Abstract
Neurogenesis declines with advancing age. The mammalian achaete-scute homologue-1 encodes a basic helix–loop– helix transcription factor, which controls neuronal differentiation. In this study, we first tested whether atorvastatin treatment enhances neurological functional outcome and neuronal differentiation after stroke in retired breeder 12 month rats. Rats were subjected to middle cerebral artery occlusion and treated with or without atorvastatin (3 mg/kg) for 7 days. Atorvastatin significantly increased expression of mammalian achaete-scute homologue-1, β-tubulin III, and vascular endothelial growth factor in the ischemic brain, and concomitantly improved functional outcome compared with middle cerebral artery occlusion control rats. Increased neurogenesis significantly correlated with functional recovery after stroke. To further investigate the mechanisms of atorvastatin-induced neuronal differentiation, experiments were performed on neurospheres derived from retired breeder rat subventricular zone cells. Atorvastatin increased neuronal differentiation and upregulated vascular endothelial growth factor and mammalian achaete-scute homologue-1 gene expression in cultured neurospheres. Vascular endothelial growth factor–treated neurospheres significantly increased mammalian achaete-scute homologue-1 and β-tubulin III expression. Inhibition of vascular endothelial growth factor decreased atorvastatin-induced mammalian achaete-scute homologue-1 and β-tubulin III expression. These data indicate that atorvastatin increases neuronal differentiation in retired breeder rats. In addition, atorvastatin upregulation of vascular endothelial growth factor expression, influences mammalian achaete-scute homologue-1 transcription factor, which in turn, facilitates an increase in subventricular zone neuronal differentiation. These atorvastatin-mediated molecular events may contribute to the improved functional outcome in retired breeder rats subjected to stroke.
Keywords: neuronal differentiation, Mash1, atorvastatin, VEGF, neurosphere, stroke
Neurogenesis and synaptic reorganization are important for functional improvement after stroke (Hallett, 2001; Rijntjes and Weiller, 2002). Administration of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) promotes neurogenesis and functional recovery after stroke in young adult animals (Chen et al., 2003, 2005). However, stroke is a major cause of mortality and morbidity, which especially afflicts the elderly population. Most experimental studies on the nature of cerebral ischemia have focused on the young adult brain, and relatively little information is available about the regulation of neurogenesis in the older brain or the potential of using pharmacological treatment on older animals.
Several signaling cascades play important roles in the regulation of neurogenesis. Transcription factors with basic helix–loop–helix (bHLH) motifs, are essential elements in neurogenesis (Jennings et al., 1994; Ma et al., 1997). The mammalian achaete-scute homologue-1 (Mash1) encodes a bHLH transcription factor, which controls the correct timing of differentiation during neuronal development (Horton et al., 1999). Neuronal differentiation is promoted by Mash1, and partially contributes to ischemia-induced neurogenesis in the adult dentate gyrus (Kawai et al., 2005). In the absence of Mash1, tissue morphology, cell proliferation and gene expression within the forebrain region are disrupted (Horton et al., 1999). In addition, suppression of Stat3 upregulates Mash1 gene expression and induces neuronal differentiation in neural stem cells (Gu et al., 2005).
The subventricular zone (SVZ) in the adult brain is an endogenous source of neuronal precursors that can be recruited to adjacent lesioned areas (Zhang et al., 2003). However, this source of neuronal precursors as well as neurogenesis declines with advancing age (Cameron and McKay, 1999; Kuhn et al., 1996), which may be regulated by a decline of growth factors. Fibroblast growth factor-2 (FGF-2), insulin-like growth factor-I (IGF-1), and vascular endothelial growth factor (VEGF) decline in middle age and remain steady between middle age and old age (Shetty et al., 2005). VEGF, an angiogenic factor, not only induces angiogenesis, but also stimulates neurogenesis and axonal outgrowth (Sondell et al., 1999). In this study, we tested the hypotheses that treatment of stroke with atorvastatin induces neuronal differentiation and promotes functional recovery in retired breeder rats, and that atorvastatin-induced neuronal differentiation may be regulated by VEGF and Mash1.
EXPERIMENTAL PROCEDURES
Middle cerebral artery occlusion (MCAo) model and atorvastatin administration
Retired breeder male Wistar rats weighing 550–650 g (n=18, age 12 months) were employed in all our experiments. All experimental procedures have been approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital. Every effort was made to minimize the number of animals used and their suffering. Rats were anesthetized with halothane. Permanent MCAo was induced by advancing a 3-0 surgical nylon suture (20–22 mm determined by body weight) with an expanded (heated) tip from the external carotid artery into the lumen of the internal carotid artery to block the origin of the MCA using a method of intraluminal vascular occlusion modified in our laboratory (Li et al., 2005). Experimental groups consisted of (n=9/group): group 1: MCAo alone for control; group 2: atorvastatin (3 mg/kg) was gavaged starting at 24 h after MCAo daily for 7 days. All animals were killed at 28 days after MCAo.
Functional tests
In all animals, a battery of behavioral tests was performed before MCAo, and at 1, 7, 14, 21 and 28 days after MCAo by an investigator who was blinded to the experimental groups. The battery of tests consisted of the adhesive-removal somatosensory test (Chen et al., 2001a,b; Schallert and Whishaw, 1984) and the modified Neurological Severity Score (mNSS, Table 1) (Chen et al., 2001a).
Table 1.
mNSS
| Motor tests | Points |
|---|---|
| Raising the rat by the tail | 3 |
| 1 Flexion of forelimb | |
| 1 Flexion of hindlimb | |
| 1 Head moved more than 10° to the vertical axis within 30 seconds | |
| Walking on the floor (normal=0; maximum=3) | 3 |
| 0 Normal walk | |
| 1 Inability to walk straight | |
| 2 Circling toward the paretic side | |
| 3 Fall down to the paretic side | |
| Sensory tests | 2 |
| 1 Placing test (visual and tactile test) | |
| 1 Proprioceptive test (deep sensation, pushing the paw against the table edge to stimulate limb muscles) | |
| Beam balance tests (normal=0; maximum=6) | 6 |
| 0 Balances with steady posture | |
| 1 Grasps side of beam | |
| 2 Hugs the beam and one limb falls down from the beam | |
| 3 Two limbs fall down from the beam, or spins on beam (>60 s) | |
| 4 Attempts to balance on the beam but falls off (>40 s) | |
| 5 Attempts to balance on the beam but falls off (>20 s) | |
| 6 Falls off: No attempt to balance or hang on to the beam (<20 s) | |
| Reflexes absent and abnormal movements | 4 |
| 1 Pinna reflex (a head shake when touching the auditory meatus) | |
| 1 Corneal reflex (an eye blink when lightly touching the cornea with cotton) | |
| 1 Startle reflex (a motor response to a brief noise from snapping a clipboard paper) | |
| 1 Seizures, myoclonus, myodystony | |
| Maximum points | 18 |
One point is awarded for the inability to perform the tasks or for the lack of a tested reflex; 13–18 severe injury; 7–12 moderate injury; 1–6 mild injury.
Histological and immunohistochemical assessment
Animals were killed at 28 days after MCAo. Rat brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde, and the brains were embedded in paraffin. The cerebral tissues were cut into seven equally spaced (2 mm) coronal blocks. A series of adjacent 6 μm-thick sections was cut from each block in the coronal plane and was stained with hematoxylin and eosin (H&E). The seven brain sections were traced using the Global Laboratory Image analysis system (Data Translation, Marlboro, MA, USA). The indirect lesion area, in which the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere, was calculated (Swanson et al., 1990). Lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere.
Immunohistochemical staining
Immunohistochemical staining was used for identification of Mash1 (1:1000 dilution, BD Biosciences Pharmingen, San Diego, CA, USA) and β-tubulin III (TUJ1), a marker of immature neuronal phenotype (1:400, Monoclonal Anti-B-Tubulin Isotype III, Covance, Berkeley, CA, USA), and VEGF (goat-polyclonal lgG antibody diluted 1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), as previously described. Briefly, standard paraffin blocks were obtained from the center of the lesion, corresponding to coronal coordinates for bregma −1~1 mm. A series of 6 μm thick sections at various levels (100 μm interval) was cut from this block and was analyzed using light and fluorescent microscopy (Olympus BH-2). Control experiments consisted of staining brain coronal tissue sections as outlined above, but omitted the primary antibodies, as previously described (Li et al., 1998).
Quantification
Mash1 positive cell number in the ipsilateral SVZ was counted using a three-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research, St. Catharines, Canada). The total positive cell numbers of Mash1 in the ipsilateral SVZ are presented. In addition, TUJ1 and VEGF positive areas in the ischemic border were digitized under a 20× objective (Olympus BX40) using a three-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system. The digitalized images were then contrast-enhanced to clearly differentiate positivity from background, and a thresholding procedure was established to determine the proportion of immunoreactive area within each fixed field of view (Calza et al., 2001; Chen et al., 2003). The data are presented as a percentage of positive immunoreactivity area in the ischemic border area.
SVZ neurosphere culture
SVZ cells were dissociated from normal retired breeder rats (12m), as reported previously (Morshead et al., 1994). Mechanically dissociated SVZ cells were plated at 3×104 cells/ml in DMEM-F-12 medium containing 20 ng/ml epidermal growth factor (EGF, R&D System, Minneapolis, MN, USA) and 20 ng/ml bFGF (R&D System). DMEM-F-12 medium containing DMEM-F-12 (R&D System), L-glutamine (2 mM), glucose (0.6%) and B27. Primary neurospheres were cultured for 7 days, after which they were collected and passaged by mechanical dissociation, resuspended in proliferation medium, and plated at 2×104 cells/ml in each well of a 24 well plate (Corning).
Real time-PCR
The SVZ neurosphere culture experimental groups include: 1): SVZ neurosphere culture alone for control; 2): SVZ neurosphere treated with 0.1 μM atorvastatin for 7 days. Cells were harvested and total RNA was isolated from treated cells with TRIzol (Invitrogen), following standard protocol (Livak and Schmittgen, 2001). Quantitative PCR was performed using SYBR Green real time PCR method. Quantitative RT-PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA, USA) using three-stage program parameters provided by the manufacturer. Each sample was tested in triplicate, and samples were obtained from three independent experiments that were used for analysis of relative gene expression data using the 2−ΔΔCT method (Livak and Schmittgen, 2001). The following primers for RT-PCR were designed using Primer Express software (ABI). VEGF: Fwd: GAAAATCACTGTGAGCCTTGTTC; Rev: TGCAAG-TACGTTCGTTTAACTCA. Mash1: Fwd: GGTCTTTGCTTCT-GTTTTCTTCAT; Rev: CCCCAAACTGGTTAGGATAGATA. TUJ1: Fwd: CCCGAGGGCTCAAGATGTC; Rev: CGCTTGAACAGCTC-CTGGAT. GAPDH: Fwd: AGAACATCATCCCTGCATCC; Rev: CACATTGGGGGTAGGAACAC.
SVZ neurosphere cell differentiation
To test whether atorvastatin increases neurosphere cell neuronal differentiation, TUJ1 immunostaining was performed. Neurospheres were mechanically dissociated to single-cells, which were plated (2×104/cm2) onto laminin-coated glass coverslips in differentiation medium (without bFGF and EGF). The SVZ neurosphere culture experimental groups include: 1): SVZ neurosphere culture alone for control; 2): SVZ neurosphere treated with 0.1 μM atorvastatin. Incubation was terminated at 7 days after plating and immunostaining for TUJ1. The number of TUJ1 positive cells was counted. The data are presented as percentage of TUJ1 positive cells in total DAPI cells.
Western blot assay
To test whether VEGF regulates Mash1 expression and neuronal differentiation, SVZ neurospheres were treated with: 1) control; 2) atorvastatin (0.1 μM); 3) neutralized VEGF antibody alone (5 μg/ml, Abcam Inc., Cambridge, MA, USA); 3) atorvastatin (0.1 μM) with neutralized VEGF antibody (5 μg/ml, Abcam Inc.) for 7 days. Mash1 and TUJ1 expression was measured using Western blot. Protein was isolated from cultured cells using Trizol (Invitrogen, Carlsbad, CA, USA), following standard protocol. Equal amounts of proteins were loaded in a 10% SDS PAGE precast gel (Invitrogen). Gel was transferred to a nitrocellulose membrane by running the transfer at 400 mA for one hour. Nitrocellulose membrane was blocked in 5% milk in 1× TBS-T for one hour, and then Mash1 (BD Pharmingen) or Tuj1 (Covance) monoclonal primary antibody was added at 1:1000 dilution in 5% milk in TBS-T, and incubated on a shaker overnight at 4C°. For detection, secondary anti-mouse antibody (1:1000, Bio Rad) was added in 5% milk in TBS-T on a room temperature shaker. Normalization of results was ensured by running parallel Western blots with β-actin antibody (Abcam, 1:5000 dilution). The membrane was again washed three times for 5 min with TBS-T. After the final wash, substrate solution was added and allowed react with the membrane for 5 min. The membranes were then exposed to Kodak BioMax MR Film for 30 s before development of the film.
Statistical analysis
For in vivo study, the retired breeder rats were enrolled into either the controls or the treatment of atorvastatin (3 mg/kg), administered at 24 h after MCAo. Functional data (Adhesive test, mNSS) were collected at day 1 before the treatment, and at days 7, 14, 21 and 28 after the MCAo and treatment. The lesion volume and histological data were collected at day 28 after MCAo. Data were evaluated for normality. Two-sample t-test was used to compare the outcome difference between the two groups, if data were normal, otherwise, the nonparametric Wilcoxon test was considered. Repeated analysis of variance (ANOVA) was used to study the treatment effect on functional recovery at days 7, 14 and 21 after MCAo. Analysis began testing for the treatment by time interaction, followed by testing the main effect of the treatment and the time if no interaction was observed at the 0.05 level or testing the treatment effect at each time point if the interaction was detected. Two-sample t-test was performed to compare the lesion volume, Mash1, TUJ1, VEGF protein and gene expression between the two groups. The data are presented as means±S.E. A value of P<0.05 was taken as significant. In addition, Spearman’s partial correlation coefficients were calculated for measurements of interest adjusting for the treatment. Functional data at the 28 day time point were used for correlation analysis. Correlation coefficient ranged from −1 (y decreases, when x increases) to 1 (y increases, as x increases) as a perfect correlation. The value zero means no correlation. A significant correlation at 0.05 indicated that the correlation among variables of interest was significantly removed from no correlation.
RESULTS
Neurological functional outcome and lesion volume
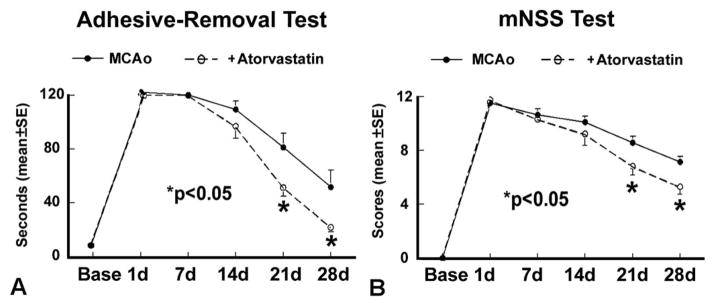
To test whether atorvastatin promotes functional recovery after stroke in older rats, retired breeder male rats (12m) were subjected to permanent MCAo and treated with or without atorvastatin (3 mg/kg) for 7 days. The treatment by time interaction was observed on the adhesive (P<0.05) and NSS tests (P<0.01). Fig. 1 shows that significant improvement in functional recovery (A: adhesive-removal test; B: mNSS) was found in atorvastatin-treated animals compared with the control group, at 21 and 28 days after MCAo (P<0.05, n=9/group) in retired breeder rats. No significant differences (P=0.81) of ischemic lesion volumes in atorvastatin treatment animals (24±2.7%) were detected compared with the control group (26±4.2%).
Fig. 1.
Atorvastatin promotes functional recovery after stroke in retired breeder rats. Retired breeder rats were subjected to MCAo and treated with or without atorvastatin starting at 24 h after stroke for 7 days (n=9/group). Functional tests were performed before MCAo and 1, 7, 14, 21, and 28 days after MCAo. (A) Adhesive-removal test; (B) mNSS test. * P<0.05 vs. MCAo control.
Atorvastatin regulates SVZ cell neuronal plasticity after stroke
To test whether atorvastatin regulates neuronal plasticity, Mash1, TUJ1 and VEGF immunostaining was performed. Fig. 2A–C shows TUJ1 expression significantly increased in the ischemic boundary area of the atorvastatin treatment group (B and C) compared with the MCAo control group (A and C, P<0.05). Fig. 2D–F shows Mash1 expressed in the nuclei of SVZ cells (D and E, arrow) and atorvastatin significantly increased Mash1 expression (E and F) in the SVZ compared with MCAo control animals (D and F). Fig. 2G–I shows VEGF expression significantly increased in the ischemic border in atorvastatin treatment animals (H and I) compared with control MCAo animals (G and I). In addition, correlation coefficient analysis shows that functional outcome (adhesive-removal test, r=−0.77, P<0.05; mNSS test, r=−0.55, P=0.05) significantly correlated with TUJ1 expression.
Fig. 2.
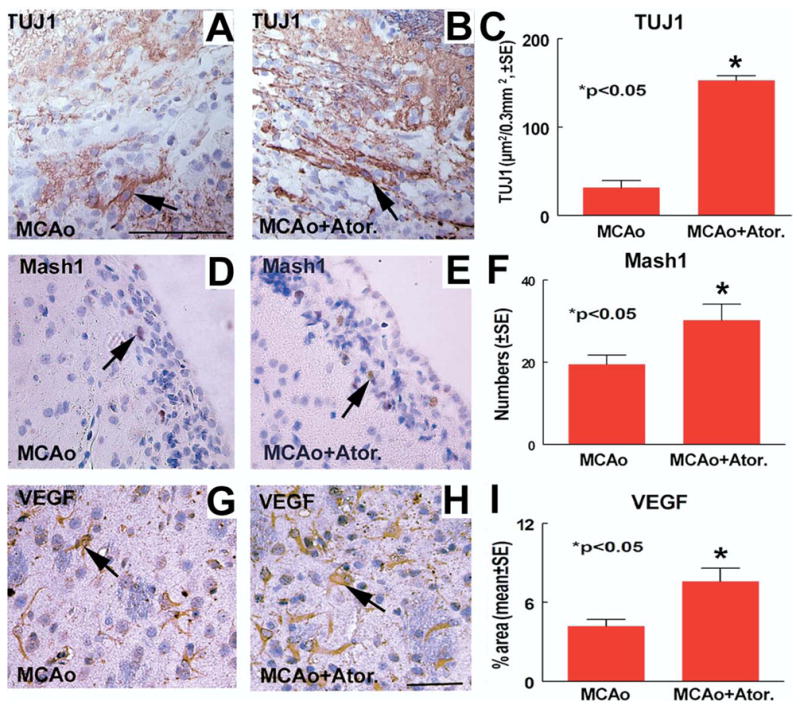
Atorvastatin increases TUJ1, Mash1 and VEGF expression after stroke. TUJ1, Mash1 and VEGF are expressed in the ipsilateral SVZ and ischemic border at 28 days after MCAo. Panels A, D and G show TUJ1 (A; arrow, TUJ1 positive cell), Mash1 (D; arrow, Mash1 positive cell) and VEGF (G; arrow, VEGF positive cell) expression in MCAo control rats, respectively. Panels B, E and H show TUJ1 (B, arrow, TUJ1 positive cell), Mash1 (E; arrow, Mash1 positive cell) and VEGF (H; arrow, VEGF positive cell) expression in atorvastatin-treated rats, respectively. Panel F shows quantitative data of Mash1 positive cells number in the ischemic SVZ. Panels C and I show quantitative data of TUJ1 (C) and VEGF (I) positive percentage of area in the ischemic border area. Scale bar=100 μm A, H=50 μm.
Atorvastatin promotes SVZ neurosphere neuronal differentiation
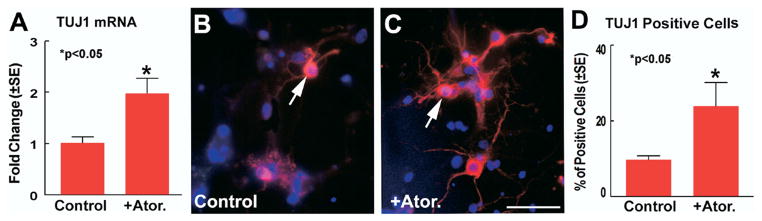
To investigate whether atorvastatin regulates SVZ cell neuronal differentiation, TUJ1 mRNA and protein expression was measured by real time PCR and immunostaining in cultured SVZ neurospheres treated with or without atorvastatin. Fig. 3 show that atorvastatin significantly promotes TUJ1 mRNA expression (A) and TUJ1 positive cell number (C and D) compared with the non-treatment control (B and D).
Fig. 3.
Atorvastatin promotes SVZ neurosphere cell neuronal differentiation. Panel A shows quantitative data of TUJ1 mRNA expression in retired breeder rat SVZ neurospheres treated with or without atorvastatin (0.1 μM) for 7 days. Panels B–C show that TUJ1 immunostaining in retired breeder rat SVZ neurospheres treated with (C; arrow: TUJ1 positive cell, red) or without (B; arrow: TUJ1 positive cell, red) atorvastatin for 7 days. Panel D shows quantitative data of percentage of TUJ1 positive cell number in retired breeder rat SVZ neurospheres treated with or without atorvastatin. Scale bar=50 μm C.
Atorvastatin upregulates SVZ neurosphere VEGF and Mash1 mRNA expression
To determine the molecular mechanisms underlying atorvastatin-regulated neuronal differentiation, Mash1 and VEGF gene expression was measured. Fig. 4A–B shows that atorvastatin treatment significantly increases VEGF (A) and Mash1 (B) gene expression compared with the non-treated control.
Fig. 4.
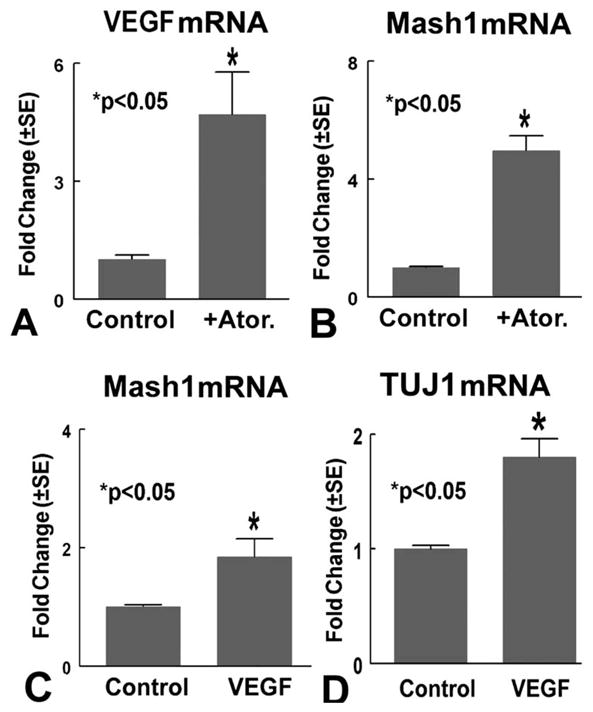
Atorvastatin regulates SVZ neurosphere VEGF and Mash1 gene expression. Panels A–B show VEGF (A) and Mash1 (B) mRNA expression in retired breeder SVZ neurosphere cells treated with or without atorvastatin (0.1 μM). Panels C–D show Mash1 (C) and TUJ1 (D) mRNA expression in retired breeder SVZ neurosphere cells treated with or without VEGF (100 ng/ml) (n=3/group).
VEGF increases Mash1 and TUJ1 mRNA expression
To further investigate whether atorvastatin upregulates VEGF expression, which may influence Mash1 and neuronal differentiation, retired breeder rat SVZ neurospheres were treated with or without VEGF (100 ng/ml, R&D Systems Inc.) for 7 days. Mash1 and TUJ1 gene expression was measured using RT-PCR. Fig. 4C–D shows that VEGF significantly increases Mash1 (C) and TUJ1 (D) gene expression.
VEGF mediates atorvastatin-induced Mash1 expression and neuronal differentiation
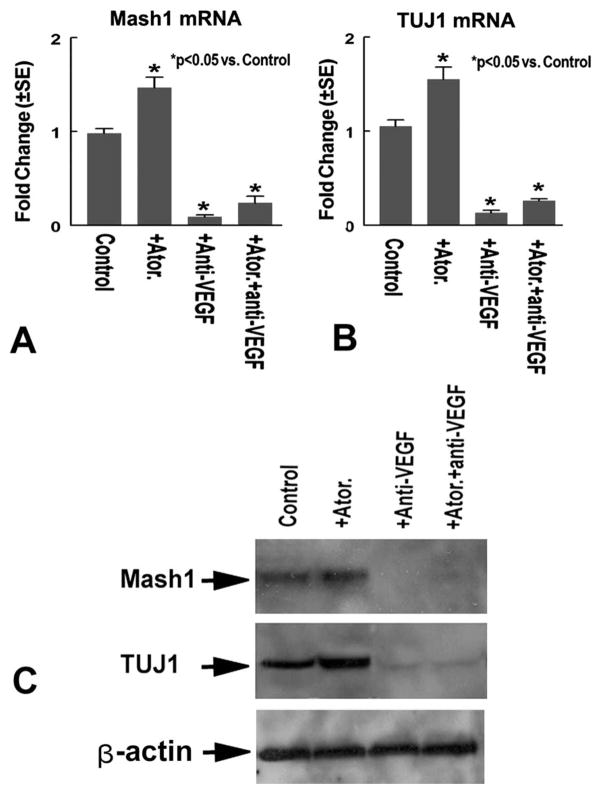
To further test whether VEGF mediates atorvastatin-induced Mash1 expression and neuronal differentiation, SVZ neurospheres were treated with: 1) control; 2) atorvastatin (0.1 μM); 3) neutralized VEGF antibody alone (5 μg/ml, Abcam Inc.); 4) atorvastatin (0.1 μM) with neutralized VEGF antibody (5 μg/ml, Abcam Inc.) for 7 days. Mash1 and TUJ1 protein expression was measured using Western blot. Fig. 5 shows that atorvastatin increases Mash1 (A) and TUJ1 (B) gene and protein (C) expression. Inhibition of VEGF significantly decreases atorvastatin-induced Mash1 and TUJ1 gene (A and B) and protein (C) expression.
Fig. 5.
VEGF mediates atorvastatin-induced Mash1 and TUJ1 expression. Panels A and B show Mash1 (A) and TUJ1 (B) mRNA expression in SVZ neurosphere cells treated with or without atorvastatin and neutralized VEGF antibody for 7 days (n=3/group). Panel C shows Mash1 and TUJ1 protein expression measured by Western blot in retired breeder rat neurospheres.
DISCUSSION
We demonstrate for the first time that a clinically employed statin, atorvastatin, when administered one day after MCAo to retired breeder rats, evokes neuronal differentiation, as well as a significant improvement in neurological functional recovery. Increased neurogenesis treatment with atorvastatin is correlated with neurological functional outcome after stroke. Atorvastatin upregulates neuronal differentiation, which parallels an increase in Mash1 and VEGF expression. VEGF promotes SVZ neurosphere Mash1 gene expression and increases neuronal differentiation. Inhibition of VEGF attenuates atorvastatin-induced Mash1 and TUJ1 expression. Thus, our data indicate that atorvastatin promotes VEGF expression, and thereby influences Mash1 expression, which in turn, may facilitate an increase in SVZ neuronal differentiation.
Retired breeder rats respond to atorvastatin-induced neurogenesis and functional recovery after stroke
Neurogenesis, the process through which precursor cells differentiate into a mature neuronal phenotype, persists in the SVZ of adult brain (Lie et al., 2004). Neurogenesis which contributes to the ability of the adult brain to function normally and adapt to disease, declines with advancing age (Cameron and McKay, 1999; Kuhn et al., 1996). The decline in neuronal plasticity proteins as well as a diminished neurogenesis potential likely impedes functional recovery from stroke in old rats (Badan et al., 2003; Marini et al., 2001). Our data show that atorvastatin promotes neuronal differentiation, which significantly correlates with increasing of neurological functional outcome after stroke. Therefore, increased neurogenesis induced by atorvastatin treatment may benefit neurological functional outcome in retired breeder rats.
Atorvastatin promotes Mash1 gene expression and promotes neuronal differentiation
Neurogenesis is promoted by bHLH transcription factors Mash1, Math1 and/or NeuroD (Ito et al., 2003). Mash1 is a proneural bHLH factor, and is responsible for the upregulation of neuronal differentiation. Mash1 is required for the generation of ventral neuroendocrine neurons (McNay et al., in press). Increased Mash1 mRNA contributes to the regulation of neurogenesis in the adult dentate gyrus after ischemia (McNay et al., in press). Our data demonstrate that atorvastatin increased neuronal differentiation coincident with increased Mash1 expression in old rats after stroke. Therefore, atorvastatin-induced neuronal differentiation may be regulated by Mash1 expression.
Atorvastatin upregulates VEGF expression and subsequently increases Mash1 expression
Neurotrophins stimulate neuronal differentiation by altering the balance of expression of various bHLH transcription factors (Ito et al., 2003). VEGF was originally identified as an angiogenic agent (Thomas, 1996). However, there is increasing evidence that VEGF acts as a neurotrophic factor. VEGF provides a significant dose-responsive increase in the neuronal microtubule markers TUJ1 and MAP-2 (Rosenstein et al., 2003). VEGF achieves its effects by acting on the neuronal microtubular content, which is involved with growth, stability and maturation. Genetically increasing hippocampal VEGF in adult rats increases neurogenesis, which associated with improved cognition (During and Cao, 2006). VEGF also promotes neurite outgrowth from cerebral cortical neurons by interacting with VEGFR2 and activating Rho/ROK signaling pathways (Jin et al., 2006). Our data demonstrate that atorvastatin not only increases Mash1 expression but also upregulates VEGF expression in the ischemic brain. Previous data have shown that atorvastatin promotes angiogenesis, and neuronal plasticity as well as increased VEGF/VEGFR2 and BDNF expression (Chen et al., 2005). Atorvastatin also increases baseline absolute cerebral blood flow after 14-day high-dose mevastatin (20 mg/kg/day) pre-treatment (before MCAo) in young adult mice (Amin-Hanjani et al., 2001). Neurogenesis occurs in close proximity to blood vessels, where VEGF expression is high and angiogenesis is ongoing (Palmer et al., 2000). The newly activated and expanded vasculature substantially increases the production and release of BDNF, which produces a vascular niche associated with neurogenesis and recruitment of new neurons (Leventhal et la., 1999). Thus, the vascular niche may contribute to statin-induced neurogenesis. To further identify the direct effect of VEGF on statin-induced neurogenesis, and to exclude the influence of the vascular niche on neurogenesis, we used in vitro SVZ neurosphere culture. Our data show that atorvastatin upregulates neuronal differentiation, which parallels an increase in Mash1 and VEGF expression in cultured neurosphere cells. VEGF promotes SVZ neurosphere Mash1 gene expression and increases neuronal differentiation. Inhibition of VEGF attenuates atorvastatin-induced Mash1 and TUJ1 expression in cultured neurosphere cells. Therefore, these data suggest that VEGF mediates atorvastatin-induced neurosphere neuronal differentiation.
CONCLUSION
In summary, atorvastatin upregulates VEGF expression and promotes neuronal differentiation by upregulation of Mash1 gene expression in retired breeder SVZ neurospheres. Atorvastatin, thus, has the ability to regulate neurogenesis in retired breeder rats, and thereby may enhance neurological functional recovery after stroke in retired breeder rats.
Acknowledgments
The authors wish to thank Qinge Lu for technical assistance. This work was supported by NINDS grants PO1 NS23393, RO1 NS047682.
Abbreviations
- bHLH
basic helix–loop–helix
- Mash1
mammalian achaete-scute homologue-1
- MCAo
middle cerebral artery occlusion
- mNSS
modified Neurological Severity Score
- SVZ
subventricular zone
- TUJ1
β-tubulin III
- VEGF
vascular endothelial growth factor
References
- Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32:980–986. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- Badan I, Platt D, Kessler C, Popa-Wagner A. Temporal dynamics of degenerative and regenerative events associated with cerebral ischemia in aged rats. Gerontology. 2003;49:356–365. doi: 10.1159/000073763. [DOI] [PubMed] [Google Scholar]
- Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci U S A. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001a;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res. 2006;3:29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- Gu F, Hata R, Ma YJ, Tanaka J, Mitsuda N, Kumon Y, Hanakawa Y, Hashimoto K, Nakajima K, Sakanaka M. Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J Neurosci Res. 2005;81:163–171. doi: 10.1002/jnr.20561. [DOI] [PubMed] [Google Scholar]
- Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain Res Brain Res Rev. 2001;36:169–174. doi: 10.1016/s0165-0173(01)00092-3. [DOI] [PubMed] [Google Scholar]
- Horton S, Meredith A, Richardson JA, Johnson JE. Correct coordination of neuronal differentiation events in ventral forebrain requires the bHLH factor MASH1. Mol Cell Neurosci. 1999;14:355–369. doi: 10.1006/mcne.1999.0791. [DOI] [PubMed] [Google Scholar]
- Ito H, Nakajima A, Nomoto H, Furukawa S. Neurotrophins facilitate neuronal differentiation of cultured neural stem cells via induction of mRNA expression of basic helix-loop-helix transcription factors Mash1 and Math1. J Neurosci Res. 2003;71:648–658. doi: 10.1002/jnr.10532. [DOI] [PubMed] [Google Scholar]
- Jennings B, Preiss A, Delidakis C, Bray S. The notch signalling pathway is required for enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Development. 1994;120:3537–3548. doi: 10.1242/dev.120.12.3537. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takagi N, Nakahara M, Takeo S. Changes in the expression of Hes5 and Mash1 mRNA in the adult rat dentate gyrus after transient forebrain ischemia. Neurosci Lett. 2005;380:17–20. doi: 10.1016/j.neulet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. discussion 1980–1991. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma Q, Sommer L, Cserjesi P, Anderson DJ. Mash1 and neurogenin1 expression patterns define complementary domains of neuroepithelium in the developing CNS and are correlated with regions expressing notch ligands. J Neurosci. 1997;17:3644–3652. doi: 10.1523/JNEUROSCI.17-10-03644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini AM, Choi J, Labutta R. Synaptic deprivation and age-related vulnerability to hypoxic-ischemic neuronal injury. A hypothesis. Ann N Y Acad Sci. 2001;939:238–253. doi: 10.1111/j.1749-6632.2001.tb03631.x. [DOI] [PubMed] [Google Scholar]
- McNay DE, Pelling M, Claxton S, Guillemot F, Ang SL. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol Endocrinol. 2006 doi: 10.1210/me.2005-0518. in press. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Weiller C. Recovery of motor and language abilities after stroke: the contribution of functional imaging. Prog Neurobiol. 2002;66:109–122. doi: 10.1016/s0301-0082(01)00027-2. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23:11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ. Bilateral cutaneous stimulation of the somatosensory system in hemidecorticate rats. Behav Neurosci. 1984;98:518–540. doi: 10.1037//0735-7044.98.3.518. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem. 1996;271:603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang L, Zhang ZG, Morris D, Jiang Q, Wang L, Zhang LJ, Chopp M. Migration and differentiation of adult rat subventricular zone progenitor cells transplanted into the adult rat striatum. Neuroscience. 2003;116:373–382. doi: 10.1016/s0306-4522(02)00696-6. [DOI] [PubMed] [Google Scholar]