Abstract
Benzo[a]pyrene (B[a]P) is a ligand for the aryl hydrocarbon receptor (Ahr). After binding ligand, Ahr dimerizes with the aryl hydrocarbon receptor nuclear translocator (Arnt) protein, and the dimer upregulates the transcription of Cyp1a1, Cyp1b1 and other enzymes involved in the metabolic activation of B[a]P. Arnt null mice die in utero. Mice in which Arnt deletion occurs constitutively in the epidermis die perinatally. In the current study, mice were developed in which the Arnt gene could be deleted specifically in adult skin epidermis. This deletion had no overt pathological effect. Homozygosity for a null reduced nicotinamide adenine dinucleotide (phosphate): quinone oxidoreductase allele was introduced into the above mouse strain to render it more susceptible to tumor initiation by B[a]P. Deletion of Arnt in the epidermis of this strain completely prevented the induction of skin tumors in a tumor initiation–promotion protocol in which a single topical application of B[a]P acted as the tumor-initiating event, and tumor promotion was provided by repeated topical applications of 12-O-tetradecanoyl phorbol-13-acetate (TPA). In contrast, deletion of Arnt did not prevent the induction of skin tumors in a protocol also using TPA as the promoter but using as the initiator N-methyl-N′-nitro-N-nitrosoguanidine, whose activity is unlikely to be affected by the activity of Ahr, Arnt or their target genes. These observations demonstrate that Arnt is required for tumor initiation by B[a]P in this system.
Introduction
The classical model of two-stage tumorigenesis in mouse skin consists of a single exposure to an initiator and multiple exposures to a promoter. Frequently, the polycyclic aromatic hydrocarbon, benzo[a]pyrene (B[a]P), is used as the initiator and 12-O-tetradecanyl phorbol-13-acetate (TPA) is used as the promoter (1). Tumor initiation by B[a]P is presumed to require its metabolism to mutagenic derivatives (2). The principal such derivative is B[a]P 7,8-diol-9.10-epoxide, which is generated by the activities of certain cytochrome P450s (principally CYP1A1 and CYP1B1) and epoxide hydrolase. The levels of CYP1A1 and CYP1B1 are markedly upregulated by B[a]P in a process mediated by the aryl hydrocarbon receptor (Ahr). After binding ligands such as B[a]P, the Ahr translocates into the nucleus and dimerizes with the aryl hydrocarbon receptor nuclear translocator (Arnt) protein. The Ahr/Arnt dimer then binds to xenobiotic-responsive elements upstream of the Cyp1a1 and Cyp1b1 proximal promoter region of these and other genes and upregulates their rates of transcription (3).
Although it has been demonstrated that Ahr is required for complete carcinogenesis by B[a]P (4), it has not been determined whether Ahr is required in the initiation or promotion stages of this process or both. It is thus formally possible that Ahr is not required for tumor initiation by B[a]P. Furthermore, even if Ahr is required for tumor initiation by B[a]P, it is conceivable that Arnt is not required since some pathological effects of Ahr ligands appear to be mediated by Ahr independently of Arnt and of mutagenic events (5–8). This study addresses the hypothesis that Arnt is required for skin tumor initiation by B[a]P, by utilizing mice in which Arnt has been deleted specifically in the epidermis.
Arnt (and Arnt2) can also dimerize with hypoxia-inducible factor (HIF)-1α or HIF-2α to form HIF, which represents the master regulator of the hypoxic response. In normal cells under normoxic conditions, HIF-1α and HIF-2α are destabilized and are thus incapable of activating transcription of target genes. Under hypoxic conditions, HIF-1α and HIF-2α are stabilized and upregulate many genes involved in glycolysis, angiogenesis and cell survival. Portions of solid tumors are generally in a hypoxic state, resulting in activation of HIF-1α and/or HIF-2α (9). Furthermore, HIF-1α and HIF-2α are also upregulated in many cancer cells even under normoxic conditions, due to the effects of activated proto-oncogenes or the inactivation of tumor suppressor genes (10). A large number of studies have been performed to investigate the role of HIF in tumor growth. With few exceptions, these studies have indicated that HIF activity enhances tumor growth (11). However, these studies have utilized tumor xenograft models, which have the inherent limitations that the tumor cells so tested are already fully malignant and that the tumor cells are exposed to an artificial cellular environment. In contrast, our experiments provided us with the potential opportunity to investigate the effect of ablation of Arnt (and thus of HIF) on the growth kinetics of endogenously arising tumors in the skin. This issue represented a further objective of our studies.
Materials and methods
Breeding and genotyping of mice
The original ArntF allele contained a neo cassette (12). This was excised as described previously (13). The mice were originally of a mixed C57BL/6, 129/Sv and FVB/N genetic background. The mice were crossed to homozygous K14-Cre-ERT2 mice (14), which were of a mixed C57BL/6 and FVB/N genetic background. Progeny from this cross were then backcrossed at least 10 successive times to the C57BL/6 strain before experiments were performed. These mice are therefore in a 100% C57BL/6 background. Genotyping of the ArntF and ArntΔ alleles was performed by polymerase chain reaction as described previously (15). The K14-Cre-ERT2 transgene was genotyped with polymerase chain reaction primers directed at the Cre gene as described previously (15). K14-Cre-ERT2+/− heterozygotes could not be distinguished from K14-Cre-ERT2+/+ homozygotes by this procedure, and these genotypes are collectively referred to as K14-Cre-ERT2+. ArntF/F: K14-Cre-ERT2+ mice were crossed with reduced nicotinamide adenine dinucleotide (phosphate): quinone oxidoreductase (Nqo1−/−) mice that were of the C57BL/6 genetic background, and the presence of the wild-type and Nqo1− alleles was assessed by polymerase chain reaction (16).
Carcinogen treatment
Mice of 11 weeks of age were injected intraperitoneally with 100 μg tamoxifen (in 100 μl sunflower oil) for five consecutive days and again for three consecutive days every month. Carcinogen treatments started 4 weeks after the first tamoxifen dose. Mice were treated on an area of shaved dorsal skin with 200 μg of B[a]P dissolved in 200 μl acetone, with one dose of 5 μmol N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) or with the vehicle, acetone. Twice weekly applications of 10 μg TPA started 1 week after initiator treatment and were continued for 25 weeks. Mice were monitored for tumors weekly for up to 40 weeks or were euthanized when tumors exceeded 1.5 cm in diameter, if they ulcerated or if the mice showed signs of distress or discomfort. Tumors >2 mm in diameter were counted. See Table I for the different types of carcinogen treatment. DNA from tumors was performed using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA) following the manufacturer's instructions.
Table I.
Carcinogen treatments
| Group | Tamoxifen | B[a]P or MNNG | TPA | Number of mice | Expected Arnt phenotype |
| I1 | + | B[a]P | + | 24 | − |
| I2 | − | B[a]P | + | 19 | + |
| I3 | + | MNNG | + | 14 | − |
| I4 | − | MNNG | + | 14 | + |
| I5 | + | Acetone | + | 14 | − |
| I6 | − | Acetone | + | 15 | + |
All mice were of the genotype K14-Cre-ERT2+: ArntF/F: Nqo1−/−.
Analysis of tumors
Diagnosis was performed by a board certified pathologist on tumor specimens fixed with formalin and stained with hematoxylin and eosin. The additional criteria for diagnosing neurofibrosarcomas was positive staining in nuclei and cytoplasm for S100 and negative staining for desmin and pancreatin. Staining of sections for Arnt2 (Santa Cruz Biotechnology antibody, M-165-sc-5581) was performed with the indicated antibody.
Isolation of epidermis and dermis from normal skin
Mouse skin was treated with 0.25% trypsin at 4°C for 18 h, and the dermis then peeled away from the epidermis with forceps. Epidermal sheets were stirred in Eagle's minimal essential medium containing 8% fetal calf serum. The resulting epidermal cell suspension was then filtered through Nytex gauze to remove the stratum corneum and remaining hair, and the epidermal cells were pelleted by centrifugation. DNA was extracted from the dermis and epidermal cell pellets using the DNeasy Blood and Tissue kit (Qiagen).
Results
Deletion of Arnt eliminates the development of tumors in a model of tumor initiation by B[a]P
Mice that are homozygous for an Arnt null allele die in utero (17,18). ArntF/F: K14-Cre mice, in which deletion of Arnt occurs in the skin epidermis, die within 1 day of birth due to loss of skin barrier function (15). In order to delete Arnt in the skin epidermis of adult mice, we constructed mice of the following genotype-ArntF/F: K14-Cre-ERT2+ (either homozygous or heterozygous for the Cre transgene). Cre-ERT2 expression in the above construct is driven by the keratin 14 promoter, which is selective for the basal layer of stratified squamous epithelia. Cre-ERT2 encodes a fusion protein between the Cre recombinase and a mutated ligand-binding domain of the human estrogen receptor α. The recombinase activity of this construct is activated by the anti-estrogen tamoxifen but not by endogenous estrogens (14). These mice were originally in a mixed C57BL/6:129/Sv: FVB/N genetic background, but we backcrossed them at least 10 times to the C57B1/6 strain, so that the experimental animals were in a pure C57BL/6 genetic background. The C57BL/6 mouse strain is particularly resistant to tumor formation in the B[a]P plus TPA protocol (19). In order to increase the number of tumors, we generated mice of the above genotype that were also homozygous for a Nqo1 null allele since Nqo-1−/− mice exhibit much greater sensitivity to B[a]P plus TPA skin tumorigenesis than wild-type C57Bl/6 mice (16,19).
We utilized a protocol involving a single topical application of B[a]P to mouse skin, followed by twice weekly applications of TPA. In this protocol, B[a]P acts as tumor initiator and TPA acts as tumor promoter. We compared tumor formation in ArntF/F: K14-Cre-ERT2+: Nqo1−/− mice that had previously been injected intraperitoneally with tamoxifen or had been left untreated. Analysis of the epidermis and dermis isolated directly from the skin of these mice showed that Arnt deletion occurred rapidly and efficiently in the epidermis but not the dermis of these mice after tamoxifen treatment (Figure 1), consistent with the original observations on the K14-Cre-ERT2 transgene by Li et al. (14). [Tamoxifen treatment had no overt detrimental effect on the skin of Cre-positive mice not treated with B[a]P (group I5).] Four weeks after the initial tamoxifen treatment, mice were treated once topically with 200 μg B[a]P and then twice weekly with TPA (groups I1 and I2 in Table I). As controls, we also examined mice untreated with B[a]P (groups I5 and I6) or mice treated with the alkylating agent MNNG (groups I3 and I4), which acts as a direct-acting tumor initiator, but whose activity is unlikely to be affected by the activity of Ahr, Arnt or their target genes (all mice received TPA).
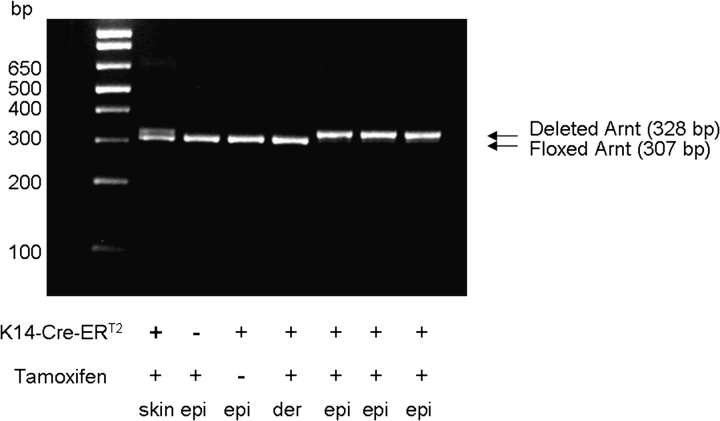
Fig. 1.
Rapid deletion of Arnt in epidermis but not dermis of ArntF/F: K14-Cre-ERT2+ mice treated with tamoxifen. Mice were injected with tamoxifen for five consecutive days, and skin excised 3 days later. The floxed Arnt allele gives a polymerase chain reaction (PCR) product of 307 bp, whereas the deleted Arnt allele gives a PCR product of 328 bp. Both ArntF and ArntΔ alleles were detected in whole skin of tamoxifen-treated Cre+ mice (lane 1). No deletion occurred in the epidermis (epi) from Cre− mice treated with tamoxifen (lane 2) or in the epidermis from Cre+ mice not treated with tamoxifen (lane 3) or in the dermis (derm) of Cre+ mice treated with tamoxifen (lane 4). Complete deletion occurred in the epidermis of tamoxifen-treated Cre+ mice (lanes 5,6 and 7, corresponding to three different mice). (The initial lane contains DNA molecular weight markers.)
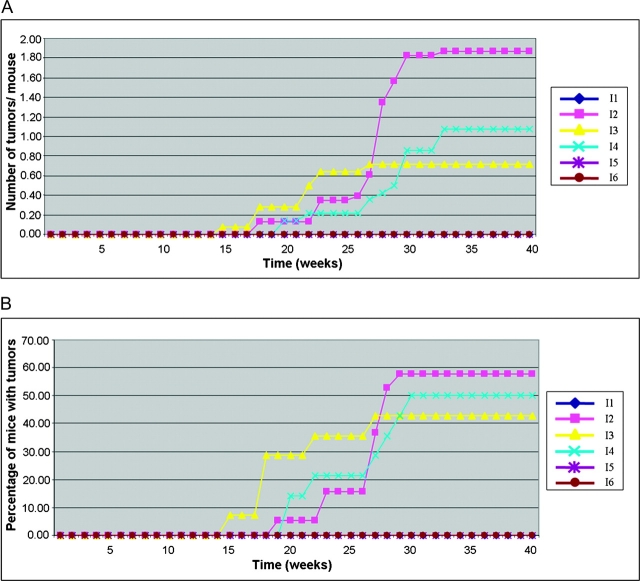
From 14 to 24 mice were treated per group (approximately the same number of males and females in each group). Fifty-eight per cent of ArntF/F: K14-Cre-ERT2+: Nqo1−/− mice untreated with tamoxifen but treated with B[a]P developed tumors, and there were an average of 1.8 tumors per mouse. No tumors were obtained in equivalent mice that were treated with tamoxifen to induce deletion in the ArntF allele (Figure 2A and B). MNNG + TPA treatment generated tumors in approximately the same proportion of mice (50 and 43%) at approximately the same frequency (1.1 and 0.70) in tamoxifen-untreated mice as in tamoxifen-treated mice, respectively. No tumors were obtained in mice treated with TPA without prior treatment with B[a]P or MNNG, irrespective of whether or not they had been treated with tamoxifen (Figure 2A and B). These results demonstrate that Arnt activity is required for tumor initiation by B[a]P in this experimental model.
Fig. 2.
Skin tumor induction in the initiation experiment. (A) Number of tumors per mouse. (B) Proportion of mice with tumors. The key to the different treatment groups (I1–I6) is given in Table I.
All eight tumors induced by B[a]P plus TPA that were analyzed were squamous cell carcinomas. Thus, all the tumors were epidermal in origin, which is consistent with the topical application of B[a]P, its greater rate of metabolism in epidermis than dermis (20,21) and its limited permeation to the dermis (22). In the case of MNNG plus TPA, 64% of the tumors were squamous cell carcinomas and 14% were papillomas and thus of epidermal origin. The remainder of the tumors were neurofibrosarcomas (Table II).
Table II.
Diagnosis of tumors (all tumors analyzed were derived from different mice)
| Group | Squamous cell carcinomas | Neurofibrosarcomas | Squamous papillomas |
| I2 | 8 | 0 | 0 |
| I3 | 6 | 1 | 1 |
| I4 | 3 | 2 | 1 |
Although Arnt2 has been reported not to collaborate with Ahr in activation of gene transcription and was previously not detected in mouse skin by gross analysis (23–25), we nevertheless thought it prudent to test for Arnt2 protein expression in skin and in the tumors. Immunohistochemical analysis failed to detect the Arnt2 protein in normal skin epidermal keratinocytes or in representative tumors, whereas the protein was readily detectable in mouse kidney (data not shown).
Role of Arnt in kinetics of tumor growth
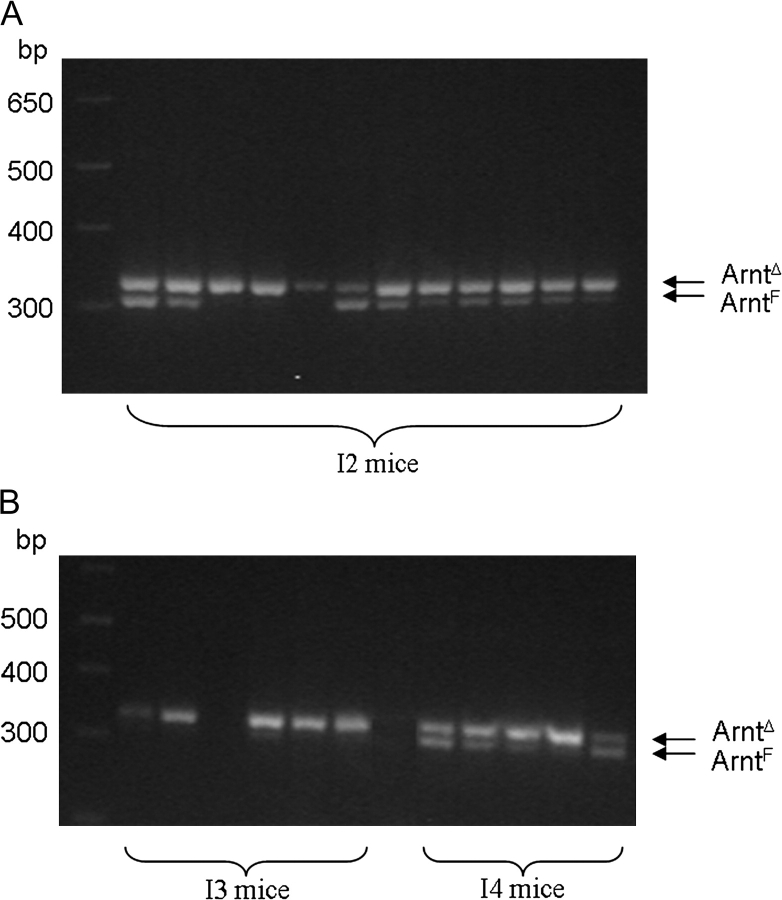
Since MNNG is presumed to act independently of Arnt, the kinetics of growth of the tumors induced by MNNG plus TPA provided a potential opportunity to investigate the effect of ablating Arnt on the growth characteristics of endogenously arising tumors. Mice treated with tamoxifen developed tumors from the MNNG plus TPA regimen (I3, Figure 2A and B) more quickly than those not treated with tamoxifen (I4). This suggested that loss of Arnt accelerated tumor growth in this system. In order to investigate this possibility further, we examined representative tumor specimens for the presence of the ArntF and ArntΔ alleles. Tumors from mice treated with tamoxifen (I3) exhibited only the ArntΔ allele. Unexpectedly, however, mice not treated with tamoxifen [whether exposed to B[a]P or MNNG] exhibited the ArntΔ allele as well as the ArntF allele (groups I2 and I4, Figure 3).
Fig. 3.
Genotyping of tumor specimens. Tumors were excised and their DNA isolated. Genotyping of the ArntΔ and ArntF alleles was performed by polymerase chain reaction (PCR) as described in ‘Materials and Methods’. The corresponding PCR products in the ethidium bromide stained gels are indicated.
The proportion of the different alleles in these last tumors was not related to the sex of the animals, indicating that the degree of deletion was not related to the levels of circulating estrogens in the animals. Deletion of Arnt in non-tamoxifen-treated mice also did not occur in mice not treated with TPA. These observations are consistent with the notion that TPA activates Cre-ERT2 either directly or indirectly and caused partial deletion of Arnt. Since deletion of Arnt presumably occurred in the non-tamoxifen-treated, MNNG plus TPA-treated mice during the development of the tumors, the interpretation of the tumor growth kinetic data with regard to the role of Arnt in the growth of these tumors is compromised and conclusions regarding whether Arnt accelerates or retards tumor growth in this model cannot be made. It should be noted, however, that the single B[a]P treatment occurred before TPA treatment, and therefore, the conclusion that Arnt is required for tumor initiation by B[a]P is not compromised.
Discussion
In our study, we used mice in which Arnt could be deleted specifically in the adult epidermis. Furthermore, we introduced homozygosity for an Nqo1 null allele into these mice to enhance their sensitivity to the tumor initiation–promotion protocol. B[a]P is metabolized by Cyp1a1 and other cytochrome P450s into the highly carcinogenic B[a]P 7,8-diol-9, 10-epoxide. Nqo1 null mice exhibit an increased sensitivity to B[a]P-induced skin cancer, probably via an altered redox state along with lack of induction of p53 and decreased apoptosis (26). The ArntF/F: K14-Cre-ERT2: Nqo1−/− did not develop tumors after exposure to B[a]P plus TPA if they had been pretreated with tamoxifen to delete Arnt in the epidermis, thus demonstrating that Arnt is required for tumor initiation by B[a]P. This is consistent with Ahr/Arnt mediating induction of CYP1A1 and CYP1B1 and subsequent metabolism of B[a]P to genotoxic derivatives by these cytochrome P450s.
Shimizu et al. (4) found that Ahr null mice were completely resistant to tumor induction when subjected to a complete carcinogenesis protocol involving once weekly topical applications of B[a]P for 25 weeks. In their protocol, B[a]P is presumed to act as both initiator and promoter. However, the experiments of Shimizu et al. do not allow determination as to whether Ahr is required for tumor initiation, tumor promotion or both processes. This is an important issue because there may be important mechanistic differences between the complete carcinogenesis and the initiation–promotion assays. For example, microsomal epoxide hydrolase null mice are completely resistant to 7,12-dimethylbenz(a)anthracene induction of skin tumors in a complete carcinogenesis protocol but only partially resistant to tumor induction in a 7,12-dimethylbenz(a)anthracene–TPA initiation–promotion protocol (27). In contrast to the experiments of Shimizu et al., our experiments specifically demonstrate that Arnt is required for initiation by B[a]P in skin. This is compatible with the notion that Ahr is also required for this stage of the carcinogenesis process.
It would also be interesting to determine whether Arnt is required for tumor promotion by B[a]P since Ahr may mediate promotion in an Arnt-independent process. The tumor initiation–promotion protocol represents a simplified model for human carcinogenesis. For most ‘naturally occurring’ human tumors, ‘initiating’ agents probably act at multiple steps of tumor progression. Thus, Arnt is probably required at several stages in the development of such tumors. It is of interest that Ahr appears to protect against tumor induction by B[a]P at sites distant from the site of application but to mediate tumor induction at the site of application (28). Our results are consistent with this generalization.
There is a considerable effort underway to develop HIF-1α and/or HIF-1β inhibitors for the treatment of cancer (reviewed in ref. 29). Since inhibition of Arnt would not only negate the hypoxic response to tumors but, as our results suggest, would also inhibit the genotoxic effects of the environmentally ubiquitous polycyclic aromatic hydrocarbons, Arnt may represent a better target for therapy than HIF-1α.
Funding
NIH (RO1 CA93471); an underrepresented minority supplement (RO1 CA93471 to K.H.B.); NIH (RO1 CA28868); National Cancer Institute Intramural Research Program to F.J.G. and S.H.-Y.
Acknowledgments
We thank Drs Daniel Metzger and Pierre Chambon, University of Strasbourg, Strasbourg, France, for the K14-Cre-ERT2 mice.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- Ahr
aryl hydrocarbon receptor
- Arnt
aryl hydrocarbon receptor nuclear translocator
- B[a]P
benzo[a]pyrene
- HIF
hypoxia-inducible factor
- MNNG
N-methyl-N′-nitro-N-nitrosoguanidine
- Nqo1
reduced nicotinamide adenine dinucleotide (phosphate): quinone oxidoreductase
- TPA
12-O-tetradecanoyl phorbol-13-acetate
References
- 1.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol. Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarti D, et al. The role of polycyclic aromatic hydrocarbon-DNA adducts in inducing mutations in mouse skin. Mutat. Res. 2008;649:161–178. doi: 10.1016/j.mrgentox.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankinson O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu Y, et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl Acad. Sci. USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouki R, et al. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581:3608–3615. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Ge N-L, et al. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. J. Biol. Chem. 1998;273:22708–22713. doi: 10.1074/jbc.273.35.22708. [DOI] [PubMed] [Google Scholar]
- 7.Haarmann-Stemmann T, et al. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem. Pharmacol. 2009;77:508–520. doi: 10.1016/j.bcp.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Tian Y, et al. Ah receptor and NF-κB interactions, a potential mechanism for dioxin toxicity. J. Biol. Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 9.Kaelin WG, Jr, et al. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Gordan JD, et al. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr. Opin. Genet. Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell PH. The HIF pathway in cancer. Semin. Cell Dev. Biol. 2005;16:523–530. doi: 10.1016/j.semcdb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Tomita S, et al. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1alpha. Mol. Endocrinol. 2000;14:1674–1681. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
- 13.Tomita S, et al. T cell-specific disruption of arylhydrocarbon receptor nuclear translocator (Arnt) gene causes resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced thymic involution. J. Immunol. 2003;171:4113–4120. doi: 10.4049/jimmunol.171.8.4113. [DOI] [PubMed] [Google Scholar]
- 14.Li M, et al. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature. 2000;407:633–636. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- 15.Takagi S, et al. Alteration of the 4-sphingenine scaffolds of ceramides in keratinocyte-specific Arnt-deficient mice affects skin barrier function. J. Clin. Invest. 2003;112:1372–1382. doi: 10.1172/JCI18513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long DJ, II, et al. NAD(P)H: quinone oxidoreductase 1 deficiency increases susceptibility to benzo(a)pyrene-induced mouse skin carcinogenesis. Cancer Res. 2000;60:5913–5915. [PubMed] [Google Scholar]
- 17.Kozak KR, et al. ARNT-deficient mice and placental differentiation. Dev. Bio. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- 18.Maltepe E, et al. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 19.Iskander K, et al. Deficiency of NAD(P)H:quinone oxidoreductase 2 increases susceptibility to 7,12-dimethylbenz(a)anthracene and benzo(a)pyrene-induced skin carcinogenesis. Cancer Res. 2004;64:5925–5928. doi: 10.1158/0008-5472.CAN-04-0763. [DOI] [PubMed] [Google Scholar]
- 20.Akin FJ, et al. Factors affecting measurement of aryl hydrocarbon hydroxylase activity in mouse skin. J. Invest. Dermatol. 1976;67:709–712. doi: 10.1111/1523-1747.ep12598592. [DOI] [PubMed] [Google Scholar]
- 21.Thompson S, et al. Mouse epidermal aryl hydrocarbon hydroxylase. J. Invest. Dermatol. 1976;66:108–111. doi: 10.1111/1523-1747.ep12481449. [DOI] [PubMed] [Google Scholar]
- 22.Kao J, et al. Skin penetration and metabolism of topically applied chemicals in six mammalian species, including man: an in vitro study with benzo[a]pyrene and testosterone. Toxicol. Appl. Pharmacol. 1985;81:502–516. doi: 10.1016/0041-008x(85)90421-1. [DOI] [PubMed] [Google Scholar]
- 23.Dougherty EJ, et al. Analysis of Ah receptor-ARNT and Ah receptor-ARNT2 complexes in vitro and in cell culture. Toxicol. Sci. 2008;103:191–206. doi: 10.1093/toxsci/kfm300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drutel G, et al. Cloning and selective expression in brain and kidney of ARNT2 homologous to the Ah receptor nuclear translocator (ARNT) Biochem. Biophys. Res. Commun. 1996;225:333–339. doi: 10.1006/bbrc.1996.1176. [DOI] [PubMed] [Google Scholar]
- 25.Sekine H, et al. Unique and overlapping transcriptional roles of arylhydrocarbon receptor nuclear translocator (Arnt) and Arnt2 in xenobiotic and hypoxic responses. J. Biol. Chem. 2006;281:37507–37516. doi: 10.1074/jbc.M606910200. [DOI] [PubMed] [Google Scholar]
- 26.Iskander K, et al. Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res. 2005;65:2054–2058. doi: 10.1158/0008-5472.CAN-04-3157. [DOI] [PubMed] [Google Scholar]
- 27.Miyata M, et al. Targeted disruption of the microsomal epoxide hydrolase gene. Microsomal epoxide hydrolase is required for the carcinogenic activity of 7,12-dimethylbenz[a]anthracene. J. Biol. Chem. 1999;274:23963–23968. doi: 10.1074/jbc.274.34.23963. [DOI] [PubMed] [Google Scholar]
- 28.Nebert DW, et al. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov. Today. 2007:853–859. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]