three orthogonal oblique directions. Additional post-contrast-enhanced DW images were obtained after intravenous administration of SPIO (Resovist; Bayer Schering Pharma AG, Berlin, Germany). The dose of SPIO was 0.016 mL/kg, corresponding to 0.45 mg/kg of Fe.
Contrast enhanced CT was conducted using the LightSpeed ultra 16-MDCT scanner (GE Healthcare, Milwaukee, WI, USA) with pre- and postcontrast triple-phase (arterial, portal venous, and equilibrium phase) scans after injection of 80 to 100 mL of Iopamidol (Iopamiro; Bayer Schering Pharma AG, Berlin, Germany) at an injection rate of 1.5 to 3.0 mL/s.
Visualization of lesions, relative contrast ratio between the lesion and surrounding liver parenchyma (RCR), and ADC values were compared between benign and malignant lesions on DWI. Analysis and measurements of DWI data was performed using the GE FUNCTOOL software. All regions of interest (ROI) were created as large as possible in each lesion. In cases with multiple lesions, only the most conspicuous lesion was selected for quantitative measurements. If different types of lesions were mixed (for example, hepatocellular carcinomas and cysts), each lesion was measured.
Visualization of lesions
All but two lesions (CT examinations were not performed in one case of metastatic liver cancer and hemangioma.) were evaluated. Two diagnostic doctors performed a visual evaluation of each selected CT image (best phase for CT examinations in individual lesions) and DWI (only before SPIO administration) based on mutual agreement. They classified the visualization of lesions on CT and DWI into three categories according to the following criteria. Grade 1: no or slight visualization and unclear margin. Grade 2: moderate visualization and clear margin. Grade 3: marked visualization and very clear margin.
RCR measurements
SPIO was administered to 59 patients (18 HCC, 26 metastatic liver cancers, 10 hemangiomas, seven cysts) and the RCR was compared before and after SPIO administration to differentiate between benign and malignant lesions. The RCR was calculated by the following equation: RCR = SIlesion/SIliver, where SI is signal intensity of the lesion and SIliver was evaluated from hepatic tissue surrounding the lesion. In this paper, the conventional contrast-to-noise ratio analysis was not employed because the standard deviation of the background could not be measured easily in the images obtained using ASSET[11].
ADC measurements
ADCs of both focal hepatic lesions and surrounding liver parenchymas (26 HCC, 32 metastatic liver cancers, four cholangiocarcinomas, 10 hemangiomas, eight cysts) were measured in 65 patients. Lesions in the lateral segments were excluded for ADC measurement because their ADC could not be correctly measured in the DWI sequences without simultaneous cardiac gating[12].
Statistical analysis
ADC and SI were measured twice and averaged. All data are expressed as mean ± SD. The visualization data was statistically analyzed by Wilcoxon’s sighed rank test, and RCR and ADC were analyzed by Student’s t test using Prism 4.0 software (GraphPad Software, Inc., San Diego, CA, USA). A P value of < 0.05 was considered statistically significant.
RESULTS
Visualization of lesions
Most malignant lesions were significantly more clearly visualized on DWI than on CT (Table 1). In particular, multiple small focal hepatic lesions were visualized clearly on DWI (Figure 1). Two malignant lesions were not detected on DWI, even though they could be easily detected on enhanced CT. These lesions were HCC just under the diaphragm of the lateral segment with liver cirrhosis. Benign lesions, by contrast, were significantly more poorly visualized on DWI than on CT. Similar to the results of several articles published previously[13], cysts showed low or no signal intensities in six of eight cases on DWI (in these cases, no signal intensity means lower signal intensities than background signal intensities.). DWI visualized most hemangiomas; however, CT was better than DWI for the visualization of hemangiomas.
Table 1.
Visual evaluation of focal hepatic lesions
| Lesions | Modalities | Grade 1 | Grade 2 | Grade 3 |
| Metaa (n = 33) | CT | 9 | 17 | 7 |
| DWI | 1 | 8 | 24 | |
| HCCa (n = 29) | CT | 4 | 19 | 6 |
| DWI | 6 | 11 | 12 | |
| Cholangiocarcinoma (n = 4) | CT | 1 | 1 | 2 |
| DWI | 0 | 0 | 4 | |
| Cysta (n = 8) | CT | 0 | 1 | 7 |
| DWI | 8 | 0 | 0 | |
| Hemangiomaa (n = 9) | CT | 0 | 8 | 1 |
| DWI | 5 | 2 | 2 |
Meta: Metastatic liver cancer; HCC: Hepatocellular carcinoma; DWI: Diffusion-weighted imaging; CT: Computed tomography.
P < 0.05.
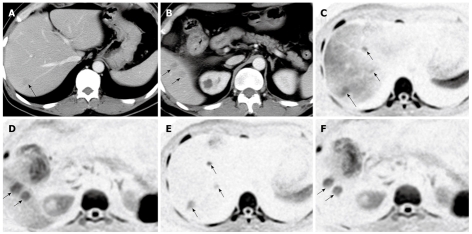
Figure 1.
A case of hepatic metastases of colon cancer. A, B: Dynamic computed tomography of the liver in the portal phase showing multiple metastatic lesions, which are indicated as low-density masses (arrows); C, D: Diffusion-weighted imaging (DWI) of the same locations as in (A) and (B), clearly showing metastatic lesions as high signal intensities; E, F: After SPIO administration, the background signal intensity of the liver parenchyma was reduced and the signal intensities of the metastatic lesions were seen more clearly.
RCR measurements
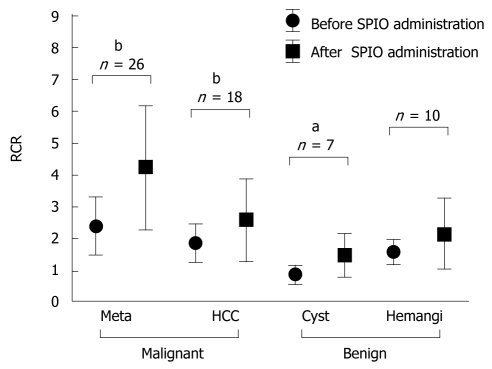
The average RCR on DWI before and after SPIO administration is shown in Figure 2. RCR seemed to be significantly improved after SPIO administration in malignant lesions that were metastatic liver cancers (before: 2.39 ± 1.28, after: 4.23 ± 1.34, n = 26) and HCC (before: 1.85 ± 0.58, after: 2.59 ± 1.28, n = 18). On the other hand, the RCR was not significantly improved in benign lesions after SPIO administration. The RCR of cysts was 0.86 ± 0.30 (n = 7) before SPIO administration, because the SI of cysts was lower than background SI on DWI. Therefore, the RCR of cysts increased after SPIO administration (1.48 ± 0.65) as a result of a decrease in the SI of the liver. The RCR of hemangiomas was 1.58 ± 0.38 (n = 10) before and 2.15 ± 1.10 after SPIO administration. This was not statistically significant. The RCRs of hemangiomas were reduced in three lesions after SPIO administration. One example is shown in Figure 3.
Figure 2.
Relative contrast ratio (RCR) of focal hepatic lesions visualized by diffusion-weighted imaging (DWI) before and after the administration of superparamagnetic iron oxide (SPIO). Meta: Metastatic liver cancer; HCC: Hepatocellular carcinoma; hemangi: Hepatic hemangioma. aP < 0.05, bP < 0.01.
Figure 3.
A case of hepatic hemangioma. A: Dynamic computed tomography in the portal phase showing a low-density mass with a marginal stain at the S7 lobe (arrow). This is a typical staining pattern for hemangiomas; B: The hemangioma (arrow) expressed high signal intensity on diffusion-weighted imaging; C: The intensity of this signal was reduced after administration of superparamagnetic iron oxide (arrow); D: The hemangioma showed a high apparent diffusion coefficient (ADC) value on the ADC map (arrow).
Figure 4 is a case in which multiple small cysts and metastatic tumors of colon cancer were colocalized in the liver. Both cysts and cancers expressed high signal intensities in T2-weighted MR imaging. In contrast, only the metastatic nodules were expressed on DWI after SPIO administration.
Figure 4.
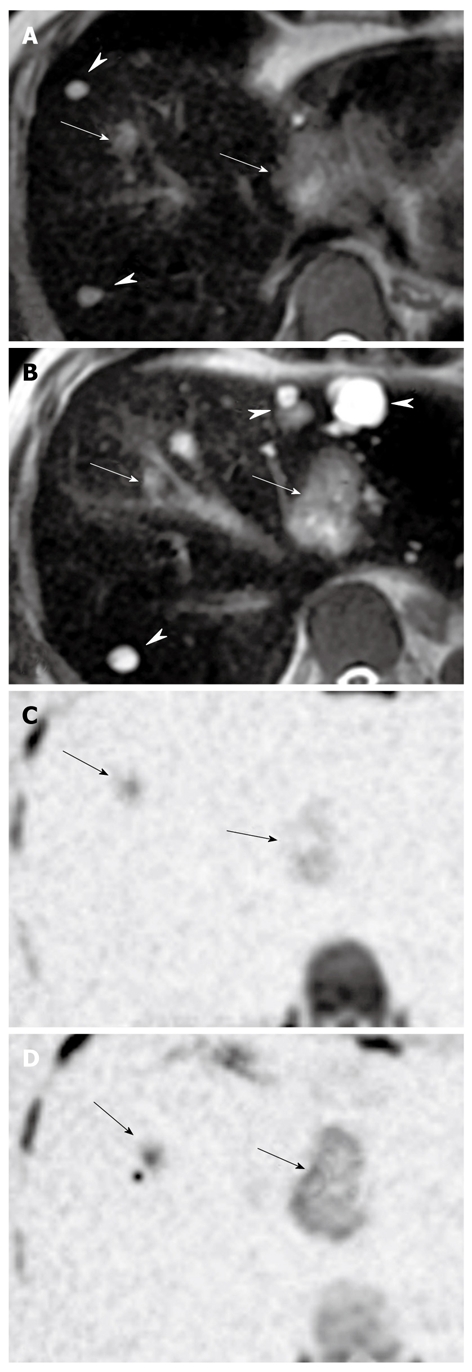
A case of multiple hepatic metastases of colon cancer with multiple hepatic cysts. A, B: T2-weighted magnetic resonance imaging showing high signal intensities on both metastatic lesions (arrows) and cysts (arrow heads); C, D: Diffusion-weighted imaging after administration of superparamagnetic iron oxide showing high signal intensities on metastatic lesions only (arrows).
ADC measurements
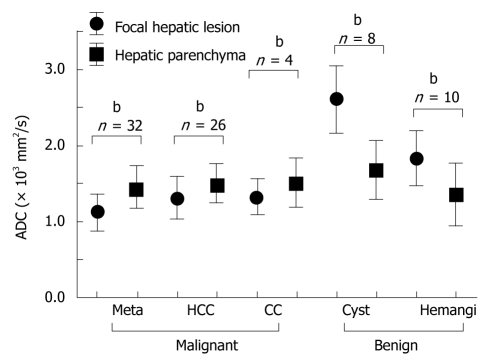
Generally speaking, the lesions showing high signal intensity on DWI demonstrate low ADCs. The average ADCs in our study are shown in Figure 5. The ADCs (mean ± SD × 10-3 mm2/s) of malignant lesions, both HCC (1.31 ± 0.28, n = 26) and metastatic liver cancer (1.11 ± 0.22, n = 32), were significantly lower than the ADCs of the surrounding hepatic tissues. The ADC of cholangiocarcinomas was 1.33 ± 0.23 (n = 4). Although this was lower than the ADC of the surrounding hepatic tissue, the difference was not significant.
Figure 5.
Comparison of apparent diffusion coefficient (ADC) between focal hepatic lesions and surrounding hepatic parenchyma. Meta: Metastatic liver cancer; HCC: Hepatocellular carcinoma; CC: Cholangiocarcinoma; Hemangi: Hepatic hemangioma. bP < 0.01.
In benign lesions, the ADCs of both cysts (2.61 ± 0.45, n = 8) and hemangiomas (1.84 ± 0.37, n = 10) were significantly higher than the ADC of the surrounding hepatic tissue. A representative hemangioma is shown in Figure 3, which expressed high signal intensity and high RCR on DWI. The ADC of the hemangioma was high even though their signal intensity was higher than the surrounding hepatic tissue on DWI. A representative hepatic cyst case is shown in Figure 6. The cyst expressed nearly no signal intensity on DWI after SPIO administration. However, the cyst revealed a higher ADC value than the surrounding hepatic tissue on the ADC map.
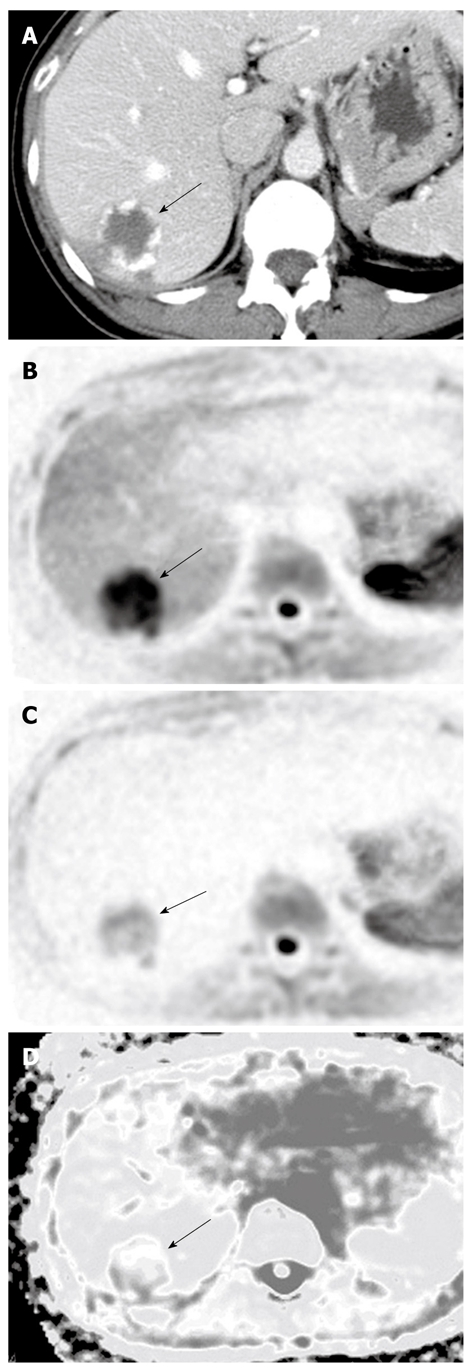
Figure 6.
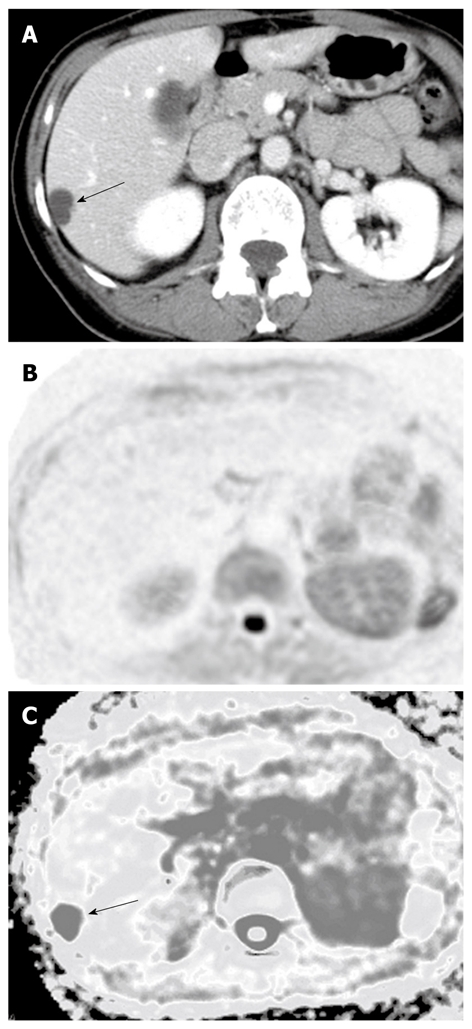
A case of hepatic cysts. A: Dynamic computed tomography in the portal phase showing the hepatic cyst as a low-density lesion (arrow); B: The lesion expressed no signal intensity on diffusion-weighted imaging; C: The lesion showed a high apparent diffusion coefficient (ADC) on the ADC map (arrow).
DISCUSSION
Surgeons need new imaging modalities that can precisely and concisely evaluate malignant lesions. DWI has recently emerged as a tool for detecting cancers in the abdominal organ field[5,13,14]. Positron emission imaging is currently used as a powerful screening tool for malignancy[15]. Some articles have reported that SPIO-enhanced T2-weighted MR imaging and CT during arterioportography have the best ability to diagnose metastatic liver cancer[8,9,16]. However, even with use of these modalities, differentiating between malignant tumors and intrahepatic vascular structures (e.g. arterioportal shunts and thin vessels) or benign lesions (e.g. small cysts and hemangiomas) is sometimes difficult. One of the purposes of the current study was to evaluate the potential of DWI for differentiating malignant from benign lesions.
Nasu et al[17] reported the sensitivity of DWI for detecting small metastases to be higher than that of SPIO-enhanced T2-weighted MR imaging because metastatic tumors tended to appear larger on DWI than on T2-weighted images, and intrahepatic vascular signals were suppressed on DWI. Parikh et al[18] also reported that DWI was better than standard breath-hold T2-weighted imaging for the detection of focal hepatic lesions. In particular, they found much higher contrast between HCC and cirrhotic liver on DWI than on T2-weighted images. The contrasts between HCC and cirrhotic liver on DWI in the present study, however, were not higher than that between metastatic liver cancer and surrounding hepatic tissue. This finding might have been because the signal intensity of HCC was not so high as metastatic tumors and the signal intensity of the cirrhotic liver was irregularly increased in our study (data not shown), probably because of marked liver fibrosis, as previously noted[19-21]. High signal intensities were found in most malignant focal hepatic lesions and DWI was useful for identifying them, as it was in previous studies[17,18].
However, the findings of benign lesions differed from malignant lesions. Cysts and hemangiomas are most frequently found in the benign lesions of the liver. In the present study, the cysts had low or no signal intensities and hemangiomas had high or low signal intensities, similar to previous studies[22]. Such lesions are usually diagnosed using major imaging modalities, such as ultrasonography and enhanced CT. However, because these lesions are small and multiple, differential diagnosis against malignant focal hepatic lesions is often difficult, even using these modalities. The characteristics of cysts on DWI, however, in which the cysts express low or no signal intensities, can enable differential diagnosis against malignant focal hepatic lesions, as shown in Figure 4.
Administration of SPIO in the present study reduced the signal intensity of liver parenchyma on DWI and increased the RCR of malignant focal hepatic lesions, but did not often increased the RCR of hepatic hemangiomas. Therefore, SPIO was quite useful for differential diagnosis, especially between small metastatic liver cancers and hepatic hemangiomas.
We used a high b value (1000 s/mm2) for DWI because a high b value allows delineation of malignant tumors with excellent conspicuity owing to the generally suppressed background noise. Furthermore, the differences in the RCR between malignant and benign lesions were increased with a high b value. However, this characteristic of DWI occasionally hinders defining the anatomical location of the abnormal signal on DWI. Therefore, the location of the signal must be correlated with ordinary T2 images by making the slice thickness, interslice gap, and fields of view uniform among these images. Recent developments in fusion software can readily resolve this problem by anatomically overlapping DWI onto ordinary T2 images. Such fused images might allow improved detection of malignant tumors[22].
ADC is a quantitative parameter calculated from DWI. ADC combines the effects of capillary perfusion and water diffusion in the extracellular and extravascular space[1]. ADC is helpful for characterizing focal and diffuse diseases in the body. Several studies have reported that ADC can contribute to the differential diagnosis of benign and malignant focal lesions in the liver[7,18,23].
Our results also revealed the contribution of ADC to the characterization of focal hepatic lesions. The ADC of malignant lesions was significantly lower than that of the surrounding hepatic tissue, whereas the ADC of benign lesions was higher than that of the surrounding hepatic tissue. Benign lesions, hemangiomas, and cysts in particular had high ADCs, even if they had high signal intensities on conventional DWI. The signal intensity of DWI includes both restricted diffusion and the effect of tissues with high T2 relaxation times, which is called the “T2 shine-through” effect. This might be why hemangiomas and cysts sometimes have high signal intensities on DWI. In any case, those results will be useful for differential diagnosis of malignant and benign focal hepatic lesions. For large lesions, however, the ADC was often markedly inhomogeneous on the locations and slices in the lesion. ADCs also seem to differ according to the machine used, the set-up conditions of the machine, and each human body[24]. Thus, ADC must be carefully used in the evaluation of lesion characteristics.
A few malignant lesions could not be visualized by DWI in our study. These lesions were mainly located on the left lobe, just under the diaphragm. DWI is known to be extremely sensitive to motion. During clinical image interpretation of MRI, a signal drop is often encountered in the lateral segment of the liver, which is assumed to be due to cardiac pulsation. Not only the signal intensity of DWI, but also ADC, is thought to be incorrect for the lateral segment just under the diaphragm[12,23,25]. Further studies need to be conducted to better understand the characteristics of DWI.
This study had some limitations. First, the subjects were a heterogeneous group, and the number of cases was relatively small. Second, histological proofs were not completely obtained, especially for the benign lesions. Therefore, the sensitivities and specificities of DWI cannot be reported here. Third, placement of the region of interest cursor might not be objective enough for measuring ADC and RCR. Additionally, inhomogeneity of hepatic lesions and motion may have affected the accuracy of the calculations, because the DWI was acquired with free breathing[25].
Despite these limitations, we believe that DWI is a very useful imaging modality for identifying malignant focal lesions in the liver. Recently, a new MRI contrast medium, gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA), has been used for diagnosis of HCC. We plan to combine both Gd-EOB-DTPA and DWI for diagnosing focal hepatic lesions.
In conclusion, DWI is a simple and sensitive method for screening focal hepatic lesions. SPIO administration can effectively improve the RCR of metastatic liver cancers. ADC measurement is occasionally helpful for differential diagnosis of malignant and benign small focal hepatic lesions.
COMMENTS
Background
Screening, accurate detection and characterization of focal hepatic lesions are important for planning treatment of malignant hepatic lesions. Recently, some studies have reported that the apparent diffusion coefficient (ADC), which is one of calculated parameters of diffusion-weighted imaging (DWI), might be useful for differential diagnosis of benign and malignant lesions in the liver. Superparamagnetic iron oxide (SPIO) has been reported to further improve the performance of DWI of the liver because SPIO reduces the signal in normal liver parenchyma. This study aimed to evaluate the usefulness of DWI in screening and differential diagnosis of benign and malignant focal hepatic lesions.
Research frontiers
In some previous studies,the ADC of benign lesions, such as hepatic cysts and hemangiomas, was reported to be higher than that of malignant lesions, such as hepatocellular carcinoma and metastasis. However, ADC seems differ according to the machine used, the set-up conditions of the machine, and each human body. In this study, the authors compared ADC of focal hepatic lesions with its surrounding hepatic parenchyma for this differential diagnosis. Only a few studies have statistically evaluated the contrast changes in focal hepatic lesions after SPIO administration.
Innovations and breakthroughs
This is the first report indicating that these simple ADC and RCR patterns are useful for the differential diagnosis of benign and malignant focal hepatic lesions.
Applications
DWI can be used for differential diagnosis of focal hepatic lesions using calculation of ADC or RCR after SPIO administration. This is a simple and concise method that is easily available, not only to the radiologist, but also the physician and surgeon. DWI also should become a good diagnostic tool for other intra-abdominal organs.
Terminology
Diffusion is the thermally induced motion of water molecules, which is also referred to as Brownian motion. DWI is a new magnetic resonance imaging (MRI) technique that provides images of the diffusion in biological tissues. The ADC is a quantitative parameter calculated from DWI and it combines the effects of capillary perfusion and water diffusion in the extracellular extravascular space. SPIO is a liver-specific particulate MRI contrast agent that is taken up by the reticuloendothelial system of the liver and improves the focal hepatic lesion-to-liver contrast-to-noise ration and hepatic tumor detection.
Peer review
This is a retrospective study. The authors presented the results of their study and they reviewed the literature related with this subject. As a conclusion; the authors recommended that DWI by MR was a simple, sensitive and useful method for screening focal hepatic lesions and their differential diagnosis.
Footnotes
Peer reviewer: Dr. Serdar Karakose, Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Wang JL L- Editor Stewart GJ E- Editor Ma WH
References
- 1.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 2.Sorensen AG, Buonanno FS, Gonzalez RG, Schwamm LH, Lev MH, Huang-Hellinger FR, Reese TG, Weisskoff RM, Davis TL, Suwanwela N, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996;199:391–401. doi: 10.1148/radiology.199.2.8668784. [DOI] [PubMed] [Google Scholar]
- 3.Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology. 1992;42:1717–1723. doi: 10.1212/wnl.42.9.1717. [DOI] [PubMed] [Google Scholar]
- 4.Moseley ME, Kucharczyk J, Mintorovitch J, Cohen Y, Kurhanewicz J, Derugin N, Asgari H, Norman D. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol. 1990;11:423–429. [PMC free article] [PubMed] [Google Scholar]
- 5.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with single-shot echo-planar imaging in the upper abdomen: preliminary clinical experience in 61 patients. Abdom Imaging. 1999;24:456–461. doi: 10.1007/s002619900539. [DOI] [PubMed] [Google Scholar]
- 6.Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275–282. [PubMed] [Google Scholar]
- 7.Gourtsoyianni S, Papanikolaou N, Yarmenitis S, Maris T, Karantanas A, Gourtsoyiannis N. Respiratory gated diffusion-weighted imaging of the liver: value of apparent diffusion coefficient measurements in the differentiation between most commonly encountered benign and malignant focal liver lesions. Eur Radiol. 2008;18:486–492. doi: 10.1007/s00330-007-0798-4. [DOI] [PubMed] [Google Scholar]
- 8.Strotzer M, Gmeinwieser J, Schmidt J, Fellner C, Seitz J, Albrich H, Zirngibl H, Feuerbach S. Diagnosis of liver metastases from colorectal adenocarcinoma. Comparison of spiral-CTAP combined with intravenous contrast-enhanced spiral-CT and SPIO-enhanced MR combined with plain MR imaging. Acta Radiol. 1997;38:986–992. doi: 10.1080/02841859709172115. [DOI] [PubMed] [Google Scholar]
- 9.Vogl TJ, Schwarz W, Blume S, Pietsch M, Shamsi K, Franz M, Lobeck H, Balzer T, del Tredici K, Neuhaus P, et al. Preoperative evaluation of malignant liver tumors: comparison of unenhanced and SPIO (Resovist)-enhanced MR imaging with biphasic CTAP and intraoperative US. Eur Radiol. 2003;13:262–272. doi: 10.1007/s00330-002-1677-7. [DOI] [PubMed] [Google Scholar]
- 10.Naganawa S, Sato C, Nakamura T, Kumada H, Ishigaki T, Miura S, Maruyama K, Takizawa O. Diffusion-weighted images of the liver: comparison of tumor detection before and after contrast enhancement with superparamagnetic iron oxide. J Magn Reson Imaging. 2005;21:836–840. doi: 10.1002/jmri.20346. [DOI] [PubMed] [Google Scholar]
- 11.Kwiat D, Einav S, Navon G. A decoupled coil detector array for fast image acquisition in magnetic resonance imaging. Med Phys. 1991;18:251–265. doi: 10.1118/1.596723. [DOI] [PubMed] [Google Scholar]
- 12.Nasu K, Kuroki Y, Sekiguchi R, Kazama T, Nakajima H. Measurement of the apparent diffusion coefficient in the liver: is it a reliable index for hepatic disease diagnosis? Radiat Med. 2006;24:438–444. doi: 10.1007/s11604-006-0053-y. [DOI] [PubMed] [Google Scholar]
- 13.Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999;210:617–623. doi: 10.1148/radiology.210.3.r99fe17617. [DOI] [PubMed] [Google Scholar]
- 14.Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393–398. doi: 10.2214/ajr.173.2.10430143. [DOI] [PubMed] [Google Scholar]
- 15.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305–332. doi: 10.1148/radiol.2312021185. [DOI] [PubMed] [Google Scholar]
- 16.Kim YK, Kwak HS, Han YM, Kim CS. Usefulness of combining sequentially acquired gadobenate dimeglumine-enhanced magnetic resonance imaging and resovist-enhanced magnetic resonance imaging for the detection of hepatocellular carcinoma: comparison with computed tomography hepatic arteriography and computed tomography arterioportography using 16-slice multidetector computed tomography. J Comput Assist Tomogr. 2007;31:702–711. doi: 10.1097/RCT.0b013e31802d0b6b. [DOI] [PubMed] [Google Scholar]
- 17.Nasu K, Kuroki Y, Nawano S, Kuroki S, Tsukamoto T, Yamamoto S, Motoori K, Ueda T. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006;239:122–130. doi: 10.1148/radiol.2383041384. [DOI] [PubMed] [Google Scholar]
- 18.Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812–822. doi: 10.1148/radiol.2463070432. [DOI] [PubMed] [Google Scholar]
- 19.Lewin M, Poujol-Robert A, Boelle PY, Wendum D, Lasnier E, Viallon M, Guechot J, Hoeffel C, Arrive L, Tubiana JM, et al. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology. 2007;46:658–665. doi: 10.1002/hep.21747. [DOI] [PubMed] [Google Scholar]
- 20.Girometti R, Furlan A, Bazzocchi M, Soldano F, Isola M, Toniutto P, Bitetto D, Zuiani C. Diffusion-weighted MRI in evaluating liver fibrosis: a feasibility study in cirrhotic patients. Radiol Med. 2007;112:394–408. doi: 10.1007/s11547-007-0149-1. [DOI] [PubMed] [Google Scholar]
- 21.Taouli B, Tolia AJ, Losada M, Babb JS, Chan ES, Bannan MA, Tobias H. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007;189:799–806. doi: 10.2214/AJR.07.2086. [DOI] [PubMed] [Google Scholar]
- 22.Koyama T, Tamai K, Togashi K. Current status of body MR imaging: fast MR imaging and diffusion-weighted imaging. Int J Clin Oncol. 2006;11:278–285. doi: 10.1007/s10147-006-0605-2. [DOI] [PubMed] [Google Scholar]
- 23.Bruegel M, Holzapfel K, Gaa J, Woertler K, Waldt S, Kiefer B, Stemmer A, Ganter C, Rummeny EJ. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477–485. doi: 10.1007/s00330-007-0785-9. [DOI] [PubMed] [Google Scholar]
- 24.Kuroki Y, Nasu K, Nawano S, Sekiguchi R. Diffusion-weighted MR imaging of the liver. Nichidoku-iho. 2007;52:72–81. [Google Scholar]
- 25.Nasu K, Kuroki Y, Sekiguchi R, Nawano S. The effect of simultaneous use of respiratory triggering in diffusion-weighted imaging of the liver. Magn Reson Med Sci. 2006;5:129–136. doi: 10.2463/mrms.5.129. [DOI] [PubMed] [Google Scholar]