Abstract
Breast cancer frequently metastasizes to the skeleton resulting in bone degradation due to osteoclast activation. Metastases also downregulate differentiation and the bone-rebuilding function of osteoblasts. Moreover, cancer cells trigger osteoblast inflammatory stress responses. Pro-inflammatory mediators such as interleukin (IL)-6, monocyte chemoattractant protein-1 (MCP-1), cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), expressed by osteoblasts (MC3T3-E1) stimulated with human breast cancer cell (MDA-MB-231) conditioned medium, are pivotal to osteoclast activation and metastasis. Given that these genes are regulated by nuclear factor-κB (NF-κB), a redox-sensitive transcription factor, we hypothesized that selenium (Se) could abrogate the inflammatory response to metastatic breast cancer cells by modulating NF-κB. Caffeic acid phenethyl ester and parthenolide inhibited NF-κB activation, as seen by gel shift assays and immunoblotting for p65 in nuclear fractions, as well as decreased production of IL-6 and MCP-1. Supplementation of MC3T3-E1 with methylseleninic acid (MSA) (0.5 μM to 4 μM) reduced the activation of NF-κB leading to a decrease in IL-6, MCP-1, COX-2 and iNOS in response to MDA-MB-231 conditioned medium. Addition of MSA to osteoblasts for as little as 15 min suppressed activation of NF-κB suggesting that short-lived active metabolites might be involved. However, brief exposure to MSA also brought about an increase in selenoprotein glutathione peroxidase 1. In summary, our data indicate that the osteoblast response to metastatic breast cancer cells is regulated by NF-κB activation, which can be effectively suppressed by MSA either through short-lived active metabolites and/or selenoproteins. Thus, Se supplementation may prevent the osteoblast inflammatory response or dampen the vicious cycle established when breast cancer cells, osteoblasts and osteoclasts interact.
Introduction
The skeleton is a preferred site for breast cancer metastasis. In spite of many rounds of chemotherapy and an often long latency period, metastases in this sanctuary grow and bring about osteolytic lesions. Bone loss is not a direct function of the cancer cells but is largely due to activated osteoclasts. According to the ‘vicious cycle’ paradigm, cancer cells activate osteoblasts, which in turn attract, differentiate and activate osteoclasts to resorb bone. Upon degradation, the matrix releases many stored growth factors including transforming growth factor (TGF)-β that maintain the cancer cells (1).
There is no cure for bone metastasis, but bisphosphonates such as Alendronate™ are used to inhibit osteoclasts. While this therapy slows lesion progression, the bone does not heal (2). One possible reason is that the cancer cells impair the function of osteoblasts, the bone forming cells (3,4). We tested this possibility using an in vitro system in which human metastatic breast cancer cells or their media were cocultured with osteoblast lines. We found that cancer cells or their conditioned media [breast cancer cell-conditioned medium (BCCM)] increased the prevalence of osteoblast apoptosis (5), prevented differentiation and mineralization, and caused a change in osteoblast morphology (6). With an in vivo study, we confirmed that osteoblasts no longer differentiated and were lost over time following arrival of the cancer cells into the femur (7).
Both direct and indirect contact (i.e. conditioned medium) of breast cancer cells with osteoblasts, result in the downregulation of the expression of osteoblast differentiation proteins such as osteocalcin, osteonectin, alkaline phosphatase and bone sialoprotein (6). TGF-β produced by the cancer cells was responsible for the suppression of differentiation (6). However, we also found that osteoblasts in the presence of BCCM greatly increased expression of several pro-inflammatory molecules such as, interleukin (IL)-6, IL-8 and monocyte chemoattractant protein-1 (MCP-1). TGF-β and possibly also parathyroid hormone-related protein in the conditioned medium were largely responsible for production of these molecules (8). The inflammatory cytokines, normally produced in low levels by osteoblasts, play a role in the activation of osteoclasts (9), and have been implicated in metastasis (10). Together, these molecules provide a potent stimulus for bone osteolysis. Their production by osteoblasts also suggests that it is not necessary to invoke cancer cells or immune cells such as macrophages or lymphocytes as the sources of inflammatory molecules, although they undoubtedly play a role in vivo. Interestingly, IL-6, IL-8 and MCP-1 also are expressed as part of the immediate/early inflammatory stress response of osteoblasts to prosthesis debris in bone implants in humans (11). This chronic inflammatory process also leads to bone resorption.
It has long been suspected from both experimental and epidemiological studies that inflammation is strongly linked to cancer (10,12). A key regulatory molecule in the inflammatory process is the redox-sensitive transcription factor nuclear factor-κB (NF-κB), which, upon activation, initiates the expression of a cascade of cytokines, including IL-6, IL-8 and MCP-1. The NF-κB recognition motifs are found in the 5′ regulatory regions of these cytokines. NF-κB represents a ubiquitously expressed family of transcription factors that participate in the regulation of diverse biological processes, including immune, inflammatory and apoptotic responses (13). For the purposes of this study, we concentrated on NF-κB regulation of the inflammatory cytokines IL-6 and MCP-1 (13). Inhibition of NF-κB activation could potentially interfere with the production of pro-inflammatory mediators as well as the osteoblast stress response and the activation of osteoclasts.
Recently, we have shown that cellular selenium (Se) status, via the incorporation of Se into proteins (selenoproteins), can modulate the activation of the NF-κB pathway in macrophages (14). Glutathione peroxidases (GPxs) and thioredoxin reductases (TRs) and other selenoproteins play a pivotal role in the maintenance of cellular redox balance (14,15). GPx1–4 exhibit high peroxidase activity toward certain reactive oxygen species, including H2O2 and fatty acid hydroperoxides (16,17). Thus, Se is an essential micronutrient required for normal physiological function. Early epidemiological studies have suggested that there is a unique relationship between Se levels and cancer (18). More recent studies have suggested that Se is protective and that administration of supranutritional levels is chemopreventive. The literature indicates that Se acts at the steps of both tumor initiation and progression. A reduction in the levels of GPx is inversely related to cancer progression and disease stage (19). Moreover, reduced GPx activity and Se levels have been found in the blood of cancer patients with metastases (20). Nonetheless, the mechanisms are not clear. It is known that Se is required for the synthesis of anti-oxidant selenoenzymes important for maintaining oxidative balance. Because oxidative stress can lead to mutations and cancer, these anti-oxidative enzymes may be critical in blocking tumor formation. Roebuck et al. (21) related oxidative stress to increased NF-κB activity in the osteoblast inflammatory response to titanium particles. However, the regulation of the osteoblast inflammatory response during metastasis via Se-dependent control of NF-κB has not been investigated.
The objective of this study was to determine if modification of the Se status of osteoblasts affected their inflammatory response to BCCM. We examined the relationships among NF-κB activation, Se status and pro-inflammatory protein production by osteoblasts exposed to BCCM from a human metastatic breast cancer cell line, MDA-MB-231. We found that IL-6, MCP-1 and additionally cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) were diminished in the presence of methylseleninic acid (MSA). MSA supplementation also effectively inhibited NF-κB activation.
Materials and methods
Cells
MC3T3-E1, an osteoblast line derived from murine calvaria that differentiates in culture (22), was a gift from Dr Norman Karin, Pacific Northwest National Laboratory. The cells were maintained in growth medium, α-modified Eagle’s medium plus 10% fetal bovine serum (FBS, Cansera, Roxdale, Ontario), 100 U/ml penicillin and 100 μg/ml streptomycin. They were passaged every 3–4 days with 0.002% pronase and not used beyond passage 20. For experiments, the cells were transferred to differentiation medium, i.e. growth medium plus 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate. The cells were usually used after ∼2 weeks in differentiation medium as indicated for individual experiments.
MDA-MB-231, a human metastatic breast cancer line originally derived from a pleural effusion (23), forms bone metastasis in immunodeficient mice following injection into the left ventricle of the heart. These cells were a gift from Dr Danny Welch, University of Alabama at Birmingham. They were maintained in Dulbecco's modified Eagle's medium containing 5% FBS and non-essential amino acids.
MDA-MB-231 BCCM preparation
The medium of MDA-MB-231 cells cultured to ∼90% confluency was replaced with serum-free α-modified Eagle’s medium for 24 h to allow accumulation of secreted molecules. The supernatant (BCCM) was collected, centrifuged at 300g for 10 min to remove cell debris, aliquoted and stored at −20°C. For comparison, we tested the same medium, vehicle control medium (VM) that was not exposed to breast cancer cells.
Se levels
Osteoblast Se levels were manipulated by the Se content of the serum and by addition of Se. The Se concentration of the lot of FBS used for most experiments was determined to be 0.363 μM by atomic absorption spectrometry. To obtain Se-deficient cells, MC3T3-E1 and MDA-MB-231 were plated in 5% FBS with a final Se concentration of 0.0182 μM. Under these conditions, normal growth and osteoblast differentiation as determined by the production of alkaline phosphatase and positive staining for von Kossa (data not shown) were seen. Cells were cultured for at least four passages in the Se-deficient medium, and Se status was measured by total GPx activity and GPx1 expression. In some experiments, osteoblast cultures were supplemented with 0.5 μM to 4 μM of MSA (Sigma, St Louis, MO) for 7 days as indicated. In other experiments, MSA was added directly to osteoblasts at the same or near the same time as addition of BCCM. MSA was preferred over sodium selenite given the possible toxicity associated with the latter (24,25).
Reagents and cell treatments
NF-κB inhibitors, caffeic acid phenethyl ester (CAPE) (26) and parthenolide (27) (Calbiochem, San Diego, CA) and were dissolved in dimethyl sulfoxide (DMSO). Osteoblasts were incubated with inhibitors for 1 or 2 h before the addition of BCCM. DMSO (0.2% vol/vol) was added to control cultures at the same time as the other compounds. Osteoblasts were washed with phosphate-buffered saline and treated with 50% BCCM or VM for the indicated times.
iNOS expression detection by reverse transcription–polymerase chain reaction
After incubation with BCCM or VM for 4 h, cells were washed one time with phosphate-buffered saline; RNA was extracted using the RNeasy kit (QIAGEN, Valencia, CA), treated with RNase-free DNase to eliminate genomic DNA contamination and quantified on the basis of A260. Equal amounts of RNA (1 μg) from each sample were reverse transcribed using Ambion Retroscript™ with iNOS-specific primer along with those for β-actin as described previously (15).
Total cell lysate preparation and western blot analysis
After treatment, cells were lysed with a buffer containing 0.5 M Tris–HCl, pH 6.8, 19% (vol/vol) glycerol and 10% (wt/vol) sodium dodecyl sulfate. Protease and phosphatase inhibitors, 2 ng/ml aprotinin, 1 μg/ml pepstatin, 7.5 mM NaF, 1 mM NaVO3 and 1 mM phenylmethylsulfonyl fluoride, were included. Total proteins (40 μg) from each sample were used for western blotting as described (28). Samples of cells treated for 4 h were used for COX-2 expression. COX-2 antibody was purchased from Cayman Chemical (Ann Arbor, MI) and β-actin and GAPDH antibodies were from Cell Signaling (Dover, MA) and Fitzgerald Industries (Concord, MA), respectively.
Nuclear extract preparation and electrophoretic mobility shift assay
After 1 h of treatment, cells were washed and processed using the NE-PER® Nuclear and Cytoplasmic Extraction kit (Pierce Biotechnology, Rockford, IL) as described (28). Nuclear extracts (50 μg) were used in the western blot assay and electrophoretic mobility shift assay (EMSA). Antibody to p65 was purchased from Santa Cruz Biologicals (Santa Cruz, CA). β-Actin was used as a loading control. Blots were also probed with GAPDH to confirm that the nuclear extracts were essentially free of cytoplasmic contamination. For EMSA, the sense strand of the NF-κB oligonucleotide, 5′-GATCCAGTTGAGGGGACTTTCCCAGGC-3′ (QIAGEN), was annealed with its complementary strand. [γ-32P]-adenosine triphosphate (3000 Ci/mol at 10 mCi/ml) end labeling, clean up of the labeled oligonucleotide, incubation with nuclear extracts (10 μg) followed by autoradiography were performed as described earlier from our laboratory (29). The NF-κB bands were confirmed by competition using a >100-fold excess of unlabeled oligonucleotide and supershift assays with anti-p50 (Santa Cruz).
Cytokine detection
After 4 h incubations with the cells, the culture media were collected to measure the expression of murine IL-6 and MCP-1 by sandwich enzyme-linked immunosorbent assays as described previously by our laboratory (8) with antibodies purchased from R & D Systems (Minneapolis, MN). Plates were coated with capture antibodies to IL-6 (2 μg/ml) or to MCP-1 (0.4 μg/ml). Detection antibodies were used at 80 ng/ml for IL-6 and 100 ng/ml for MCP-1. All enzyme-linked immunosorbent assays were performed twice, each time with duplicate samples.
Detection of GPx1 and TR1
Se-deficient osteoblasts were either cultured in 4 μM MSA for at least 2 passages or exposed to MSA for up to 60 min as indicated. The cells were lysed with Mammalian Protein Extraction Reagent (Pierce) containing protease inhibitor cocktail and 1 mM phenylmethylsulfonyl fluoride, centrifuged (14 000g) for 10 min and total protein was determined (BCA Reagent, Pierce). The samples were assayed immediately for total GPx activity, using H2O2 as a substrate (30). The GPx activity was expressed as nanomoles of NADPH oxidized per minute per milligram of protein. Western blots were carried out for GPx1 and TR1.
Results
NF-κB was activated during stimulation with BCCM and was critical for cytokine induction
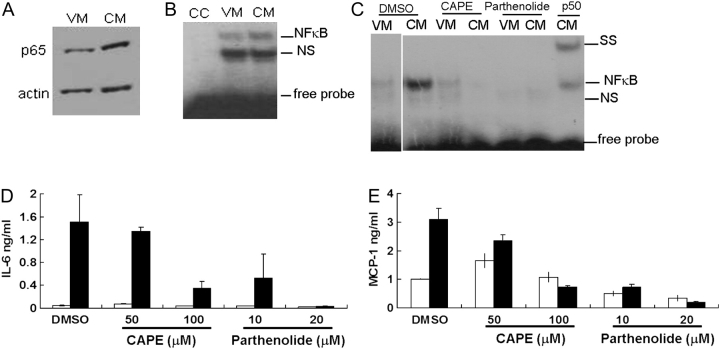
To confirm that NF-κB was activated, we tested for p65 nuclear translocation following osteoblast stimulation by BCCM. Pilot experiments indicated that 1 h following stimulation was optimal for NF-κB activation (data not shown). The presence of nuclear, activated NF-κB was evaluated both by western blotting for p65 and by NF-κB EMSA (Figure 1A–C). We observed an increase in p65 translocation and an increase in DNA-bound NF-κB at 1 h posttreatment indicating that NF-κB was activated by BCCM. In order to demonstrate the importance of NF-κB, we treated MC3T3-E1 cells with NF-κB inhibitors, CAPE and parthenolide in separate experiments, to determine if blocking NF-κB would result in the reduction of the BCCM-cytokine response (Figure 1D and E). Both IL-6 and MCP-1 were reduced by the inhibitors in a dose-dependent manner, whereas vehicle (0.2% DMSO)-treated samples showed no response. Both inhibitors blocked NF-κB translocation into the nucleus (Figure 1C). Thus, both NF-κB translocation and DNA-binding activity were clearly abrogated by both CAPE and parthenolide. To verify that the reduction of cytokines was not due to cell death, viability was tested by trypan blue staining. After 5 h of incubation with parthenolide or 6 h with CAPE and 0.2% DMSO, viability remained nearly 100% even with the highest concentration of inhibitor (data not shown). Together, the data indicated that NF-κB was a common regulator for IL-6 and MCP-1 and was critical for their induction in osteoblasts by BCCM.
Fig. 1.
NF-κB activation in osteoblasts exposed to breast cancer cell-conditioned medium (BCCM). MC3T3-E1 were cultured and differentiated for 2 weeks before stimulation with 50% BCCM for different times. (A) p65 translocation was detected by western blot using nuclear extracts prepared from MC3T3-E1 1 h after treatment with BCCM. (B) NF-κB gel mobility shift analysis (EMSA) of samples prepared as in (A); CC, cold oligonucleotide competitor; NS, non-specific binding; SS, super-shift with anti-p50. (C) Inhibition of NF-κB binding. Osteoblasts were pretreated with inhibitors CAPE for 2 h or with parthenolide for 1 h before addition of BCCM for 1 h. NF-κB–DNA-binding activity was detected by an EMSA p50 supershift. (D) IL-6 and (E) MCP-1 production by osteoblasts treated with NF-κB inhibitors CAPE for 2 h or with parthenolide for 1 h before incubation with BCCM for 4 h. DMSO (0.2%) treatment was used as control. Cytokines in the culture media were detected by enzyme-linked immunosorbent assay. Open bars indicate treatment with VM; closed bars indicate treatment with BCCM. All experiments were performed twice and each sample was assayed in duplicate.
BCCM stimulates NF-κB-regulated COX-2 and iNOS expression
In addition to testing for IL-6 and MCP-1, we evaluated COX-2 and iNOS expression in MC3T3-E1 after treatment with BCCM. After 4 h, COX-2 protein and iNOS messenger RNA both increased with BCCM treatment compared with VM (see Figure 2C and D). These data as well as the induction of IL-6, MCP-1 and IL-8 that we reported earlier (8) suggest that NF-κB controls a wide range of pro-inflammatory reactions in osteoblasts in response to BCCM.
Fig. 2.
Response of osteoblasts to MSA supplementation. Osteoblasts were cultured and differentiated in a Se-deficient culture medium (0.0182 μM Se) for 7 days before supplementation with MSA for another 7 days. After 2 weeks of culture, osteoblasts were treated with 50% BCCM for 4 h. (A) IL-6, (B) MCP-1, (C) COX-2 and (D) iNOS expression were used as indicators of the effects of Se addition. Cytokines were measured by enzyme-linked immunosorbent assay [open bars indicate VM; closed bars indicate BCCM (CM)]; COX-2 expression was detected by western blot and iNOS expression was evaluated by reverse transcription–polymerase chain reaction. β-Actin was used as a loading control in COX-2 and iNOS detection. Cell viability was not affected by 7 days of MSA supplementation as seen by trypan blue staining (data not shown). Indicated are the concentrations of MSA added to the cultures.
Osteoblast response to MSA
There are many reports that Se can decrease NF-κB activation under different situations. However, the responses are cell type and Se compound specific (31,32). For example, Li et al. (33) demonstrated that inorganic Se, selenite, and organic Se, MSA, activated different apoptotic pathways in the same cell type. Therefore, we tested the less toxic form, MSA. Osteoblasts cultured in Se-deficient differentiation medium for 7 days before addition of MSA for another 7 days were treated with BCCM for 4 h. IL-6 and MCP-1 concentrations were measured in the culture media (Figure 2A and B). With MSA supplementation, both IL-6 and MCP-1 declined in a dose-dependent manner. Trypan blue staining ensured that the reduction in cytokines was not due to cell death. These data indicated that MSA was a biologically effective Se compound capable of reducing the cytokine response of osteoblasts toward BCCM. Furthermore, we examined the effect of MSA on the expression of iNOS and COX-2 induction by BCCM (Figure 2C and D). We saw that both were reduced in the presence of MSA suggesting that a wide range of inflammatory responses could be regulated by MSA. Interestingly, a comparison of the dose responses of iNOS, COX-2 and IL-6 indicated that >1 μM MSA was required.
GPx1 and TR1 may be markers of adequate Se status but might not reflect the inflammatory status
Selenium, required for the maintenance of intracellular redox status, is critical to both biosynthesis and function of reactive oxygen species-scavenging enzymes, e.g. GPx. Because the MSA-supplemented osteoblasts produced a reduced inflammatory response to BCCM, we tested if the effect was related to the level of GPx activity, which in turn, affects intracellular oxidative stress.
Total cell lysates from osteoblasts cultured in MSA-containing medium were used to measure GPx activity (Figure 3A). In the absence of added Se, osteoblasts showed no detectable GPx activity. With the gradual increase of MSA supplementation, the activity was restored. An obvious increase in GPx activity was detected with as little as 0.05 μM MSA supplementation, which implied that osteoblasts could absorb and use MSA efficiently. However, GPx activity appeared to be almost saturated when osteoblasts were supplemented with 1 μM MSA (Figure 3A); higher concentrations of MSA caused no additional increase in GPx activity (data not shown). On the other hand, we found that MSA supplementation of 1 μM had little or no effect on IL-6 and MCP-1 production. In addition, we examined the expression of both GPx1 and TR1 (Figure 3B and C). Both proteins increased with the addition of as little as 0.05 μM MSA. Saturation occurred by ∼0.5–1 μM. These results suggested that while GPx and TR synthesis were sensitive to Se supplementation, additional Se-containing ‘factors’ could probably be involved in the downregulation of IL-6 and MCP-1 production.
Fig. 3.
Intracellular GPx1 and TR1 in MSA-supplemented osteoblasts. Osteoblasts were cultured in Se-deficient medium in the absence or presence of MSA (A and C) for 2 passages at the MSA concentration indicated or (B) Osteoblasts were cultured in Se-deficient medium for 1 week and then in the presence of MSA for another week as described in Materials and Methods. Cell lysates were used to measure GPx activity (A), with hydrogen peroxide as substrate. The activity is presented as nanomoles of NADPH oxidized per minute per milligram of protein. Western blot was used to assay the levels of GPx1 (B) or TR1 (C). GAPDH was used as a loading control. The results were quantified using ImageQuant 5.1 software and the ratio between the protein and corresponding GAPDH is shown in the bar graphs.
MSA supplementation blocked the activation of NF-κB by BCCM in osteoblasts
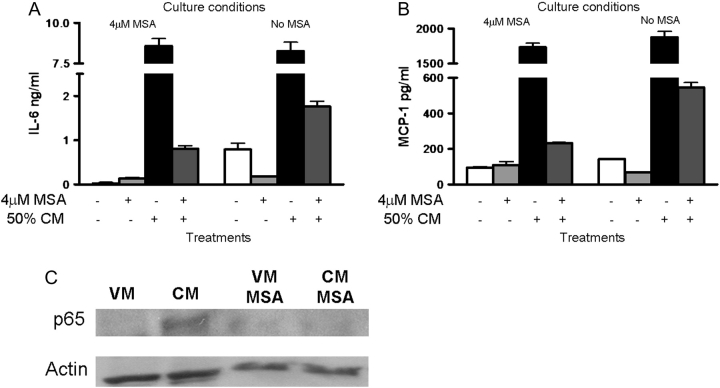
To determine if MSA regulated IL-6 and MCP-1 expression through inhibition of NF-κB, we examined the activation of NF-κB after MSA treatment. The translocation of p65 into the nuclear fraction was detected by western blot of osteoblasts supplemented with MSA (0.1 μM to 4 μM) and treated with VM or BCCM for 1 h (Figure 4A). Accumulation of p65 in the nuclear fraction was apparent in cells without MSA treatment. With MSA supplementation as low as 1 μM, less p65 translocated. With 4 μM MSA, no nuclear p65 was detectable. The EMSA findings also supported this result (Figure 4B). DNA-bound NF-κB was inversely correlated to the amount of MSA added to the osteoblasts and treated with BCCM. However, when osteoblasts were supplemented with 4 μM MSA, BCCM failed to activate NF-κB. These data suggested that MSA supplementation of the cells inhibited the activation of NF-κB and consequently reduced the expression of its target genes.
Fig. 4.
NF-κB inhibition by MSA supplementation. Osteoblasts were cultured in Se-deficient medium (0.0182 μM) for 7 days before adding MSA to the culture system for another 7 days. On day 14, osteoblasts were treated with BCCM for 1 h and nuclear extracts were prepared. NF-κB activation was measured by (A) p65 translocation and (B) NF-κB gel mobility shift analysis (EMSA). Actin was used as a loading control; CC, cold oligonucleotide competitor; NS, non-specific binding.
Because of the discordance between the dose response of MSA for GPx activity and the expression of GPx and TR proteins and the expression of inflammatory cytokines, we asked if MSA might act through a mechanism that did not involve a selenoprotein (34). MC3T3-E1 were grown for 2 weeks with Se-deficient medium either with or without supplementation with 4 μM MSA for the last week. Concurrent with the addition of BCCM, 4 μM MSA was added to osteoblasts that were or were not supplemented with MSA. As seen previously, addition of BCCM to cells supplemented for 7 days with MSA showed an attenuated (<10%) cytokine response (Figure 5A and B, left). However, when 4 μM MSA was added at the same time as BCCM to osteoblasts that had not previously seen MSA, there was a similar reduction in IL-6 (<20%) (Figure 5A and B, right). We also noticed that the basal levels of cytokines in cells that were exposed to VM and MSA were lower than those with VM alone (21 versus 792 pg/ml) suggesting that MSA acted directly on the cells and not on factors in the BCCM. We also found that addition of MSA together with BCCM prevented the activation of NF-κB (Figure 5C).
Fig. 5.
Osteoblasts response to BCCM following a short exposure to MSA. Osteoblasts were cultured in Se-deficient medium for 7 days before the addition of 4 μM MSA to the culture system for another 7 days. On day14, osteoblasts were treated for 2 h with VM or 50% BCCM in the presence or absence of 4 μM MSA. The supernatants were collected for enzyme-linked immunosorbent assay for (A) IL-6 and (B) MCP-1. Shown are the average values for 2 cultures carried out for each condition as indicated. (C) Some cells were treated for 1 h and the nuclear extracts were collected to assay translocation of p65 by western blotting. β-Actin was used as a loading control.
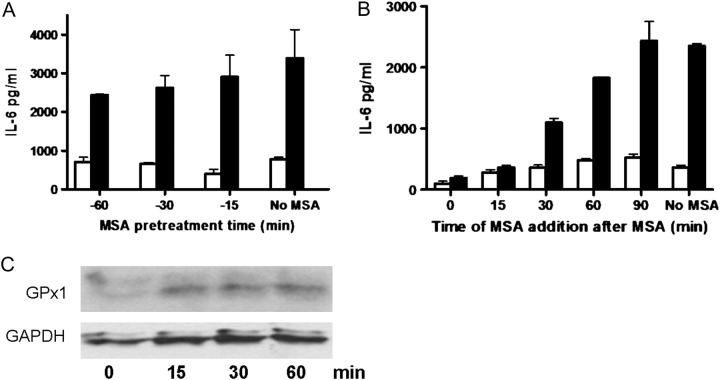
To further eliminate the possibility that MSA interacted with components in the BCCM as opposed to acting on the cells, we added MSA prior to the addition of BCCM. The MSA incubation was carried out for various times up to 60 min before the cells were washed and BCCM was added. MSA pretreatment for as little as 15 min was effective in reducing the osteoblast cytokine response (Figure 6A) although to a lesser extent than when MSA and BCCM were added together (15% inhibition versus 97.2% inhibition). Longer times of pretreatment showed greater inhibition; i.e. 60 min pretreatment with MSA brought about a 28.2% reduction in IL-6. These data indicated that MSA might be acting through short-lived metabolites as reported by others (34). Therefore, we tested the effect of adding MSA following the addition of BCCM. Addition of MSA at 15 min post BCCM held the production of IL-6 to the same levels as when MSA was added simultaneously with the BCCM (Figure 6B). After 30 and 60 min, the levels of IL-6 increased in proportion to the time of delay of MSA addition. By 90 min there was no inhibition.
Fig. 6.
The effect of exposure to MSA prior to or after treatment with BCCM on IL-6 production. Osteoblasts were cultured in Se-deficient medium for 14 days. (A) On day 14, the medium was removed and fresh medium containing 4 μM MSA was added and allowed to remain for 15, 30 or 60 min and then removed and the cultures were washed once with phosphate-buffered saline. VM or 50% BCCM without MSA was added for 2 h and the supernatants were collected for cytokine measurement. Shown are the average IL-6 concentrations (±SD) for 3 plates of cells. (B) On day 14, VM or 50% BCCM was added to the osteoblasts in the absence of MSA. At 0, 15, 30, 60 or 90 min, 4 μM MSA was added to the cultures. Cells were incubated for a total of 2 h after addition of BCCM before the supernatants were collected for cytokine measurements. Shown are the average IL-6 values for 2 cultures. One set of cultures was never exposed to MSA (no MSA). Open bars indicate treatment with VM; closed bars indicate treatment with BCCM. (C) Lysates of cells treated as in (A) were examined for GPx1 expression by western blot. GAPDH was used as a loading control.
In order to determine if selenoproteins were involved in this short-term response, we examined GPx expression levels. Western blot analysis indicated that even 15 min exposure of MC3T3-E1 to 4 μM MSA was sufficient to increase the levels of GPx (Figure 6C).
Discussion
In this study, we investigated the ability of Se to alleviate the NF-κB-dependent regulation of the inflammatory response of osteoblasts to BCCM. We found that not only the production of IL-6 and MCP-1 but also other inflammatory molecules, COX-2 and iNOS, were significantly enhanced. These findings indicated that a wide range of inflammatory responses, triggered upon interaction with the BCCM, was primarily driven by the activation of NF-κB. The response was greater in cells grown in Se-deficient medium than in cells grown in medium supplemented with MSA. MSA effectively inhibited activation of NF-κB and subsequently the production of IL-6, MCP-1, iNOS and COX2. Surprisingly to us, short (minute to hour) exposure to MSA was sufficient to suppress the inflammatory response. This result implied that MSA possibly produced short-lived active metabolites. However, during this time MSA also increased the synthesis of major selenoproteins, GPx1 and possibly others. Thus, MSA may suppress NF-κB and the osteoblast stress response through metabolites and/or selenoproteins yet to be identified.
Se, an essential element in the mammalian diet, provides protection from oxidative damage (35). Severe Se deficiency observed primarily in certain areas in China is associated with Kashin–Beck, an endemic osteoarticular disease with degenerative and necrotic lesions of chondrocytes (35). Several reports indicate that bone tissues in humans are affected by Se (36,37). For example, in healthy infants, there is a correlation between the Se level in the urine and bone resorption (37) suggesting a link between Se and bone turnover. However, the importance and detailed mechanism of Se action remain unclear.
Se is also known to prevent degenerative diseases, such as atherosclerosis, arthritis, central nervous system pathologies, altered immunological function and cancers (18). Epidemiological studies describe an inverse correlation between Se levels and cancers, such as stomach, pancreas, lung and breast (18). In the Nutritional Prevention of Cancer Trial (38), researchers tested the effect of Se supplementation on cancer incidence in a large population. The results indicated an impressive preventative effect of Se supplementation, especially for lung, colorectal and prostate cancers (39). However, only very few of the subjects’ (6/1312) plasma levels were below 80 ng/ml Se (40), the minimum requirement needed to produce maximum selenoproteins. This finding suggests that the preventive mechanism of Se is not through well-known selenoproteins, such as GPx, but through other selenoproteins or active metabolites. In a more recent study, the selenium and vitamin E cancer prevention trial, seleno-methionine or vitamin E supplementation did not prevent the occurrence of new cases of prostate cancer in a relatively healthy population (41). While there may be design questions with this trial (42), for our purposes, this study did not deal with metastasis, specifically mestastasis to bone, a common site for prostate cancer.
Organic Se is present in foods in the form of seleno-methionine, selenocysteine and methylselenocysteine, whereas inorganic Se is usually in the form of selenate or selenite in the soil (43). After ingestion, these compounds follow different metabolic pathways but eventually form a common intermediate, selenide (Se2−), which is incorporated into proteins (44). Organo-Se compounds, metabolized through lyases, more probably form methylselenol (CH3SeH) first. When cells generate too much selenide, it reacts with oxygen to produce superoxide radicals (O2−), which are toxic (44). On the other hand, it is a well accepted that CH3SeH is involved in the anticancer effects of Se (44). Taken together, different forms of Se compounds may enter the metabolic pathway at different points and may have different effects on different types of cells (31,32). In this regard, MSA, unlike selenite, has been demonstrated not to cause oxidative stress and is therefore less toxic (24). Thus, we restricted our investigation to MSA. Data (Figures 5 and 6) suggested that the NF-κB inhibitory activity of MSA may be attributed, in part, to the production of ‘bioactive metabolites’ as described by Juliger et al. (34), in addition to the synthesis of selenoproteins. Based on many papers in the literature, saturation of GPx1 expression in most eukaryotic cells occurs at 0.05–0.075 μM of sodium selenite as opposed to that shown here with high concentrations (0.1–0.5 μM) of MSA. Such a lag in the concentration required to saturate Gpx1 lends credence to the idea that bioactive metabolites formed from MSA may partake an important role in the inactivation of NF-κB. These intriguing possibilities need to be elucidated in detail in future studies to account for the anti-inflammatory property of Se.
The data strongly indicated that NF-κB plays an important role in the inflammatory responses of osteoblasts to BCCM. Therefore, the inhibition of NF-κB activation, through active Se metabolites and selenoproteins, is a possible approach to reduce cytokines and other inflammatory molecules. The importance of alleviating the bone inflammatory response becomes even more critical because bone-remodeling process shares similarities with an inflammatory response (45). Many inflammatory molecules that are secreted by immune cells are produced by osteoblasts. Many, such as IL-1, IL-6, IL-8, MCP-1, prostaglandin E2 and COX-2, are also osteoclastogenic (46) i.e. they attract and activate osteoclasts, resulting in bone matrix degradation and the release of growth factors including TGF-β (1) from the matrix. The abundance of growth factors makes the bone microenvironment more affable to metastases. IL-6 receptors are expressed by osteoclasts, and when stimulated, initiate osteoclast differentiation and the bone resorption process (9). Some reports indicate that increased plasma IL-6 is an indicator of metastasis (47). IL-6 is also associated with increased breast cancer cell migration(46). MCP-1 regulates bone resorption by stimulating the migration of monocyte–osteoclast progenitor cells to the bone (8,9), in addition to increasing angiogenesis and cancer cell survival (46). COX-2, through prostaglandin E2 production, is associated with growth, invasion, apoptosis and angiogenesis in breast cancer (46). Taken together, their increased expression by osteoblasts may affect the equilibrium in the bone microenvironment and promote osteolytic bone metastasis. Currently there is no way to restore existing osteolytic lesions; however, limiting the effect of bone metastasis using MSA may significantly improve the quality of life of individuals with breast cancer bone metastasis.
Metastatic lesions often originate from tumor cells after a long period of dormancy in the target tissue (48). In breast cancer, tumor cells may disseminate early from non-invasive tumors (49) but remain dormant in the secondary tissue or in the lymph nodes for years (50). All together, it may be impossible to totally prevent metastasis since it is difficult to define the time or the critical stages of this event. However, reducing the damage from the metastases is still beneficial. Dietary Se supplementation can increase intracellular Se levels sufficient to produce useful ‘bioactive’ intermediates and selenoproteins. By the time dormant micrometastases in the bone begin to proliferate, the higher Se levels in osteoblasts may prevent or reduce their responses to cancer cells, which in turn may slow the metastatic growth and break the vicious cycle. There are many reports that Se functions in both cancer initiation and progression. Our results offer the possibility that Se can also affect the metastasis process. We plan to use a mouse breast cancer model to evaluate the effect of MSA on metastasis in vivo.
In summary, we have demonstrated that Se supplementation significantly reduced the inflammatory response of osteoblasts to metastatic breast cancer cells by inactivating the nuclear translocation of NF-κB. The expression of NF-κB-dependent genes, which play an important role in metastasis, were downregulated implying that Se supplementation may negatively impact the metastasis process.
Funding
American Institute for Cancer Research (06027); with added support from National Foundation for Cancer Research, Center for Metastatic Research, University of Alabama at Birmingham to A.M.M.; National Institutes of Health (R01 DK 077152 to K.S.P.); The President's Fund for Undergraduate Research to L.N.
Acknowledgments
We thank Drs Karam El-Bayoumy and Arun Das for measuring the Se content of the serum.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BCCM
breast cancer cell-conditioned medium
- CAPE
caffeic acid phenethyl ester
- COX-2
cyclooxygenase-2
- DMSO
dimethyl sulfoxide
- EMSA
electrophoretic mobility shift assay
- FBS
fetal bovine serum
- GPx
glutathione peroxidase
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- MCP-1
monocyte chemoattractant protein-1
- MSA
methylseleninic acid
- NF-κB
nuclear factor-κB
- Se
selenium
- TGF
transforming growth factor
- TR
thioredoxin reductase
- VM
vehicle control medium
References
- 1.Guise TA, et al. Cancer and bone. Endocr. Rev. 1998;19:18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 2.Lipton A. Bisphosphonates and breast carcinoma: present and future. Cancer. 2000;88:3033–3037. doi: 10.1002/1097-0142(20000615)88:12+<3033::aid-cncr20>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Delmas PD, et al. Serum bone gamma carboxyglutamic acid-containing protein in primary hyperparathyroidism and in malignant hypercalcemia. Comparison with bone histomorphometry. J. Clin. Invest. 1986;77:985–991. doi: 10.1172/JCI112400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart AF, et al. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J. Clin. Endocrinol. Metab. 1982;55:219–227. doi: 10.1210/jcem-55-2-219. [DOI] [PubMed] [Google Scholar]
- 5.Mastro AM, et al. Breast cancer cells induce osteoblast apoptosis: a possible contributor to bone degradation. J. Cell. Biochem. 2004;91:265–276. doi: 10.1002/jcb.10746. [DOI] [PubMed] [Google Scholar]
- 6.Mercer RR, et al. Metastatic breast cancer cells suppress osteoblast adhesion and differentiation. Clin. Exp. Metastasis. 2004;21:427–435. doi: 10.1007/s10585-004-1867-6. [DOI] [PubMed] [Google Scholar]
- 7.Phadke PA, et al. Kinetics of metastatic breast cancer cell trafficking in bone. Clin. Cancer Res. 2006;12:1431–1440. doi: 10.1158/1078-0432.CCR-05-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinder M, et al. Metastatic breast cancer induces an osteoblast inflammatory response. Exp. Cell Res. 2008;314:173–183. doi: 10.1016/j.yexcr.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Manolagas SC. Role of cytokines in bone resorption. Bone. 1995;17:63S–67S. doi: 10.1016/8756-3282(95)00180-l. [DOI] [PubMed] [Google Scholar]
- 10.Balkwill F, et al. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 11.Fritz EA, et al. Titanium particles induce the immediate early stress responsive chemokines IL-8 and MCP-1 in osteoblasts. J. Orthop. Res. 2002;20:490–498. doi: 10.1016/S0736-0266(01)00154-1. [DOI] [PubMed] [Google Scholar]
- 12.Taranova AG, et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res. 2008;68:8582–8589. doi: 10.1158/0008-5472.CAN-08-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baud V, et al. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamamiri-Davis F, et al. Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency. Free Radic. Biol. Med. 2002;32:890–897. doi: 10.1016/s0891-5849(02)00775-x. [DOI] [PubMed] [Google Scholar]
- 15.Prabhu KS, et al. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: role of nuclear factor-kappaB in up-regulation. Biochem. J. 2002;366:203–209. doi: 10.1042/BJ20020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornstedt M, et al. Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols. J. Biol. Chem. 1995;270:11761–11764. doi: 10.1074/jbc.270.20.11761. [DOI] [PubMed] [Google Scholar]
- 17.Saito Y, et al. Selenoprotein P in human plasma as an extracellular phospholipid hydroperoxide glutathione peroxidase. Isolation and enzymatic characterization of human selenoprotein p. J. Biol. Chem. 1999;274:2866–2871. doi: 10.1074/jbc.274.5.2866. [DOI] [PubMed] [Google Scholar]
- 18.Patrick L. Selenium biochemistry and cancer: a review of the literature. Altern. Med. Rev. 2004;9:239–258. [PubMed] [Google Scholar]
- 19.Mantovani G, et al. Reactive oxygen species, antioxidant mechanisms and serum cytokine levels in cancer patients: impact of an antioxidant treatment. J. Cell. Mol. Med. 2002;6:570–582. doi: 10.1111/j.1582-4934.2002.tb00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guven M, et al. Lipid peroxidation and antioxidant system in the blood of cancerous patients with metastasis. Cancer Biochem. Biophys. 1999;17:155–162. [PubMed] [Google Scholar]
- 21.Roebuck KA, et al. Down-regulation of procollagen alpha1[I]] messenger RNA by titanium particles correlates with nuclear factor kappaB (NF-kappaB) activation and increased rel A and NF-kappaB1 binding to the collagen promoter. J. Bone Miner Res. 2001;16:501–510. doi: 10.1359/jbmr.2001.16.3.501. [DOI] [PubMed] [Google Scholar]
- 22.Sudo H, et al. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Biol. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cailleau R, et al. Breast tumor cell lines from pleural effusions. J. Natl Cancer Inst. 1974;53:661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ip C, et al. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- 25.Li GX, et al. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis. 2008;29:1005–1012. doi: 10.1093/carcin/bgn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haddad JJ. Nuclear factor (NF)-kappa B blockade attenuates but does not abrogate LPS-mediated interleukin (IL)-1 beta biosynthesis in alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2002;293:252–257. doi: 10.1016/S0006-291X(02)00213-9. [DOI] [PubMed] [Google Scholar]
- 27.Hehner SP, et al. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J. Immunol. 1999;163:5617–5623. [PubMed] [Google Scholar]
- 28.Palempalli UD, et al. Gambogic acid covalently modifies IkappaB kinase-beta subunit to mediate suppression of lipopolysaccharide-induced activation of NF-kappaB in macrophages. Biochem. J. 2009;419:401–409. doi: 10.1042/BJ20081482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vunta H, et al. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J. Biol. Chem. 2007;282:17964–17973. doi: 10.1074/jbc.M703075200. [DOI] [PubMed] [Google Scholar]
- 30.Paglia DE, et al. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 31.Park JM, et al. Methylseleninic acid inhibits PMA-stimulated pro-MMP-2 activation mediated by MT1-MMP expression and further tumor invasion through suppression of NF-kappaB activation. Carcinogenesis. 2007;28:837–847. doi: 10.1093/carcin/bgl203. [DOI] [PubMed] [Google Scholar]
- 32.Jiang C, et al. Monomethyl selenium–specific inhibition of MMP-2 and VEGF expression: implications for angiogenic switch regulation. Mol. Carcinog. 2000;29:236–250. doi: 10.1002/1098-2744(200012)29:4<236::aid-mc1006>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.Li GX, et al. Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells. Int. J. Cancer. 2007;120:2034–2043. doi: 10.1002/ijc.22480. [DOI] [PubMed] [Google Scholar]
- 34.Juliger S, et al. Chemosensitization of B-cell lymphomas by methylseleninic acid involves nuclear factor-kappaB inhibition and the rapid generation of other selenium species. Cancer Res. 2007;67:10984–10992. doi: 10.1158/0008-5472.CAN-07-0519. [DOI] [PubMed] [Google Scholar]
- 35.Turan B, et al. Selenium combined with vitamin E and vitamin C restores structural alterations of bones in heparin-induced osteoporosis. Clin. Rheumatol. 2003;22:432–436. doi: 10.1007/s10067-003-0809-z. [DOI] [PubMed] [Google Scholar]
- 36.Moreno-Reyes R, et al. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Bone Miner Res. 2001;16:1556–1563. doi: 10.1359/jbmr.2001.16.8.1556. [DOI] [PubMed] [Google Scholar]
- 37.Tsukahara H, et al. Selenium status and skeletal tissue metabolism in young infants. Eur. J. Pediatr. 1996;155:148–149. doi: 10.1007/BF02075776. [DOI] [PubMed] [Google Scholar]
- 38.Clark LC, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 39.Combs GF, Jr, et al. An analysis of cancer prevention by selenium. Biofactors. 2001;14:153–159. doi: 10.1002/biof.5520140120. [DOI] [PubMed] [Google Scholar]
- 40.Duffield-Lillico AJ, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol. Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 41.Lippman SM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kristal AR. Are clinical trials the “gold standard” for cancer prevention research? Cancer Epidemiol. Biomarkers Prev. 2008;17:3289–3291. doi: 10.1158/1055-9965.EPI-08-1066. [DOI] [PubMed] [Google Scholar]
- 43.Rikiishi H. Apoptotic cellular events for selenium compounds involved in cancer prevention. J. Bioenerg. Biomembr. 2007;39:91–98. doi: 10.1007/s10863-006-9065-7. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki KT, et al. Metabolic transformation of methylseleninic acid through key selenium intermediate selenide. Toxicol. Appl. Pharmacol. 2006;215:189–197. doi: 10.1016/j.taap.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Rodan GA. The development and function of the skeleton and bone metastases. Cancer. 2003;97:726–732. doi: 10.1002/cncr.11147. [DOI] [PubMed] [Google Scholar]
- 46.Bussard KM, et al. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- 47.Yoneda T, et al. Neutralizing antibodies to human interleukin 6 reverse hypercalcemia associated with a human squamous carcinoma. Cancer Res. 1993;53:737–740. [PubMed] [Google Scholar]
- 48.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schardt JA, et al. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell. 2005;8:227–239. doi: 10.1016/j.ccr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Karrison TG, et al. Dormancy of mammary carcinoma after mastectomy. J. Natl Cancer Inst. 1999;91:80–85. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]