Abstract
The epidermal growth factor receptor (EGF-R) constitutes one of the most broadly targeted antigens in tumor therapy since it is commonly expressed on many epithelial cancers, as well as on glioblastomas. Both EGF-R-directed tyrosine kinase inhibitors and monoclonal antibodies have been approved, but clinical response rates are often limited. A more detailed understanding of the mechanisms underlying sensitivity or resistance against EGF-R inhibitors may assist in identifying patient populations who optimally benefit from currently available reagents. In addition, these insights may guide the development of more effective molecules. In this short review, we will summarize some of the current knowledge in this rapidly evolving field with particular emphasis on EGF-R-directed antibodies.
Key words: EGF-R, ADCC, TKI, antibody engineering, therapeutic antibodies
EGF-R as Target for Cancer Therapy
Scientific background and clinical status of EGF-R inhibitors.
The epidermal growth factor receptor (EGF-R) belongs to the family of ErbB molecules, which constitute type I transmembrane tyrosine kinases. Upon binding of ligand, the receptor undergoes a conformational change from a tethered to an un-tethered configuration, which allows homo- or heterodimerization with other members of the family,1 and leads to receptor activation and recruitment of downstream signaling molecules.2 Transgenic expression of mutated EGF-R in mice induced tumors that resembled their human counterparts in many respects.3,4 Overexpression or gene amplification of EGF-R is indeed found in many human tumor types.5 Results such as these have underscored the importance of EGF-R to tumorigenesis, and have lead to wide-spread study of EGF-R inhibitors as anti-cancer agents.
So far, two classes of EGF-R inhibitors have obtained regulatory approval for the treatment of cancer patients: orally available, small molecule tyrosine kinase inhibitors (TKI) and monoclonal antibodies (mAbs) (Tables 1 and 2).6 Many other molecules, and also different approaches (e.g., immunotoxins, vaccination), are under clinical evaluation. To date, TKI have been particularly effective in non small-cell lung cancer (NSCLC), but ineffective in colorectal cancer (CRC). On the other hand, EGF-R antibodies are approved for treatment of CRC, as well as head and neck cancer, but evidence for efficacy in NSCLC is just emerging. Interestingly, several studies to combine TKI with cytotoxic chemotherapy failed, while the approved mAb therapeutic cetuximab is regularly combined with chemo- or radiotherapy. Here, we focus on EGF-R antibodies cetuximab and panitumumab as treatments for NSCLC and CRC. More detailed discussion about the clinical status of EGF-R inhibitors can be found in the literature.7–9
Table 1.
EGF-R-directed antibodies in clinical development
| Antibody | Isotype | Type | Target | Epitope/Domain | Status |
| Panitumumab | IgG2 | Human | ErbB1 | III | Approved |
| Cetuximab | IgG1 | Chimeric | ErbB1 | III | Approved |
| Nimotuzumab | IgG1 | Humanized | ErbB1 | III | Approved |
| Zalutumumab | IgG1 | Human | ErbB1 | III | Phase II/III |
| IMC-11F8 | IgG1 | Human | ErbB1 | III | Phase I/II |
| Ch806 | IgG1 | Chimeric | Mutated ErbB1 | II/III | Phase I |
| Matuzumab | IgG1 | Humanized | ErbB1 | III | Terminated |
Table 2.
Tyrosine kinase inhibitors targeting the EGF-R pathway
| Drug name | Target | Status |
| Gefitinib | EGF-R | Approved |
| Erlotinob | EGF-R | Approved |
| Vandetanib | EGF-R, VEGFR-2, VEGFR-3, RET | Phase III |
| BMS-690514 | Pan-HER; VEGFR-2 | Phase II |
| XL647 | VEGFR-2, EGF-R, ERbB-2, EPHB4 | Phase I/II |
| AEE-788 | EGF-R, ErbB-2, VEGFR-2 | Phase I |
| PKI-166 | EGF-R, ErbB-2 | Phase I |
RET, Rearranged during transfection; EPHB4, ephrin receptor B4.
Predictors of Response or Resistance to EGF-R-Directed Therapies
The concept of personalized medicine is based on the identification of patient-specific tumor characteristics that can be targeted by highly selective drugs.10 Considering side effects and the high costs of these targeted therapies, the identification of markers that predict response or resistance to these novel reagents is crucial. Typically, prognostic and predictive markers are distinguished. According to this concept, prognostic markers should relate to intrinsic characteristics of the tumor and should be independent from specific therapy. In contrast, predictive markers are defined for a specific type of therapy, and may vary depending on the therapy under evaluation. In clinical practice, distinguishing these two types of markers may be difficult and identification and verification of predictive markers constitutes a considerable task.11
Although the need to identify biomarkers for EGF-R-directed therapy is widely acknowledged, there is considerable discord among studies regarding the suitability of individual markers (see below). Discrepancies between different studies may, in part, be explained by variations in assays and technologies, which make comparisons difficult. In the future, these problems may be overcome by standardization of biomarker.12
Clinical Parameters
Clinical markers for response to tumor therapy typically include parameters like tumor stage and grading, histology, gender, ethnicity, co-morbidities and performance status. The impact of these parameters for conventional tumor therapy is well established. Analyses of early trials with EGF-R-directed TKI indicated that Asian origin, female gender, non-smoker status and adenocarcinoma/bronchoalveolar carcinoma (BAC) histology were strong predictors for response to these molecules in NSCLC.13 Interestingly, selected patients with mutated EGF-R responded to gefitinib, even if they had a low performance status that rendered them poor candidates for conventional chemotherapy.14 This latter observation suggested that conventional parameters for response prediction may not apply for targeted therapies.
Both EGF-R-directed TKI and antibodies induce a typical “acneiform” skin rash, but several other skin reactions are also commonly observed. These dermatologic side effects negatively affect the “quality of life” of patients treated with EGF-R inhibitors. On the other hand, their occurrence has repeatedly been reported to correlate with better response rates (reviewed in ref. 15). Interestingly, nimotuzumab, an EGF-R antibody against a specific EGF-R epitope,16 appeared not to trigger this type of toxicity.
Immune Activation Markers.FcγR Polymorphisms
Contribution of the immune system to the efficacy of several mAb therapeutics has been suggested by studies in mice lacking activating Fcγ receptors.17 Further analyses of the underlying mechanisms revealed macrophages to be the relevant effector cell population for B cell depletion after anti-CD20 therapy.18 Interestingly, similar studies in animals have not been reported for EGF-R antibodies. Clinical evidence for the contribution of the immune system is derived from studies that correlated Fcγ receptor polymorphisms with the therapeutic outcome of antibody therapy. For the EGF-R antibody cetuximab, two studies reported associations between the expression of certain Fcγ receptor allo-forms and therapeutic outcome. In one of these studies, cetuximab was reported to have superior activity in patients carrying FcγRIIa-131H or FcγRIIIa-158V alleles, a result that has previously been reported for other therapeutic antibodies.19 Similar correlations for FcγRIIa, but surprisingly different results with respect to FcγRIIIa, were reported in a second study.20 Since FcγRIIa is predominantly expressed by myeloid effector cells, i.e., monocytes, polymorphonuclear leukocytes (PMN), and natural killer (NK) cells predominantly express FcγRIIIa, these results may suggest that both NK cells and myeloid cells serve as effectors for EGF-R antibodies. Alternatively, Fcγ receptor alleles may be genetically linked, an issue that is controversially discussed.21,22 Importantly, panitumumab, a human IgG2 antibody that has been considered not to mediate antibody-dependent cellular cytotoxicity (ADCC), also effectively triggered cytotoxicity by myeloid, but not by NK cells.23 Studies on the impact of FcγR polymorphisms for the outcome of panitumumab therapy have not been reported.
Tumor Biomarkers
Tumor biomarkers may assist in the selection of patients who will maximally benefit from appropriate targeted therapies, and identification of relevant effector mechanisms of these agents. Biomarkers may also provide insight into the pathophysiology of the treated tumors. Since most of these markers need to be evaluated directly in tumor cells, sufficient amounts of freshly biopsied or archived tumor material are required for these analyses. This imposes a significant challenge for many retrospective studies because often only relatively small sample numbers can be analyzed, which may lead to statistical problems with the data.12 If clinical decisions depend on the results of these analyses, then turn-around times become a critical issue as well, since many of the presenting patients require timely treatment. The development of novel reagents—such as mutation-specific EGF-R antibodies,24 or novel technologies—like determination of EGF-R mutations in circulating tumor cells,25 may assist in overcoming these practical problems. Recent results for some of these biomarkers are discussed in the following sections.
Immunohistochemistry and fluorescence in situ hybridization.
An important question is whether EGF-R gene or protein expression levels are correlated with response to EGF-R inhibitors. Two methods are commonly used to evaluate expression levels: immunohistochemistry (IHC), which measures EGF-R protein expression in tumor tissue, and fluorescence in situ hybridization (FISH), which analyses EGF-R gene amplification by measuring gene copy numbers in situ. Both methods are technically demanding and difficult to standardize between various laboratories.12 These technical obstacles may in part explain discrepancies between results from different studies designed to address similar questions. Alternatively, EGF-R downmodulation by EGF-R ligands may represent another explanation.26
In contrast to trastuzumab therapy, where increased HER-2/neu expression levels and amplified gene copy numbers were correlated with increased treatment benefit,27 study results for EGF-R-directed therapy have been more controversial.
While some studies using IHC for analysis reported an impact,13 other studies did not observe similar correlations (reviewed in ref. 12). As analyzed by IHC, colorectal cancer patients apparently even responded to cetuximab in the absence of EGF-R expression.28 If these unexpected results were not explained by technical problems of IHC or sampling artefacts within different tumor areas, then they may suggest that targeting EGF-R on non-tumor cells may represent a novel “non-tumor-related” mechanism of action for EGF-R antibodies. For example, cetuximab-mediated suppression of endogenously produced vascular endothelial growth factor (VEGF) levels has been proposed.29 Alternatively, IHC may be too insensitive to detect low EGF-R expression levels that are already sufficient to trigger ADCC.
In lung cancer patients, EGF-R gene amplification analyzed by FISH appeared to be a predictor for response to TKI13 or EGF-R antibody therapy.30 Similarly, EGF-R gene amplification was associated with an improved response rate to cetuximab31 or panitumumab in colorectal cancer patients.32 However, conflicting results have also been reported in both tumor types, and the relevance of FISH for predicting response to EGF-R inhibitors has not been clearly established.33,34
Since both EGF-R-directed TKI and antibodies target the EGF-R protein, it remains difficult to understand why gene amplification, but not protein expression levels should predict responses to EGF-R inhibitors. The discrepancies in study results may again suggest methodological problems. Furthermore, animal studies demonstrated that tumor load and EGF-R expression levels may significantly affect the pharmacokinetics of EGF-R antibodies.35 These observations may have important impact for clinical dosing schedules.
EGF-R kinase mutations.
EGF-R sequencing from lung cancer samples identified the occurrence of somatic mutations in the kinase domain of EGF-R.36–38 These mutations, which were most commonly exon 19 deletions (E746_A750del) or exon 21 point mutations (L858R), increased the affinity of EGF-R for ATP, and thereby significantly altered the biology of mutated compared to wild-type receptors.39 For example, mutated, but not wild-type, EGF-R triggered ligand-independent cell proliferation. Importantly, kinase mutated receptors were significantly more sensitive to EGF-R-directed TKI like gefitinib and erlotinib than wildtype EGF-R. Furthermore, site-directed expression of mutated EGF-R in transgenic mice induced murine tumors that resembled human lung cancer, while wildtype EGF-R was not oncogenic under these conditions.3,4 In vivo, these differences translated into improved responses to TKI in animal models of EGF-R mutated compared to wild-type tumors. Together, these studies suggested that mutated EGF-R may be an “oncogenic driver”, while wild-type EGF-R appeared to rather act as a “passenger” under these conditions.40 Interestingly, the mutational status of EGF-R did not affect effector mechanisms of EGF-R antibodies in vitro41 or in animal models.42
Clinically, EGF-R kinase mutations have been demonstrated to be strong predictors for response to therapy with EGF-R-directed TKI.43 Interestingly, exon 19 deletions were associated with better clinical outcomes compared with L858R mutations,44 as suggested by their respective sensitivity against TKI in vitro.41 These mutations have been found in lung cancer patients, but were very rare in colorectal or other EGF-R expressing tumor types. In lung cancer patients, mutations are more prevalent in Asian compared to non-Asian populations, in non-smokers, in females and in tumors with adenocarcinoma or BAC histology. Retrospective analyses of large TKI trials in lung cancer patients revealed a significant positive correlation between the presence of these mutations and response to TKI therapy.45 This correlation was stronger for gefitinib than for erlotinib,46,47 which may be explained by different dosing schedules and sensitivities of the different receptors for the two drugs. In fact, remarkable response rates in the range of 50–80% have been reported from prospective trials with EGF-R-directed TKI in EGF-R-mutated lung cancer patients.48
However, as expected from clinical experience with TKI in other tumor entities, these responses were not durable.49 After a median of 12 months, patients typically develop secondary resistance to currently approved EGF-R-directed TKI.50 As a common mechanism of resistance, a secondary EGF-R kinase mutation (T790M) that inhibited TKI binding to the active site of the enzyme analogous to mutations in other kinases was described.51 In addition, amplification of MET with subsequent recruitment of ErbB3 signaling has frequently been identified.52 Together, T790M and MET amplification account for 60–70% of tumors with secondary TKI resistance. In addition, alterations in IGF-R signaling have been observed to mediate TKI resistance in cell lines.53
In comparison to TKI, experience with EGF-R antibodies in lung cancer is more limited.54 To date, the largest trial of EGF-R antibodies in NSCLC patients, the ‘First-line in lung cancer with Erbitux’ (FLEX) study, demonstrated a significant advantage in overall survival for patients receiving cetuximab in combination with chemotherapy compared to patients receiving chemotherapy alone.55 However, no biomarkers that would predict resistance or sensitivity to cetuximab have been identified from this study.56 In another large lung cancer study, neither EGF-R kinase mutations, nor EGF-R IHC or FISH were predictive for response to cetuximab therapy.57
KRAS mutations.
Ras molecules belong to the family of small GTPases that are critically involved in cell signaling and activation.58 Among the different Ras molecules, the KRAS4b isoform commonly carries activating exon 12 or 13 mutations in solid tumors. These mutations lead to receptor-independent activation of downstream signaling events.59
Activating KRAS mutations were reported in 30–40% of colorectal cancers. A definite impact of KRAS mutations as prognostic factor—independent of treatment—has not been convincingly demonstrated.60 Importantly, several large studies in colorectal cancer patients reported that the presence of somatic KRAS mutations was correlated with a lack of benefit from EGF-R antibody therapy;61–63 some investigators even suggested a negative impact of EGF-R inhibitors in these patients.64 These observations lead to the recommendation that patients with KRAS-mutated colorectal cancers should not be treated with EGF-R antibodies because these patients were considered unlikely to derive benefit from this treatment.65
In lung cancer patients, KRAS mutations are less common, and have been associated with resistance to EGF-R-directed TKI.66,67 However, it is interesting to note that response rates to the EGF-R antibody cetuximab were not affected by the absence or presence of KRAS mutations.56,57 These discrepancies between results for TKI and antibody therapies in lung cancer suggest that antibodies may recruit additional effector mechanisms compared to TKI. Differences between colorectal and lung cancers in response to EGF-R antibodies could suggest distinct functions of KRAS in both tumor types, or different modes of action of EGF-R antibodies depending on the tumor site.
Other signaling molecules.
Mutations in other downstream signaling molecules were expected to lead to similar phenotypes as KRAS mutations. For example, mutations in BRAF were reported to be associated with resistance to cetuximab or panitumumab therapy in colorectal cancer patients.68 This clinical study was supported by experimental evidence demonstrating that introduction of the typical V600E BRAF mutation rendered EGF-R expressing DiFi cells less sensitive to EGF-R antibodies. Interestingly, KRAS and BRAF mutations appeared to be mutually exclusive in this patient population.
Conflicting results have been reported concerning the impact of PIK3CA on resistance to EGF-R antibodies. While one study reported that the presence of PIK3CA mutations was correlated with a lack of response to cetuximab,69 a larger study did not confirm this observation.70 The activity of PI3KCA is inhibited by phosphatase and tensin homolog (PTEN). Thus, loss of PTEN expression leads to continuous activation of PI3K. In glioblastomas, loss of PTEN expression has been reported to mediate resistance to EGF-R-directed TKI.71 Similar results have been reported for lung cancer,72 and for cetuximab therapy in colon cancer.73
EGF-R ligands.
Human EGF-R binds at least seven different ligands, EGF, transforming growth factor, amphiregulin, epiregulin, betacellulin, epigen and heparin-binding epidermal growth factor-like growth factor, that are proteolytically cleaved from membrane-expressed precursors.74 Several studies addressed the impact of individual ligands or their respective sheddases under physiological conditions75 or for tumor development.76 For example, amphiregulin appeared to be particularly relevant for enhancing resistance to nematodes in the gut.77
Interestingly, higher levels of mRNA expression for amphiregulin and epiregulin were correlated with improved response rates to cetuximab in colorectal cancer patients.26 These provoking results have recently been confirmed by similar studies.57,73 Together, these observations may suggest that EGF-R ligand expression may play a pathophysiological role in the progression of a subgroup of colorectal cancers, which could be successfully inhibited by EGF-R blockade.
Approaches to Enhance the Efficacy of EGF-R Antibodies
In the future, a more detailed understanding of the mechanisms underlying sensitivity and resistance against EGF-R inhibitors should assist in identifying patient populations who optimally benefit from currently available reagents. In addition, these insights may guide the rational development of more effective molecules such as novel TKI or antibodies with enhanced effector functions. Potential strategies are discussed in the following paragraphs.
Patient Selection
Results from clinical trials with EGF-R-directed TKI in NSCLC constitute a classic example of biomarker-guided personalized medicine: while an unselected population of patients derived minimal benefit from TKI compared to best supportive care,46 TKI induced significant response rates if patients were selected for the presence of EGF-R mutations.48 Interestingly, patients with mutated EGF-R responded to gefitinib even if they had a low performance status that rendered them poor candidates for conventional chemotherapy.14 So far, no tumor biomarkers have been identified to predict response to cetuximab in NSCLC patients.56,57 Importantly, response to cetuximab in NSCLC was not negatively affected by the presence of KRAS mutations, which predict primary resistance against EGF-R-directed TKI. Furthermore, in vitro41 and in vivo42 studies demonstrated that EGF-R antibodies were effective against tumor cells carrying the T790M mutation, which confers secondary TKI resistance. Thus, patients unsuitable for TKI may be candidates for EGF-R antibodies. In particular, patients developing secondary resistance during TKI therapy appear to be promising candidates for EGF-R antibodies, since these tumors should be driven by EGF-R.
In colorectal cancer, the clinical relevance of KRAS mutations was demonstrated in large retrospective studies investigating the response to cetuximab or panitumumab therapy.61–64 Similarly, evidence for the predictive value of EGF-R ligand expression26,57,73 or Fcγ receptor polymorphisms19,20 was derived from retrospective analyses. Future prospective studies need to further address the predictive value of these biomarkers. With respect to the underlying effector mechanisms of EGF-R antibodies, it will be interesting to analyze whether patients with favorable Fcγ receptor allotypes (FcγRIIIa-158V/V and/or FcγRIIa-158H/H) will respond to cetuximab even if their tumors carry mutated KRAS, as indicated by results from individual patients.19 In addition, other biomarkers, such as EGF-R ligands, PIK3CA mutations or loss of PTEN, may become relevant in the future if their impact on EGF-R-directed therapy is validated.
Combinations with Other Agents
Combining individually effective drugs together is a standard approach in oncology, and has considerably improved response rates in many tumors. Interestingly, cetuximab can be combined with chemo- or radiotherapy without causing unexpected toxicities.8 However, combining EGF-R-directed TKI with chemotherapy did not further improve treatment results in NSCLC patients.8 Negative results from at least four different trials were unexpected since preclinical studies suggested synergy between TKI and chemotherapy.
Targeting Other Pathways
During the last few years, it has became evident that networks of interconnected, and in some cases redundant, signal transduction pathways are responsible for maintaining many solid tumors. Dual inhibition of VEGF and EGF-R may be especially important for optimal inhibition of tumor growth because these pathways are often linked in malignantly transformed cells.78 For example, activation of EGF-R can increase the production of VEGF in human cancer cells. Conversely, VEGF expression was decreased after inhibition of EGF-R signaling pathways. Furthermore, cetuximab in combination with VEGF-R-2 antibodies was more effective compared to single agent treatment in xenograft tumor models.79 Based on promising preclinical and early clinical data, a large randomized phase III trial tested the combination of chemotherapy and bevacizumab with or without cetuximab.80 Unexpectedly, addition of cetuximab decreased the progression-free survival in this study,81 which again demonstrated that preclinical and early clinical studies may be misleading concerning the benefit of combinations. Nevertheless, reasonable novel combinations include EGF-R inhibitors and therapeutic agents targeting c-MET- or ErbB-3 receptors, but inhibitors of these latter receptors are currently in more experimental stages of development.
Enhancing Antibodies' Effector Functions
Antibodies' effector functions can be divided into those that are mediated by binding of the F(ab) regions to their respective target antigen, and those that are recruited by the Fc region. For the purpose of the discussion here, we refer to the former mechanisms as direct, and Fc-mediated functions as indirect mechanisms of action. While direct effector functions of antibodies are predominantly determined by the fine epitope of specific antibodies, indirect effector functions are amenable to antibody Fc engineering.82 The clinical observation that EGF-R antibodies of human IgG1 (cetuximab) and IgG2 (panitumumab) isotype were effective in CRC was interpreted to suggest that Fc-independent effector mechanisms were particularly relevant because human IgG2 is a poor activator of complement, and does not bind to the FcγRIIIa receptor. However, human IgG2 effectively binds to FcγRIIa, and recruits myeloid cells (monocytes and PMN) for ADCC.23 Thus, the relative contribution of these two types of mechanisms to the efficacy of EGF-R antibodies is unknown, and may also depend on variables such as tumor type, location, Fcγ receptor polymorphisms, recruitment of immune effector cells to the tumor site or other undefined genetic alterations. Considering the high incidence of EGF-R expressing tumors and the often limited therapeutic options for these patients, EGF-R appears to be a particularly interesting target antigen for exploring optimized antibodies in clinical studies.
Enhancing direct effector functions.
Currently approved EGF-R antibodies and many of those in clinical development have been selected for blocking ligand binding to the receptor. Thus, cetuximab, panitumumab, and zalutumumab completely block EGF binding, while matuzumab and nimotuzumab demonstrate partial receptor blockade. Thus, it may not be surprising that these EGF-R antibodies are all directed against domain III, the ligand-binding region of EGF-R. Interestingly, structural analyses revealed that cetuximab, panitumumab and zalutumumab bind to distinct, but overlapping epitopes,83,84 while the binding of matuzumab and nimotuzumab is distinct from the former group.16,85 Despite overlapping epitopes, zalutumumab differed from cetuximab and panitumumab in the recruitment of direct effector functions.23,86
Interestingly, the therapeutic antibodies trastuzumab and pertuzumab bind to domains IV or II of ErbB-2, respectively.84 Based on the structural homology between EGF-R and ErbB-2, which are both members of the ErbB family, targeting other domains of EGF-R might allow isolation of EGF-R antibodies with interesting new mechanisms of action. These might include prevention of homo- or heterodimerization with ErbB family members, or inhibition of interaction with other membrane molecules interacting with EGF-R. As an example of the latter, the extracellular domain of EGF-R was demonstrated to interact and stabilize membrane expression of the sodium/glucose co-transporter SGLT1, which maintains intracellular glucose levels and thereby prevents autophagic tumor cell death.87
Besides targeting antibodies to distinct epitopes on wild-type receptor that confer novel mechanisms of action, targeting mutant forms of EGF-R represents an interesting approach. This would allow more tumor-specific targeting, the ultimate goal of targeted therapies. The type III EGF-R deletion-mutant (EGF-RvIII) is the most common variant, and was first identified in primary human glioblastomas.88 EGF-RvIII has an in-frame deletion of 801 base pairs, corresponding to exons 2–7 in the mRNA, resulting in the deletion of amino acids 6–273 in the extracellular domain and the introduction of a glycine at the fusion point. This new tumor-specific epitope is situated near the amino terminus of the receptor extracellular domain.89 Several EGF-RvIII-specific antibodies have been reported, and these demonstrated potent anti-tumor activity in vitro and in animal models.89 Promising results from a phase I trial with chimeric 806, an antibody recognizing EGF-RvIII and overexpressed wild-type receptor, have been reported.90
Enhancing indirect effector functions.
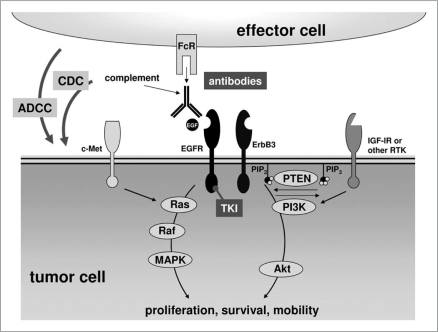
Lack of response to EGF-R targeting agents in a substantial proportion of patients can be attributed to several general mechanisms: occurrence of specific EGF-R mutations (e.g., T790M), constitutive activation of downstream mediators in the EGF-R pathway (e.g., KRAS or BRAF mutations, loss of PTEN), or activation of alternative tyrosine kinases that bypass or crosstalk with the EGF-R pathway (e.g., ErbB-3, c-MET, IGF-1R). Detailed analyses of the “mutational status” for each patient together with knowledge from clinical experience might allow identification of patients who could optimally benefit from EGF-R antibodies. Alternatively, EGF-R antibody candidates with improved ability to recruit indirect effector functions might demonstrate improved efficacy compared to the currently marketed products. By this approach, EGF-R would merely serve as a docking site for EGF-R antibodies with enhanced cytolytic potential, which ideally would kill tumor cells irrespective of the mutational status of their intracellular signaling molecules (Fig. 1).
Figure 1.
EGF-R related signaling pathways and their inhibition by TKI and antibodies. RTK, receptor tyrosine kinase; PIP2, phosphatidylinositolbisphosphate; PIP3, phosphatidylinositoltrisphosphate; Fcr, Fc receptor; ADCC, antibody-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity; EGF-R, epidermal growth factor receptor; IGF-1R, insulin-like growth factor 1 receptor; TKI, tyrosine kinase inhibitor; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinases.
Enhancing ADCC.
Antibody isotypes. The selection of distinct target epitopes may critically affect the efficacy of EGR-R-directed antibodies, and could encourage the development of antibodies with novel effector mechanisms. On the other hand, the choice between different Fc isotypes allows the tuning of indirect effector functions, resulting in molecules that optimally trigger combinations of direct and indirect effector mechanisms. Today, most clinically approved antibodies are of the human IgG1 isotype, but an IgG2 antibody against EGF-R (panitumumab) has also demonstrated clinical efficacy and is approved for the treatment of CRC patients. Interestingly, panitumumab has been reported to trigger ADCC by myeloid cells (monocytes and PMN), but not by NK cells.23 Cetuximab's efficacy was critically affected by polymorphisms in FcγRIIa and FcγRIIIa, suggesting that both myeloid and NK cells contribute to its efficacy. Surprisingly, other antibody isotypes that could be considered for clinical applications have not been carefully analyzed. For example, human IgG3 is particularly potent in triggering complement deposition, while IgG1 is more effective in ADCC by NK cells.91,92 Recently, mixed isotypes of IgG1 and IgG3 generated by genetic fusion of different domains of both isotypes have been reported, and these demonstrated potent ADCC activity comparable to IgG1 and efficient complement-dependent cytotoxicity (CDC) activity in the range of IgG3 antibodies.93 Thus, the rational choice of effector functions, which depends on tumor type, availability of effector cells or effector molecules such as complement, may further improve the efficacy of EGF-R antibodies.
In addition, non-IgG isotypes like IgA antibodies display features distinct from IgG antibodies, which make them attractive for immunotherapy. Two subclasses—IgA1 and IgA2—are distinguished. After covalent binding to plasma cell produced joining (J)-chain, IgA antibodies form natural dimers. Binding of these dimers to the polymeric immunoglobulin receptor (pIgR) leads to the directed transcellular secretion of IgA onto mucosal surfaces. At the luminal surface, secretory IgA (sIgA) is released, which consists of IgA dimers, J-chain and the proteolytically cleaved extracellular part of the pIgR. Thereby, pharmacokinetic properties of IgA are fundamentally different from those of IgG. In contrast to IgG, IgA does not bind to FcRn, and is therefore not protected from degradation, and its serum half life of approx. 5 days is significantly shorter than that of IgG.94 On the other hand, IgA, but not IgG, is actively transported to mucosal surfaces of the gut, the airways and the urogenital tract. This offers the potential advantage that intravenously applied IgA could target common tumors such as lung or colon cancers from the luminal surface, which is often enriched in neutrophilic effector cells. In vitro experiments have revealed that EGF-R-directed IgA1 and IgA2 activate human neutrophils more effectively than IgG antibodies by engagement of the myeloid IgA receptor (FcαR; CD89).95 In summary, EGF-R-directed IgA may allow potent recruitment of neutrophils, the most numerous phagocytic cell population in vivo, that are modestly activated by IgG antibodies.
Fc engineering. The contribution of ADCC to the in vivo efficacy of therapeutic antibodies was supported by elegant work in animal models and clinical studies that correlated certain FcγR polymorphisms with improved clinical performance of trastuzumab and cetuximab.20,96 Together these studies suggested the importance of FcγR engagement for the clinical efficacy of EGF-R-directed antibodies. As these polymorphisms are also clinically relevant in KRAS-mutated CRC, an important role of ADCC in cetuximab's efficacy is presumed. Indirectly, these observations may indicate that KRAS mutations have no impact on indirect Fc-mediated effector functions of therapeutic antibodies, and that the likelihood for patients to respond to antibody therapy does not rely on the KRAS status, but rather on efficient recruitment of FcγR expressing immune effector cells. Therefore, strategies to optimize effector cell recruitment by enhancing FcγRIIIa binding might represent promising approaches to enhance EGF-R directed antibody therapy. Two strategies are most advanced in clinical development at the moment: glyco-engineering and protein-engineering of the human IgG1 Fc part.97 Several reports have demonstrated superior ADCC activity in vitro and enhanced anti-tumor activity in animal models.98 Importantly, ADCC activity with effector cells from donors with the unfavorable F/F genotype and glyco-engineered Abs was more effective than ADCC with effector cells from V/V donors and non-engineered antibodies,99 suggesting that patients carrying an unfavorable FcγRIIIa-F/F genotype may also benefit from these optimized antibody variants. However, clinical trials will have to demonstrate whether these promising preclinical observations will translate into clinical benefit. To date, only limited data is available for engineered EGF-R antibodies. Low-fucosylated variants of the fully human EGF-R antibody 2F8 demonstrated a five-fold higher ADCC activity compared to fully fucosylated variants with MNC effector cells, but proved less effective with PMN.100 Protein-engineered antibody variants directed against EGF-R have not been reported so far.
Enhancing CDC.
Antibody-triggered CDC constitutes a powerful mechanism of tumor cell killing. However, most solid tumor cells proved rather complement-resistant, probably explained by high expression levels of complement regulatory proteins. Thus, individual IgG1 antibodies against EGF-R did not trigger CDC.101 Therefore, complement is currently not considered a major effector mechanism of EGF-R antibodies. However, complement resistance may be overcome by EGF-R antibody combinations that bind to non-overlapping receptor epitopes as described for cetuximab and matuzumab, or other antibody combinations.101 Interestingly, the cetuximab/matuzumab combination also demonstrated additive effects in mediating direct effector functions.102 Whether CDC by combinations of EGF-R antibodies is affected by mutations of intracellular signaling molecules discussed here is currently unknown.
Besides antibody combinations, Fc engineering may represent a potent approach to enhance CDC. Mutational analyses of human IgG1 revealed that the C1q-binding region of human IgG1 is centered on D270, K322, P329 and P331 in the CH2 domain. Two mutants, D270A and P329A, were particularly ineffective in binding C1q and activating human complement, but still retained some complement-activating capacity at higher complement concentrations.103 Further studies revealed that amino acids K326 and E333, located at the edges of the C1q binding region, profoundly influenced complement activation by IgG1 mutants.104 A K326W substitution provided the highest increase in C1q binding (3-fold) and complement-mediated killing (2-fold). At amino acid position 333, the E333S substitution resulted in the highest increase in C1q binding (2-fold) and CDC activity (1.6-fold). The K326W/E333S double mutant demonstrated additive increases in C1q binding (5-fold), but CDC activity was not further increased compared to the single mutants. Importantly, these enhancing mutations were analyzed and tested in the background of rituximab, an antibody that triggers potent CDC already in its wild-type form. Therefore, it would be interesting to assess whether these mutations also enhance CDC triggered by EGF-R-directed antibodies. So far, these mutations have not been reported in the background of EGF-R antibodies. Assuming that engineering of antibodies directed against wild-type EGF-R results in potent complement activation, toxic side effects in normal tissues such as liver might be a matter of concern.
Enhancing recruitment of the immune system.
Antibodies also offer the potential to generate tumor-specific immune responses, particularly when they are combined with immunostimulatory cytokines like interleukin-2.105 Antigen presentation was demonstrated to be particularly effective when tumor antigens were directly targeted to activating Fcγ receptors, e.g., via bispecific antibodies. These innovative molecules are making progress in clinical development,106–108 and have also been investigated to target EGF-R.109
Conclusions
In addition to clinical parameters, molecular biomarkers offer the potential of identifying patients who have optimal chances to benefit from EGF-R antibodies. Among others, lack of mutations in KRAS or BRAF, and expression of EGF-R ligands have repeatedly been identified as biomarkers for antibody responses in colorectal cancer patients. The impact of these biomarkers is consistent with the notion that inhibition of EGF-R signaling is a major mechanism of EGF-R antibodies in CRC. However, Fcγ receptor polymorphisms have also been demonstrated to impact responses to cetuximab in these patients. Surprisingly, biomarkers that predict responses to EGF-R-directed TKI in lung cancer (EGF-R kinase mutations, KRAS mutations) appeared to be irrelevant for EGF-R antibodies in this tumor type. This may suggest that Fc-mediated effector mechanisms like ADCC and CDC play a predominant role for the currently moderate efficacy of cetuximab in lung cancer. If these hypotheses were supported by results from additional studies, Fc engineered EGF-R antibodies may have the potential to significantly improve response rates in these common and difficult to treat tumors.
Acknowledgements
We gratefully acknowledge funding from the Deutsche Forschungsgemeinschaft (Va 124/7-1, Va124/6-3 and De1478/1-1), from Genmab (Utrecht, NL) and from intramural grants of the Christian-Albrechts-University, Kiel. We apologize to all investigators whose important work in the field could not be cited due to space limitations.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/10051
Conflict of interest
M.D. and T.V. receive research grants from Genmab, which has an EGF-R antibody in clinical development.
References
- 1.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 2.Citri A, Yarden Y. EGF-ERBB signalling: towards the system level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 3.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to downregulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn J. Targeting the epidermal growth factor receptor. J Clin Oncol. 2002;20:1–13. [PubMed] [Google Scholar]
- 6.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312:1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 8.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Petri ET, Halmos B, Boggon TJ. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol. 2008;26:1742–1751. doi: 10.1200/JCO.2007.12.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green MR. Targeting targeted therapy. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMe048101. [DOI] [PubMed] [Google Scholar]
- 11.Yeatman TJ. Predictive biomarkers: identification and verification. J Clin Oncol. 2009;17:2743–2744. doi: 10.1200/JCO.2008.21.5087. [DOI] [PubMed] [Google Scholar]
- 12.Eberhard DA, Giaccone G, Johnson BE. Biomarkers of response to epidermal groath factor receptor inhibitors in non-small-cell lung cancer working group: standardization for use in the clinical trial setting. J Clin Oncol. 2008;26:983–994. doi: 10.1200/JCO.2007.12.9858. [DOI] [PubMed] [Google Scholar]
- 13.Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 14.Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations wihtout indication for chemotherapy. J Clin Oncol. 2009:27. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 15.Giovannini M, Gregorc V, Belli C, Roca E, Lazzari C, Vigano MG, et al. Clinical significance of skin toxicity due to EGFR-targeted therapies. J Oncol. 2009;2009:849051. doi: 10.1155/2009/849051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talavera A, Friemann R, Gomez Puerta S, Martinez-Fleites C, Garrido G, Rabasa A, et al. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res. 2009;69:5851–5859. doi: 10.1158/0008-5472.CAN-08-4518. [DOI] [PubMed] [Google Scholar]
- 17.Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–245. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Minard-Colin V, Xiu Y, Poe JC, Horikawa M, Magro CM, Hamaguchi Y, et al. Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcγRI, FcγRIII and FcγRIV. Blood. 2008;112:1205–1213. doi: 10.1182/blood-2008-01-135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of FcγRIIa-FcγRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus iriniotecan. J Clin Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Gordon MS, Schultheis AM, Yang DY, Nagashima F, Azuma M, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor-expressing metastatic coloreactal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 21.Lejeune J, Thibault G, Ternant D, Cartron G, Watier H, Ohresser M. Evidence for linkage disequilibrium between FcγRIIIa-V158F and FcγRIIa-H131R polymorphisms in white patients, and for an FcγRIIIa-restricted influence on the response to therapeutic antibodies. J Clin Oncol. 2008;26:5489–5491. doi: 10.1200/JCO.2008.19.4118. [DOI] [PubMed] [Google Scholar]
- 22.Lehrnbecher T, Foster CB, Zhu S, Leitman SF, Goldin LR, Huppi K, et al. Variant genotypes of the low-affinity Fcγ receptors in two control populations and a review of low-affinity Fcγ receptor polymorphisms in control and disease populations. Blood. 1999;94:4220–4232. [PubMed] [Google Scholar]
- 23.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, Beyer T, Lohse S, et al. Human IgG2 antibodies against epidermal growth factor effectively trigger ADCC but, in contrast to IgG1, only by cells of myeloid lineage. doi: 10.4049/jimmunol.0900847. Submitted. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Kane S, Wu J, Benedettini E, Li D, Reeves C, et al. Mutation-specific antibodies for the detection of EGFR mutations in Non-small-cell lung cancer. Clin Cancer Res. 2009;15:3023–3028. doi: 10.1158/1078-0432.CCR-08-2739. [DOI] [PubMed] [Google Scholar]
- 25.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 27.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpress HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 28.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 29.Vincenzi B, Santini D, Tonini G. New issues on cetuximab mechanism of action in epidermal growth factor receptor-negative colorectal cancer: the role of vascular endothelial growth factor. J Clin Oncol. 2006;24:1957–1958. doi: 10.1200/JCO.2005.05.0450. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch FR, Herbst RS, Olsen C, Chansky K, Crowley J, Kelly K, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients traeted with cetuximab and chemotherapy. J Clin Oncol. 2008;26:3351–3357. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cappuzzo F, Finocchiaro G, Rossi E, Jänne PA, Carnaghi C, Calandri C, et al. EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients. Ann Oncol. 2008;19:717–723. doi: 10.1093/annonc/mdm492. [DOI] [PubMed] [Google Scholar]
- 32.Sartore-Bianchi A, Moroni M, Veronese S, Carnaghi C, Bajetta E, Luppi G, et al. Epidermal grwoth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol. 2007;25:3238–3245. doi: 10.1200/JCO.2007.11.5956. [DOI] [PubMed] [Google Scholar]
- 33.Cappuzzo F. EGFR FISH versus mutation: different tests, different end-points. Lung Cancer. 2008;60:160–165. doi: 10.1016/j.lungcan.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Takano T, Ota S, Hori A, Seki N, Eguchi K. Can epidermal growth factor receptor-fluorescent in situ hybridization predict clinical benefit from cetuximab treatment in patients with non-small-cell lung cancer. J Clin Oncol. 2009;27:464–465. doi: 10.1200/JCO.2008.20.0105. [DOI] [PubMed] [Google Scholar]
- 35.Lammerts van Bueren JJ, Bleeker WK, Bogh HO, Houtkamp M, Schuurman J, van de Winkel JGJ, et al. Effect of target dynamics on pharmacokinetics of a novel therapeutic antibody against the epidermal growth factor receptor: implications for the mechanisms of action. Cancer Res. 2006;66:7630–7638. doi: 10.1158/0008-5472.CAN-05-4010. [DOI] [PubMed] [Google Scholar]
- 36.Paez JGFJ, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 37.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 39.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 40.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 41.Peipp M, Schneider-Merck T, Dechant M, Beyer T, Lammerts van Bueren J, Bleeker W, et al. Tumor cell killing mechanisms of EGF-R antibodies are not affected by lung cancer-associated EGF-R kinase mutations. J Immunol. 2008;180:4338–4345. doi: 10.4049/jimmunol.180.6.4338. [DOI] [PubMed] [Google Scholar]
- 42.Doody JF, Wang YF, Patel SN, Joynes C, Lee SP, Gerlak J, et al. Inhibitory activity of cetuximab on epidermal growth factor receptor mutations in non-small cell lung cancers. Mol Cancer Ther. 2007;6:2642–2651. doi: 10.1158/1535-7163.MCT-06-0506. [DOI] [PubMed] [Google Scholar]
- 43.Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol. 2005;23:2556–2568. doi: 10.1200/JCO.2005.07.799. [DOI] [PubMed] [Google Scholar]
- 44.Jackman DM, Miller VA, Cioffredi LA, Yeap BY, Janne PA, Riely GJ, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 46.Thatcher N, Chang A, Parikh P, Pereira JR, Cuiuleanu T, von Pawel J, et al. Gefitinib plus best supprotive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomized, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 47.Shepherd FA, Rodriguez Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 48.Langer CJ. The “Lazarus response” in treatmentnaive, poor performance status patients with non-small-cell lung cancer and epidermal growth factor receptor mutation. J Clin Oncol. 2009;27:1350–1354. doi: 10.1200/JCO.2008.20.4859. [DOI] [PubMed] [Google Scholar]
- 49.Riely GJ, Pao W, Pham DK, Li AR, Rizvi N, Venkatraman ES, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefinitinb or erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 50.Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi K, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 52.Engelmann JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 53.Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgensztern D, Govindan R. Is there a role for cetuximab in non-small cell lung cancer ? Clin Cancer Res. 2007;13:4602–4605. doi: 10.1158/1078-0432.CCR-07-0545. [DOI] [PubMed] [Google Scholar]
- 55.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomized phase III trial. The Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 56.O'Byrne KJ, Bondarenko I, Barrios C, Eschbach C, Martens U, Hotko Y, et al. Moelcular and clinical predictors of outcome for cetuximab in non-small cell lung cancer: data from the FLEX study. Proc Am Soc Clin Oncol. 2009 [Google Scholar]
- 57.Ford S, Harbison CT, Woytowitz D, Award M, Horak C, Xu LA, et al. KRAS mutation, EGFR-related and exploratory markers as response predictors of cetuximab in first-line advanced NSCLC: retrospective analyses of the BMS099 trial. Proc Am Soc Clin Oncol. 2009 In press. [Google Scholar]
- 58.Downward J. Prelude to an anniversary for the RAS oncogene. Science. 2006;314:433–434. doi: 10.1126/science.1134727. [DOI] [PubMed] [Google Scholar]
- 59.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol. 2009;27:1130–1136. doi: 10.1200/JCO.2008.19.8168. [DOI] [PubMed] [Google Scholar]
- 61.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-Ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 62.Amado RG, Wolf M, Peeters M, van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 63.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chien CRC, Makhson A, et al. Cetuximab chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 64.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leukovorin and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2008;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 65.Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 66.Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Medicine. 2005;2:57–61. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and KRAS are predictive and prognostic indicators in patients with Non-Small-Cell Lung Cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 68.di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 69.Perrone F, Lampis A, Orsenigo M, Di Bartolomeo M, Gevorgyan A, Losa M, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- 70.Prenen H, de Schutter J, Jacobs B, de Roock W, Biesmans B, Claes B, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184–3188. doi: 10.1158/1078-0432.CCR-08-2961. [DOI] [PubMed] [Google Scholar]
- 71.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 72.Sos ML, Koker M, Weir BA, Heynck S, Rabinovsky R, Zander T, et al. PTEN loss contributes to erlotinib resistance in EGF-R-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69:3256–3261. doi: 10.1158/0008-5472.CAN-08-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loupakis F, Pollina L, Stasi I, Ruzzo A, Scartozzi M, Santini D, et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:2622–2629. doi: 10.1200/JCO.2008.20.2796. [DOI] [PubMed] [Google Scholar]
- 74.Harris RC, Chung EA, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 75.Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol. 2007;8:245–257. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]
- 76.Hynes NE, Schlange T. Targeting ADAMS and ERBBs in lung cancer. Cancer Cell. 2006;10:7–11. doi: 10.1016/j.ccr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosemann TR. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314:1746. doi: 10.1126/science.1133715. [DOI] [PubMed] [Google Scholar]
- 78.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 79.Tonra JR, Deevi DS, Corcoran E, Li H, Wang S, Carrick FE, et al. Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clin Cancer Res. 2006;12:2197–2207. doi: 10.1158/1078-0432.CCR-05-1682. [DOI] [PubMed] [Google Scholar]
- 80.Punt CJ, Tol J, Rodenburg CJ, Cats A, Creemers G, Schrama JG, et al. Randomized phase III study of capecitabine, oxaliplatin and bevacizumab with or without cetuximab in advanced colorectal cancer (ACC), the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG) J Clin Oncol. 2008:26. doi: 10.1093/annonc/mdm607. [DOI] [PubMed] [Google Scholar]
- 81.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 82.Carter PJ. Potent antibody therapeutics by design. Nat Review Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 83.Schmitz KR, Ferguson KM. Interaction of antibodies with ErbB receptor extracellular regions. Exp Cell Res. 2009;315:659–670. doi: 10.1016/j.yexcr.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peipp M, Dechant M, Valerius T. Effector mechanisms of ErbB antibodies. Curr Opin Immunol. 2008;20:436–443. doi: 10.1016/j.coi.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 85.Schmiedel J, Blaukat A, Li S, Knöchel T, Ferguson KM. Matuzumab binding to EGFR prevents the conformational rearrangement required for dimerization. Cancer Cell. 2008;13:365–373. doi: 10.1016/j.ccr.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lammerts van Beuren JJ, Bleeker WK, Brännström A, von Euler A, Jansson M, Peipp M, et al. The therapeutic antibody zalutumumab inhibits epidermal growth factor receptor signaling by limiting intra- and inter-molecular flexibility. Proc Natl Acad Sci USA. 2008;105:6109–6114. doi: 10.1073/pnas.0709477105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Weihua Z, Tsan RZ, Huang WC, Wu Q, Chiu CH, Fidler IJ, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13:385–393. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20:267–275. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuan CT, Wikstrand CJ, Bigner DD. EGF mutant receptor vIII as a molecular target in cancer therapy. Endocr Relat Cancer. 2001;8:83–96. doi: 10.1677/erc.0.0080083. [DOI] [PubMed] [Google Scholar]
- 90.Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci USA. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dechant M, Vidarsson G, Stockmeyer B, Repp R, Glennie MJ, Gramatzki M, et al. Chimeric IgA antibodies against HLA class II effectively trigger lymphoma cell killing. Blood. 2002;100:4574–4580. doi: 10.1182/blood-2002-03-0687. [DOI] [PubMed] [Google Scholar]
- 93.Natsume A, In M, Takamura H, Nakagawa T, Shimizu Y, Kitajima K, et al. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res. 2008;68:3863–3872. doi: 10.1158/0008-5472.CAN-07-6297. [DOI] [PubMed] [Google Scholar]
- 94.Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 95.Dechant M, Beyer T, Schneider-Merck T, Weisner W, Peipp M, van de Winkel JG, et al. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J Immunol. 2007;179:2936–2943. doi: 10.4049/jimmunol.179.5.2936. [DOI] [PubMed] [Google Scholar]
- 96.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 97.Desjarlais JR, Lazar GA, Zhukovsky EA, Chu SY. Optimizing engagement of the immune system by anti-tumor antibodies: an engineer's perspective. Drug Discov Today. 2007;12:898–910. doi: 10.1016/j.drudis.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68:8049–8057. doi: 10.1158/0008-5472.CAN-08-2268. [DOI] [PubMed] [Google Scholar]
- 99.Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, et al. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcγRIIIa functional polymorphism. Clin Cancer Res. 2004;10:6248–6255. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 100.Peipp M, Lammerts van Bueren J, Schneider-Merck T, Bleeker W, Dechant M, Beyer T, et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112:2390–2399. doi: 10.1182/blood-2008-03-144600. [DOI] [PubMed] [Google Scholar]
- 101.Dechant M, Weisner W, Berger S, Peipp M, Beyer T, Schneider-Merck T, et al. Complement-dependent tumor cell lysis triggered by combinations of EGF-R antibodies. Cancer Res. 2008;68:4998–5003. doi: 10.1158/0008-5472.CAN-07-6226. [DOI] [PubMed] [Google Scholar]
- 102.Kamat V, Donaldson JM, Kari C, Quadros MR, Lelkes PI, Chaiken I, et al. Enhanced EGFR inhibition and distinct epitope recognition by EGFR antagonistic mAbs C225 and 425. Cancer Biol Ther. 2008;7:726–733. doi: 10.4161/cbt.7.5.6097. [DOI] [PubMed] [Google Scholar]
- 103.Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178–4184. doi: 10.4049/jimmunol.164.8.4178. [DOI] [PubMed] [Google Scholar]
- 104.Idusogie EE, Wong PY, Presta LG, Gazzano-Santoro H, Totpal K, Ultsch M, et al. Engineered antibodies with increased activity to recruit complement. J Immunol. 2001;166:2571–2575. doi: 10.4049/jimmunol.166.4.2571. [DOI] [PubMed] [Google Scholar]
- 105.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 107.Peipp M, Valerius T. Bispecific antibodies targeting cancer cells. Biochem Soc Trans. 2002;30:507–511. doi: 10.1042/bst0300507. [DOI] [PubMed] [Google Scholar]
- 108.Repp R, van Ojik HH, Valerius T, Groenewegen G, Wieland G, Oetzel C, et al. Phase I clinical trial of the bispecific antibody MDX-H210 (anti-FcγRI × anti-HER-2/neu) in combination with Filgrastim (G-CSF) for treatment of advanced breast cancer. Br J Cancer. 2003;89:2234–2243. doi: 10.1038/sj.bjc.6601367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stadick H, Stockmeyer B, Kuhn R, Schrott KM, Kalden JR, Glennie MJ, et al. Epidermal growth factor receptor and g250: useful target antigens for antibody mediated cellular cytotoxicity against renal cell carcinoma? J Urol. 2002;167:707–712. doi: 10.1016/S0022-5347(01)69131-6. [DOI] [PubMed] [Google Scholar]