Abstract
We tested whether the nitric oxide donor, (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) aminio] diazen-1-ium-1,2-diolate (DETA-NONOate), increases expression of Angiopoietin (Ang1)/Tie2, which may play a role in regulating angiogenesis and vascular integrity after stroke in rats. Wistar rats were subjected to middle cerebral artery occlusion and treated with or without DETA-NONOate. Stroke rats treated with DETA-NONOate show significantly increased Ang1, Tie2 and Occludin expression in the ischemic border compared with control stroke animals (p < 0.05). Consistent with in vivo data, DETA-NONOate promotes capillary tube formation in cultured brain endothelial cells. Neutralizing Ang1 antibody attenuates DETA-NONOate-induced capillary tube formation. The data suggest that the Ang1/Tie2 axis promotes DETA-NONOate-induced angiogenesis and stabilizes of angiogenic vessels after stroke.
Keywords: Angiogenesis, DETA-NONOate, Angiopoietin1, Tie2, Occludin, Stroke
Nitric oxide (NO) regulates vascular tone, vascular remodeling and angiogenesis [20,23]. Molecules able to increase NO levels induce endothelial cell proliferation and migration in vivo and in vitro [27]. Increased NO production enhances endothelial cell function, and helps preserve the arteriogenic potential of preexisting collateral networks [21]. DETA-NONOate, an NO donor, promotes angiogenesis and improves neurological outcome after stroke in young adult rats [25]. Endothelial cell-derived NO induces mural cell recruitment and stabilization of angiogenic vessels [15]. Decreased plasma NO concentration by treatment with L-NAME decreases tight junction protein Occludin expression in the vessel walls of the brain [14]. However, the mechanisms of NO-induced stabilization of angiogenic vessels have not been fully elucidated.
Angiopoietin 1 (Ang1) is a family of endothelial growth factors that function as ligands for the endothelial-specific receptor tyrosine kinase, Tie2. The Ang1/Tie2 system controls pericyte recruitment, endothelial cell survival, and is implicated in blood vessel formation and vascular stabilization [13]. Transgenic overexpression of Ang1 increases vascularization [24], prevents plasma leakage in the ischemic brain, and consequently decreases ischemic lesion volume [19,26]. Ang1 acts directly on mural cells or their precursors to facilitate their recruitment to new blood vessels [19]. A combination of the submaximal doses of Ang1 and VEGF enhance these effects, and are more potent than the maximal dose of either alone [3]. In addition, Ang1-induced angiogenesis requires endothelium-derived NO [1]. Exposure to Ang1 also increases endothelial cell migration and eNOS phosphorylation [11].
In this study, we test whether DETA-NONOate promotes stabilization of angiogenic vessels and the mechanisms of DETA-NONOate-induced angiogenesis after stroke. We hypothesize that DETA-NONOate regulates Ang1/Tie2 and tight junction protein expression, which plays an important role in DETA-NONOate-induced angiogenesis after stroke in rats [25].
Adult male Wistar rats (n = 20) weighing 270–300 g were employed in all our experiments. Transient right middle cerebral artery occlusion (MCAo) was induced for 2 h by advancing a 4-0 surgical nylon suture (18.5–19.5 mm) with an expanded (heated) tip from the external carotid artery into the lumen of the internal carotid artery to block the origin of the MCA [5]. After MCAo, rats were randomly divided into two groups: Group 1 (n = 10), injected with phosphate buffered saline (PBS 1 ml) via tail the vein as control; and Group 2 (n = 10), injected with DETA-NONOate via the tail vein (0.4 mg/kg). Both groups of rats were treated 24 h after MCAo. Rats (n = 3/group) were sacrificed after 3 days for isolation of brain endothelial cells, with additional rats (n = 7/group) sacrificed after 14 days for immunohistochemical staining. Our previous study demonstrated that DETA-NONOate treatment of stroke in the rat initiated at 24 h after stroke does not alter infarct size compared to control animals [4].
Rats were sacrificed 14 days after stroke. The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. A standard paraffin block was obtained from the center of the lesion (bregma −1 to +1 mm). A series of 6 μm thick sections were cut from the block. Every 10th coronal section for a total five sections was used for immunohistochemical staining. Antibody against Ang1 (rabbit polyclonal IgG, 1:2000, Abcam), Tie2 (rabbit polyclonal IgG antibody, 1:80 dilution, Santa Cruz) and Occludin (Mouse monoclonal IgG, 1:200 dilution, Zymed) were employed. Control experiments consisted of staining brain coronal tissue sections as outlined above, but omitted the primary antibodies, as previously described [16].
For quantitative measurements of Ang1, Tie2 and Occludin, five slides from each brain, with each slide containing eight fields from the ischemic penumbra zone (IBZ) were digitized under a 20× objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research, St. Catharines, Canada) [7]. The digitalized images were contrast-enhanced to clearly differentiate positively labeled cells from background, and a thresholding procedure was established to determine the proportion of immunoreactive area within each fixed field of view [2,6]. The data are presented as a percentage of positive immunoreactivity area in the ischemic border area.
Rats were sacrificed 3 days after MCAo [28]. The ischemic brain cortex was isolated and placed in 6 ml of buffer A (20 mM NaHCO3, 10 mM Glucose, 1 mM Na Pyruvate, 0.5% BSA) and 30% dextran solution. The mixture was then spun at 6000 × g for 15 min at 4 °C, and the supernatant was removed. The remaining pellet was passed though a 100 um nylon mesh, (Becton Dickinson), collecting the filtrate. This filtrate was passed though a 40 um nylon mesh (Becton Dickinson). The microvessels collected on the mesh were washed and spun down, then stored at −80 °C until used for RNA and/or protein isolation.
Brain endothelial cells were harvested and total RNA was isolated from treated cells with TRIzol (Invitrogen), following a standard protocol [17]. Quantitative PCR was performed using the SYBR Green real time PCR method. Quantitative PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA, USA) using three-stage program parameters provided by the manufacturer, as follows: 2 min at 50 °C, 10 min at 95 °C, and then 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Each sample was tested in triplicate, and analysis of relative gene expression data using the 2−ΔΔCT method. The following primers for real-time PCR were designed using Primer Express software (ABI). Ang1: FWD, TATTTTGTGATTCTGGTGATT; REV, GTTTCGCTTTATTTTTGTAATG; Tie2: FWD, CGGCCAGGTACATAGGAGGAA; REV, TCACATCTCCGAACAATCAGC.
Protein was isolated from brain endothelial cells with TRIzol (Invitrogen) following standard protocol. Protein concentrations were determined by a DC protein assay kit (Bio-Rad, Hercules, CA, USA). Protein samples were electrophoresed on gradient sodium dodecyl sulfate–polyacrylamide gel (Bio-Rad) and subsequently electrotransferred to nitrocellulose membranes. Membranes were treated with blocking buffer (5% skimmed milk in 25 mm Tris–HCl pH 8.0, 125 mm NaCl, 0.1% Tween 20) for 1 h at room temperature, followed by incubation with primary antibodies for anti-β-actin (1:2000; Sigma, St. Louis, MO, USA), anti-Ang1 (1 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-Tie2 (1 μg/mL; Santa Cruz Biotechnology) for 16 h at 4 °C. The membranes were washed with blocking buffer without milk, and then incubated with horseradish peroxidase-conjugated secondary antibody in blocking buffer.
To examine if DETA-NONOate induces angiogenesis, a capillary-like tube formation assay was performed [10]. Briefly, 0.1 ml of 4 °C growth factor reduced Matrigel (Becton Dickinson) was added per well of a 96 well plate, and allowed to polymerize at 37 °C for 30 min. Mouse brain endothelial cells (MBECs, ATCC, CRL-2299, 2 × 104 cells) were incubated in (1) regular cell culture medium (DMEM) for control; (2) DETA-NONOate (0.4 μM); (3) neutralized Ang1 antibody (0.3125 μg/ml, Rabbit anti-angiopoietin-1 affinity purified polyclonal antibody, Chemicon); and (4) DETA-NONOate (0.4 μM) with neutralized Ang1 antibody (0.3125 μg/ml). All assays were performed in triplicate. The total length of tube formation was measured at 5 h after treatment. For quantitative measurements of capillary tube formation, Matrigel wells were digitized under a 2.5× objective (Olympus BX40) for measurement of total tube length of capillary tube formation using a video camera (Sony DXC-970MD) interfaced with MCID image analysis system. Tracks of endothelial cells organized into networks of cellular cords (tubes) were counted and averaged in randomly selected three microscopic fields [22].
Two-sample t-test was used to test for Ang1, Tie2 and Occludin expression and tube formation between groups with and without DETA-NONOate treatment at the significance level of 0.05. Data are presented as mean ± S.E.
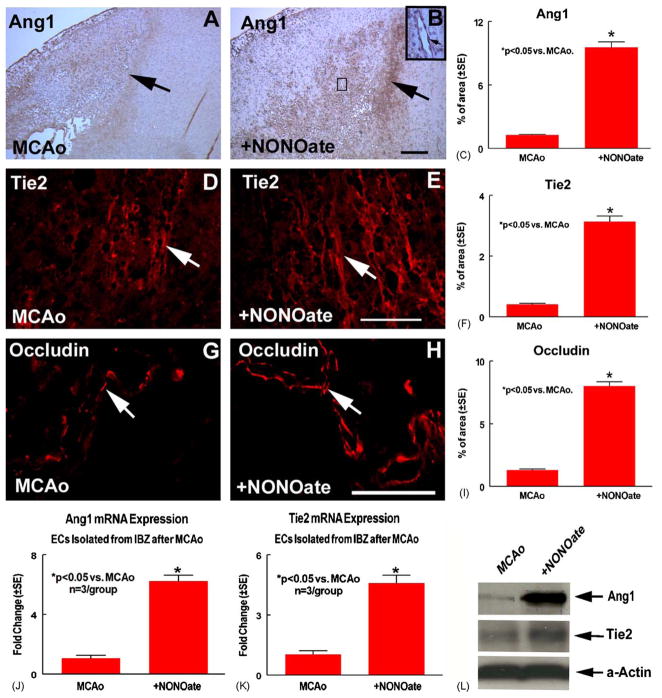
The Ang1/Tie2 pathway promotes angiogenesis [13] and induces the expression of the tight junction protein Occludin [12]. Fig. 1 shows that Ang1 (B, arrow shows Ang1 positive cell), Tie2 (E, arrow shows Tie2 positive cell and F) and Occludin (H, arrow shows Occludin positive cell) expression significantly increased in the ischemic border in the DETA-NONOate treatment group compared with the MCAo control group (A for Ang1; D for Tie2 and G for Occludin), respectively. Fig. 1C, F and I present quantitative data of Ang1(C), Tie2 (F) and Occludin (I) expression in the ischemic border.
Fig. 1.
DETA-NONOate treatment of stroke rats increase Ang1, Tie2 and Occludin expression. MCAo rats were treated with or without DETA-NONOate after stroke. Panels A, D and G show Ang1 (A), Tie2 (D) and Occludin (G) expression in MCAo control rats, respectively. Panels B, E and H show Ang1 (B), Tie2 (E) and Occludin (H) expression in the DETA-NONOate-treated rats, respectively. Panels C, F and I show quantitative data of Ang1 (C), Tie2 (F) and Occludin (I) positive cells percentage of area in the ischemic border. DETA-NONOate treatment animals should significant increases in expression of Ang1, Tie2 and Occludin compared to control animals. Scale bar B = 100 μm; E and H = 50 μm. Panels J–K show Ang1 (J) and Tie2 (K) gene expression in the ischemic brain endothelial cell were significantly increased compared to control stroke rats (n = 3/group). Panel L shows that Ang1 and Tie2 protein expression in the ischemic brain endothelial cells were increased in DETA-NONOate treated rat compared to control stroke rat.
DETA-NONOate treatment increases Ang1 and Tie2 expression in the ischemic border. The increased Ang1 and Tie2 expression may due to (1) truly upregulation of Ang1/Tie expression or (2) a higher vascular density, thus more Ang1/Tie2 signal overall. To determine whether DETA-NONOate increases Ang1/Tie2 expression or whether the increased Ang1/Tie2 expression is due to increased vascular density, brain microvessels were isolated from the ischemic border after stroke. Ang1 and Tie2 gene and protein expression were measured. Fig. 1J–L show that DETA-NONOate treatment of stroke rats significantly increases Ang1 (J) and Tie2 (K) gene expression and Ang1 and Tie2 protein expression (L) in the same number of amount of vessels in the DETA-NONOate treated group compared to the control MCAo animals. These data suggest that DETA-NONOate truly upregulates vessel Ang1/Tie2 expression in the ischemic border after stroke, and this increase is not attributed to an increase in vascular density.
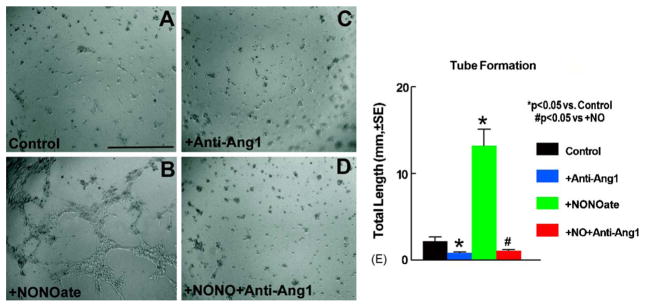
We have previously shown that treatment of stroke with DETA-NONOate induces angiogenesis [25]. To further test whether Ang1 plays a role in DETA-NONOate-induced angiogenesis, we performed a tube formation assay. Fig. 2 shows that 5 h with DETA-NONOate treatment significantly induces MBECs to form a network of capillary-like structures with numerous intercellular contacts (B and E) compared with control medium (A and E). Ang1 neutralizing antibody partially decreases DETA-NONOate-induced tube formation (C, D and E). These data suggest that Ang1 may contribute to DETA-NONOate induced angiogenesis.
Fig. 2.
Capillary-like tube formation. Panels A–D show tube formation. When MBECs were incubated with a regular medium, capillary-like tube formation was not detected (A). However, incubation with DETA-NONOate (0.4 μM) significantly induced capillary-like tube formation (B). A neutralizing antibody to Ang1 significantly inhibited DETA-NONOate-induced capillary-like tube formation (C and D). Panel E shows quantitative analysis of capillary-like tube formation. Scale bar in A = 200 μm.
In our previous study, we have shown that DETA-NONOate upregulates VEGF expression which mediates DETA-NONOate-induced angiogenesis [25]. To test whether DETA-NONOate-induces Ang1 expression in parallel with the DETA-NONOate-induced VEGF pathway, MBECs were cultured with VEGF 50 ng/ml) for 24 h. Fig. 3 shows that DETA-NONOate increases MBECs Ang1 gene and protein expression (A and B). VEGF treatment endothelial cell did not significantly increase Ang1 gene and protein expression (A and B). These data suggest that DETA-NONOate-induces Ang1 expression in parallel, and not subsequent to DETA-NONOate-induced VEGF expression.
Fig. 3.
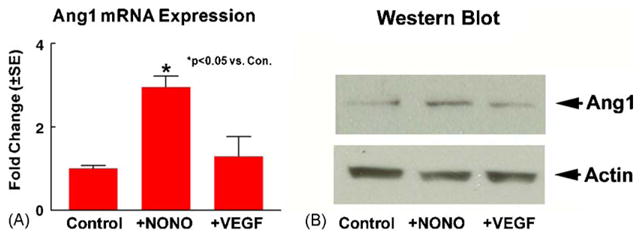
DETA-NONOate induces MBECs Ang1 expression. MBECs cultured with or without DETA-NONOate (0.4 μM) or VEGF (50 ng/ml) for 24 h. Ang1 mRNA and protein expression were measured using real time PCR and Western blot. Panel A shows Ang1 gene expression. Panel B shows Ang1 protein expression.
The present study demonstrated that treating stroke rats with DETA-NONOate induces Ang1/Tie2 expression and increases tight junction protein Occludin expression. Inhibition of Ang1 partially attenuates DETA-NONOate induced angiogenesis. This is the first direct evidence that after stroke, the Ang1/Tie2 pathway may play a role in DETA-NONOate-induced angiogenesis.
Ang1 promotes remodeling, maturation, and stabilization of blood vessels [13]. Ang1-induced Tie2 phosphorylation is an essential process for vasculogenesis and maintaining vascular endothelial integrity [13]. Soluble Ang1 chimeric protein induced vascular remodeling is mediated mainly through Tie2 activation [8]. Our data show that the NO donor, DETA-NONOate, promotes Ang1/Tie2 gene and protein expression, and increases angiogenesis. Anti-Ang1 antibody attenuates DETA-NONOate-induced angiogenesis. Angiogenesis induced by DETA-NONOate may arise from both the upregulation of Ang1/Tie2 and VEGF expression.
Tight junctions provide barriers between adjacent brain capillary endothelial cells at the blood–brain barrier (BBB), and stabilize angiogenic vessels [12]. The Ang1/Tie2 pathway augments tight junction protein Occludin gene and protein expression in brain capillary endothelial cells [12]. Long term L-NAME treatment decreases the intensity of tight junction proteins ZO-1 and Occludin expression in brain endothelial cells [14]. Pharmacological inhibition or genetic disruption of the NO synthase pathway interferes with angiogenesis [9]. Our data indicate that DETA-NONOate not only increases Ang1/Tie2, but also upregulates the expression of the tight junction proteins Occludin in the ischemic border. Ang1 regulation of tight junction protein thereby reduces endothelial permeability, which may have a role in vascular stabilization and maturation [18]. Our data suggest that DETA-NONOate-induced tight junction protein expression may be regulated through Ang1/Tie2 pathway.
In conclusion, our data indicate that DETA-NONOate upregulates the Ang1/Tie2 pathway, which promotes DETA-NONOate-induced angiogenesis and tight junction protein expression after stroke.
Acknowledgments
The authors wish to thank Qinge Lu for technical assistance. This work was supported by NINDS grants PO1 NS23393, RO1 NS047682.
References
- 1.Babaei S, Teichert-Kuliszewska K, Zhang Q, Jones N, Dumont DJ, Stewart DJ. Angiogenic actions of angiopoietin-1 require endothelium-derived nitric oxide. Am J Pathol. 2003;162:1927–1936. doi: 10.1016/S0002-9440(10)64326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci USA. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chae JK, Kim I, Lim ST, Chung MJ, Kim WH, Kim HG, Ko JK, Koh GY. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–2578. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005:21–28. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 8.Cho CH, Kim KE, Byun J, Jang HS, Kim DK, Baluk P, Baffert F, Lee GM, Mochizuki N, Kim J, Jeon BH, McDonald DM, Koh GY. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res. 2005;97:86–94. doi: 10.1161/01.RES.0000174093.64855.a6. [DOI] [PubMed] [Google Scholar]
- 9.Dulak J, Jozkowicz A, Dembinska-Kiec A, Guevara I, Zdzienicka A, Zmudzinska-Grochot D, Florek I, Wojtowicz A, Szuba A, Cooke JP. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:659–666. doi: 10.1161/01.atv.20.3.659. [DOI] [PubMed] [Google Scholar]
- 10.Haralabopoulos GC, Grant DS, Kleinman HK, Lelkes PI, Papaioannou SP, Maragoudakis ME. Inhibitors of basement membrane collagen synthesis prevent endothelial cell alignment in matrigel in vitro and angiogenesis in vivo. Lab Invest. 1994;71:575–582. [PubMed] [Google Scholar]
- 11.Harfouche R, Abdel-Malak NA, Brandes RP, Karsan A, Irani K, Hussain SN. Roles of reactive oxygen species in angiopoietin-1/Tie2 receptor signaling. FASEB J. 2005 doi: 10.1096/fj.04-3621fje. [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 13.Iurlaro M, Scatena M, Zhu WH, Fogel E, Wieting SL, Nicosia RF. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–3643. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- 14.Kalayci R, Kaya M, Elmas I, Arican N, Ahishali B, Uzun H, Bilgic B, Kucuk M, Kudat H. Effects of atorvastatin on blood-brain barrier permeability during L-NAME hypertension followed by angiotensin-II in rats. Brain Res. 2005;1042:184–193. doi: 10.1016/j.brainres.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. (discussion 1980–1981) [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka-Sakata A, Tamura H, Asada H, Miwa I, Taketani T, Yamagata Y, Sugino N. Changes in vascular leakage and expression of angiopoietins in the corpus luteum during pregnancy in rats. Reproduction. 2006;131:351–360. doi: 10.1530/rep.1.00947. [DOI] [PubMed] [Google Scholar]
- 19.Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Direct chemotactic action of angiopoietin-1 on mesenchymal cells in the presence of VEGF. Microvasc Res. 2004;68:221–230. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prior BM, Lloyd PG, Ren J, Li Z, Yang HT, Laughlin MH, Terjung RL. Arteriogenesis: role of nitric oxide. Endothelium. 2003;10:207–216. doi: 10.1080/10623320390246388. [DOI] [PubMed] [Google Scholar]
- 22.Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, Inoue N, Yokoyama M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–114. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- 23.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 27.Ziche M, Morbidelli L. Nitric oxide and angiogenesis. J Neurooncol. 2000;50:139–148. doi: 10.1023/a:1006431309841. [DOI] [PubMed] [Google Scholar]
- 28.Zlokovic BV, Wang L, Sun N, Haffke S, Verrall S, Seeds NW, Fisher MJ, Schreiber SS. Expression of tissue plasminogen activator in cerebral capillaries: possible fibrinolytic function of the blood-brain barrier. Neurosurgery. 1995;37:955–961. doi: 10.1227/00006123-199511000-00015. [DOI] [PubMed] [Google Scholar]