Abstract
Previous research has implicated dopamine as a modulating factor in choice behavior based on effort. The purpose of the present study was to determine the individual contribution of different dopamine receptors to effort-based decision-making in rats. Rats were trained in a T-maze to choose a large-reward arm that contained eight pellets of food over a small-reward arm that contained two pellets of food. The rats then were trained to climb progressively higher barriers in order to obtain the food from the large-reward arm. Using a discounting procedure on each test day, it was found that rats were more likely to choose the small-reward arm after treatment with the D1 antagonist, SCH-23390, or the D2 antagonist, haloperidol. The dopamine agonist, D-amphetamine, biased the rats towards choosing the large-reward arm and blunted the effects of SCH-23390 or haloperidol. Treatment with the D3 receptor antagonist, U99194, or the D3 receptor agonist, 7-OH-DPAT, did not alter choice behavior. These data indicate that D1 and D2 receptors are required for decisions based on effort.
Keywords: Dopamine receptor, choice behavior, T-maze, amphetamine, haloperidol, SCH-23390
Decision-making is influenced by many factors. A multitude of empirical work has characterized developmental, cultural, and environmental factors that modify decision-making. Moreover, research has started to reveal the neurobiological determinants of decision-making. To date, most of the neurobiological work has focused on how the probability of a particular outcome or the delay between a given response and outcome can influence choice behavior (see Cardinal, 2006, for review). The amount of effort required to achieve a particular outcome also constrains choice behavior, and the biological basis of such effort-based decision-making has begun to receive attention over the last several years.
In most studies of effort-based decision-making, animals are provided with a choice between a large food reward and a small food reward, but the animals are required to exert greater effort to obtain the large reward. Research over the past several years has endeavored to identify the brain regions responsible for such decision-making. Initial work by Salamone and colleagues (Cousins, Atherton, Turner, & Salamone, 1996; Salamone, Cousins, & Bucher, 1994) found that dopamine depletion in the nucleus accumbens biased rats towards making less effortful choices in a T-maze cost-benefit procedure. Walton, Bannerman, and Rushworth (2002) later showed that relatively large lesions of the medial prefrontal cortex in rats also reduced the likelihood of effortful choices. This same group (Walton, Bannerman, Alterescu, & Rushworth, 2003; Rudebeck, Walton, Smyth, Bannerman, & Rushworth, 2006) also demonstrated that relatively small lesions of the anterior cingulate cortex decreased effortful choices, whereas lesions to the prelimbic/infralimbic cortex and the orbitofrontal cortex did not (although lesions to the latter brain region increased preference for rewards associated with shorter delays vs. longer ones). Finally, the amygdala may also serve as a locus of effort-based decision making in the brain since bilateral inactivation of the basolateral amygdala, or unilateral inactivation of the basolateral amygdala concurrent with inactivation of the contralateral anterior cingulate cortex, decreases effortful behavior driven by food reward (Floresco & Ghods-Sharifi, 2007).
All brain regions currently implicated in effort-based decision-making utilize dopamine released from neurons in the ventral tegmental area as a neurotransmitter. This observation suggests a central role for dopamine in effort-based decision-making. Such an idea gains support from studies demonstrating that effortful choices can be reduced by 6-hydroxydopamine (6-OHDA) lesions of the nucleus accumbens (Salamone et al., 1994), as well as by systemic treatment with the D2 receptor antagonist, haloperidol (Salamone et al., 1994; Walton, Croxson, Rushworth, & Bannerman, 2005). Dopaminergic tone in the nucleus accumbens appears to be critical for effort-based decision-making, but it is less clear if dopamine in the anterior cingulate cortex also is needed. Walton et al. (2005) reported that 6-OHDA lesions of the anterior cingulate cortex did not alter the likelihood of effort-based responses, whereas Schweimer, Saft, and Hauber (2005) found that such lesions reduced this type of responding. The discrepancy in the outcomes may be related to methodological differences between the two studies. It is noteworthy, however, that Schweimer and Hauber (2006) demonstrated that blockade of D1, but not D2, receptors in the anterior cingulate cortex biased rats towards less effortful choices.
On balance, the data strongly support a role for dopamine in effort-based decision-making, yet the specific dopamine receptor subtypes required for such responding have not been identified. At present, there are five known dopamine receptor subtypes, D1 through D5, with the D1 and D5 subtypes thought to represent one category of dopamine receptors, and the D2, D3, and D4 receptors considered a second category of dopamine receptors (Civelli, 1995). The former group alters the activity of receptor-linked G proteins and second messengers in a manner distinct from the latter group of receptors (Civelli, 1995), although it also should be noted that activity at D1 and D2 receptors can have synergistic effects on cellular signal transduction (LaHoste, Ruskin, & Marshall, 1996; Wirtshafter & Asin, 1994). D1 and D2 receptors are densely distributed throughout the frontal and temporal cortex, limbic system, and striatum, whereas the D3, D4, and D5 receptors have a more restricted distribution in the brain (Meador-Woodruff, 1994). Studies have shown that region-specific dopamine depletion, systemic treatment with the D2 antagonist, haloperidol, and D1 receptor blockade in the anterior cingulated cortex all bias rats towards less effortful choices, but there are no studies that systematically compare the effects of systemically administered antagonists at the different dopamine receptors on effort-based decision-making.
The purpose of the present study was to determine the individual contribution of three different dopamine receptors (D1, D2, and D3) to effort-based decision-making in rats. Rats were trained and tested using a T-maze effort-based cost/benefit procedure originally developed by Salamone et al. (1994) and used later by Walton et al. (2002), although in the present study, a discounting procedure was used during test trials. In the discounting procedure, every time a rat chose the large-reward arm, the amount of food in that arm was reduced by one pellet on the next trial. This adjusting-amount procedure allows one to determine an indifference point for each rat and may provide a more sensitive measure of the reward value for each rat (see Green, Myerson, Holt, Slevin, & Estle, 2004; Richards, Mitchell, de Wit, & Seiden, 1997).
In order to identify the dopamine receptors involved in effort-based decision-making, rats received combinations of specific dopamine receptor antagonists and agonists prior to testing. Specifically, we chose to study the selective D1/D5 receptor antagonist, SCH-23390, which has a 1000-fold greater affinity for D1 receptors over D2 receptors (Hartman, Monsma, & Civelli, 1996; Kozell, Machida, Neve, & Neve, 1994) and the selective D2 receptor antagonist, haloperidol, which possesses a three- to fiftyfold greater affinity for D2 receptors relative to other dopamine receptors (Hartman et al., 1996). We also chose to study the role of D3 receptors in choice behavior by recording the effects of the D3-preferring agonist, 7-OH-DPAT, which possesses a four- (Svensson, Carlsson, Huff, Kling-Petersen, & Waters 1994) to one hundred-fold (Levesque et al., 1992) preference for D3 receptors over D2 receptors, and the D3-preferring antagonist, U99194, which possesses a fourteen-fold preference for D3 receptors over D2 receptors (Audinot et al., 1998). Given the wider distribution and greater density of the D1 and D2 receptors in areas important in effort-based decision making relative to D3 receptors, we predicted that blockade of the former receptors would have a greater impact upon choice behavior. We also predicted that treatment with D-amphetamine, a drug that increases dopamine release, would enhance preference for large rewards that can be obtained through greater effort.
Method
Subjects
Nine adult male Long-Evans rats (250–300 grams) purchased from Harlan (Indianapolis, IN) were used in these experiments. The rats were group-housed three per cage with free access to food and water except where noted. Lighting in the animal colony was maintained on a 12-hour light/dark schedule with lights on at 06:00. All procedures were performed in a different room between 08:00–20:00. All experimental procedures were performed according to the Current Guide for the Care and Use of Laboratory Animals (USPHS) under a protocol approved by the Northern Kentucky University Institutional Animal Use and Care Committee.
Apparatus
A T-maze was used that matched the dimensions listed in Walton et al. (2002) and was based upon the original apparatus described by Salamone et al. (1994). It was made of wood and wire mesh, and painted gray, with 30-cm-high walls and 10-cm-wide corridors. Each arm had a length of 60 cm. The T-maze was located on a table in a well-lit room. The barriers were constructed of wood and wire mesh in the shape of a right triangle. The rats were required to climb up the 90° face of the triangle barrier and down the 45° angle to obtain food from the large-reward arm. One barrier was 15 cm × 20 cm on the right sides of the triangle, and the other was 25 cm × 30 cm on the right sides of the triangle. The use of these two triangle barriers allowed for testing with four progressively higher barriers (i.e., 15-, 20-, 25-, and 30-cm-high barriers). When used during testing, a barrier was placed in a goal arm approximately 10 cm from the intersection of the start and goal arms of the T-maze. On forced trials, a box was used to block access to one of the goal arms. The box was 23.75 cm long, 12.5 cm wide, and 8.75 cm high.
Habituation and Training
The rats were habituated, trained, and tested using a procedure that was nearly identical to that originally described by Salamone et al. (1994) and used later by Walton et al. (2002) (see Figure 1A). During habituation, access to food was restricted until the rats were at 90% of their free-feeding weight. On the first day of habituation, the rats were placed into the T-maze in groups of three and allowed to explore the maze and eat 20-mg Noyes food pellets. Two food cups were placed at the end of each arm of the T-maze, and each cup contained nine food pellets. The food cups could be replenished up to three times and the rats were removed from the maze after 20 minutes.
Figure 1.
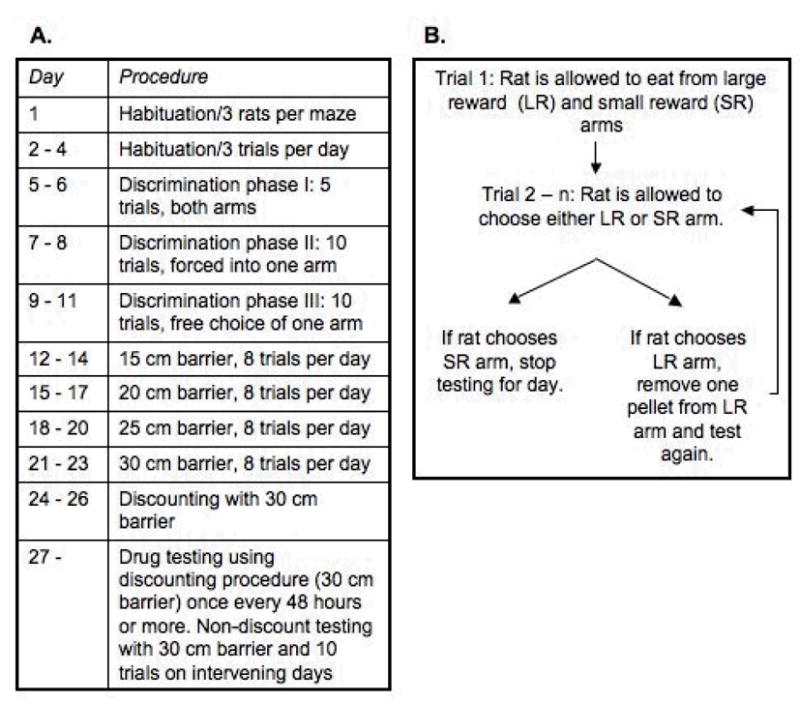
A. Timeline of training procedures. B. Diagram of discounting procedure.
One day later, each trial began by placing a single rat at the beginning of the start arm. A food cup was placed at the end of each goal arm in the T-maze and contained a single food pellet. The trial ended when the rat had eaten both pellets or 150 seconds had elapsed. Rats were given three trials a day for three days. By the end of the three days, all rats were eating both food pellets on all three trials within 60 seconds.
Discrimination Training
Discrimination training consisted of three phases. During phase one, each rat received five trials per day for two days. A food cup was placed at the end of each goal arm. There were eight pellets in one cup and two in the other. Six of the rats were trained with eight pellets in the right arm, and three were trained with eight pellets in the left arm. This side designation was maintained throughout the remaining training and test trials. The arm with the eight pellets was designated as the “large reward” or LR arm, and the arm with the two pellets was designated as the “small reward” or SR arm. Each trial began by placing the rat at the beginning of the start arm. The trial ended when the rat had eaten from both food cups or 150 seconds elapsed. The intertrial interval was approximately five minutes with the other two rats from the rat’s home cage being run during the interval.
In phase two of discrimination training, each rat received ten trials per day for two days. A box was used to block access to the left or right goal arm prior to testing. Rats were forced into the LR or SR arm five times each. They were not forced into the same arm more than two times in a row. The trial ended after the rat ate from the food cup or 150 seconds elapsed.
During the final phase of discrimination training, each rat received ten trials per day. On trials five and ten, access to the previously chosen arm was blocked with the box in order to prevent rats from adopting a side bias. The trial ended immediately after the rat ate the food from the cup or 150 seconds elapsed. Rats were tested in this phase for three days. By the end of the three days of testing, all rats chose the LR arm on seven out of the eight free-choice trials.
Barrier Training
Throughout barrier training, each rat received eight trials per day. For the first three days, a 15 cm, 90° barrier, as described above, was placed in the LR arm. The barrier was placed in the goal arm 10 cm from the intersection of the start and goal arms of the T-maze in the LR arm, and the food cup was placed 5 cm from the end of the LR arm. On the first five trials of the first day of testing, the trial ended only after the rat had climbed the barrier and eaten the eight Noyes pellets from the LR arm or 300 seconds had elapsed. On the last three trials of the first day and all remaining trials, the trial ended immediately after the rat chose one of the arms and consumed the pellets or 150 seconds elapsed. The height of the barrier was increased by 5 cm every three days until the 30-cm barrier was used. The 30-cm barrier was used in all subsequent experiments.
Discounting Procedure
A discounting procedure was used for all subsequent test trials. For this procedure, each rat received up to nine trials per day. Each rat received three days of testing with the discounting procedure prior to any drug testing. Prior to each discounting trial, the 30-cm barrier was placed in the LR arm. On the first trial of each discounting test day, the rat was allowed to eat the pellets in each arm. On all subsequent trials on a given day, the rat was only allowed to eat from the first arm chosen. If the rat chose the SR arm on the second daily trial or on any trial thereafter, testing was stopped (see Figure 1B). If the rat chose the LR arm, the LR arm was baited with one less food pellet on the next trial. This procedure was repeated after each subsequent choice of the LR arm. The number of trials until the rat chose the SR arm was recorded. Time to complete each trial also was recorded, defined as the time elapsed from placing the rat in the maze until it first ate a food pellet.
Drug Treatments
Once the rats were tested under the discounting procedure, studies were conducted that determined the effects of dopamine antagonists and agonists on effort-based choice behavior. Each study involved a within-subjects design wherein each rat was tested once under each treatment condition. In each study, treatments were administered in a counter-balanced manner. All rats were tested 2–3 times a week with at least a 48-hour interval between administration of any dopamine agonist or antagonist, except where noted below. On the days between drug testing and as noted below, all rats were given ten test trials using a non-discounting procedure. On these days, rats were tested in a manner identical to the final phase of discrimination training, except that the 30-cm barrier was placed in the LR arm, and the LR and SR arms contained eight and two pellets, respectively, on all ten trials. The rats were not tested on the weekends.
Drug treatments were studied in the following order. An initial study compared the effects of haloperidol to saline, and the interactive effects of haloperidol and D-amphetamine were assessed in a subsequent study. Following these two studies, the rats received non-discounting testing at least once a week over a period of 40 days. After that time, a third study was performed to compare the effects of SCH-23390 to saline, and a fourth study was conducted to assess the interactive effects of SCH-23390 and D-amphetamine. Two days after the completion of the latter study, the effects of the D3 antagonist, U99194, were compared to the effects of saline. A final study was initiated nine days after the completion of the U99194 study that compared the effects of two doses of the D3 agonist, 7-OH-DPAT, to saline.
All drugs were purchased from Sigma (St. Louis, MO) and, with the exception of haloperidol, were dissolved in saline. Haloperidol was dissolved in 10% glacial acetic acid, brought to volume with saline, and the pH was adjusted to ~6.3 with 6M NaOH. SCH-23390 (0.0125 mg/kg), haloperidol (0.1 mg/kg), 7-OH-DPAT (0.1 & 0.3 mg/kg), and U-99194 (6.25 mg/kg) were injected subcutaneously 30 minutes prior to testing in their respective studies. In the studies assessing the interactions between SCH-23390 or haloperidol with D-amphetamine (0.75 mg/kg), the latter drug was injected subcutaneously 10 min before testing. Drug doses were chosen based on their previously reported effects on locomotor activity. The selected doses of haloperidol, SCH-23390, and 7-OH-DPAT approximated those previously reported to reduce locomotor activity by 50% (Bardgett & Henry, 1999; Svensson, Carlsson, Huff, Kling-Petersen, & Waters, 1994). The chosen dose of U-99194 had been reported previously to produce a two-fold increase in locomotor activity (Carr, Yamamoto, Omura, Cabeza de Vaca, & Krahne, 2002). Likewise, the dose of D-amphetamine used approximated the dose previously reported to double rates of locomotor activity (Arnt 1995; Bardgett & Henry, 1999).
Data Analyses
A repeated-measures, one-way analysis of variance (ANOVA) was used to compare the percentage of LR arm choices over the six days of no barrier/barrier testing. A paired t-test was used to compare the number of trials until the SR arm was chosen between the discounting/no discounting trials. A paired t-test also was used to compare the effects of SCH-23390 or haloperidol alone to the effects of saline on the number of trials until the SR arm was chosen and the time to complete a trial. In the SCH-23390/D-amphetamine or haloperidol/D-amphetamine experiments, a two-way, repeated measures ANOVA was used to compare drug effects (i.e. antagonist vs. saline and D-amphetamine vs. saline) on the number of trials until the SR arm was chosen, as well as the time to complete the trial. In the studies involving U99194 or 7-OH-DPAT, a paired t test and a repeated-measures ANOVA were used, respectively, to compare the effects of each drug to saline on the number of trials until the SR arm was chosen and the time to complete a trial. Post-hoc comparisons were performed using Fisher’s Protected Least Squares (PLSD) test.
Results
Barrier and Discounting Effects on Choice Behavior
Over training days (i.e., Discrimination phase III), the rats came to choose the arm containing the large reward (LR arm) on a majority of trials in the absence of a barrier (Day effect: F(5, 40) = 4.7, p < .0017) (see Figure 2A, left). Without the barrier, the rats chose the LR arm more often on the third day of training in comparison to the first training day (Fisher’s PLSD, p < .01). When the 15-cm barrier was placed in the LR arm, the number of LR arm choices significantly decreased. Specifically, the rats chose the LR arm less on the first day of training with the barrier in comparison to the second and third days of training without the barrier (Fisher’s PLSD, p < .01 and .001, respectively). On the third day of training with the 15-cm barrier, the rats chose the LR arm with greater frequency than they did on the first day of such training (Fisher’s PLSD, p < .02), but with less frequency than they did on the third day of training without the barrier (Fisher’s PLSD, p < .02).
Figure 2.
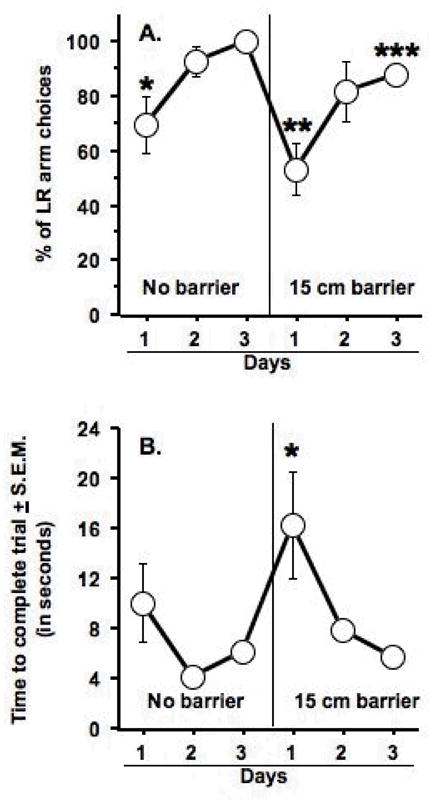
Effects of the barrier procedure on arm choice and time to complete the trial. A. Rats were significantly less likely to choose the arm containing eight pellets (LR arm) on the first day of training in comparison to the third day as indicated by the single asterisk. Placing a barrier in the LR arm significantly reduced the % of LR arm choices in comparison to the third day of training without the barrier, as indicated by the double asterisks. Rats made significantly more LR arm choices after the third day of training with the barrier in comparison to their choice behavior on the first day of training with the barrier. However, they also made significantly fewer LR choices as compared to the third day of training without the barrier, as indicated by the triple asterisks. In B. as indicated by the single asterisk, rats took significantly longer to complete the trial on the first day of barrier training in comparison to all other training days, except the first one without the barrier. Data represent mean ± S.E.M. n = 9.
As shown in Figure 2B, there was a significant test day effect on the time to complete the trial (i.e., the time elapsed between placing the rat in the start arm and the rat beginning to consume the food) (Day effect: F(5, 40) = 3.9, p < .006). On the first day of testing with the 15-cm barrier, the rats took significantly longer to complete the trial as compared to all other test days except for the first test day without the barrier (Fisher’s PLSD, p < .05 – .0002).
After achieving a stable baseline of choice behavior with a 30-cm barrier, performance was compared under two conditions: 1) “No discount” where the LR arm always contained eight pellets, and 2) “Discount” where, if the rat chose the LR arm, it contained one less pellet on the subsequent trial. Data from three test sessions under each condition were compared. By comparing the average number of trials until the rat made their first SR arm choice under each condition, it was found that that rats chose the SR arm after fewer trials under the “Discount” condition than under the “No discount” condition (paired t(8) = 3.3, p < .01) (see Figure 3A). As can be seen in Figure 3B, the rats also took longer to complete the trials when tested under the discounting procedure in comparison to the non-discounting procedure (paired t(8) = 3.2, p < .01).
Figure 3.
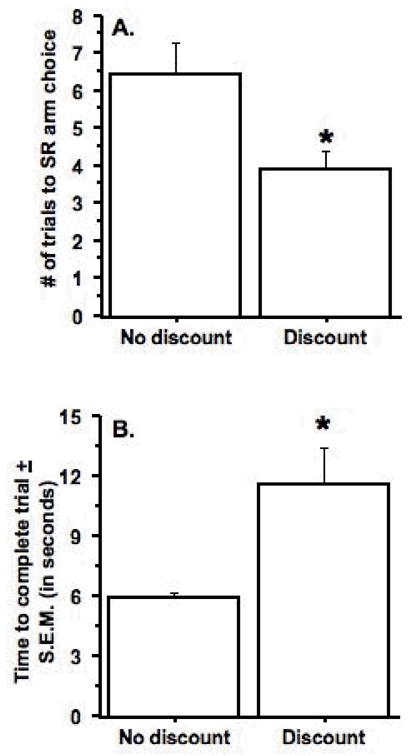
Effects of the discounting procedure on arm choice and time to complete the trial. A. Rats took significantly fewer trials to choose the small-reward (SR), non-barrier arm that contained two pellets when tested under the discounting versus the non-discounting procedure, as indicated by the asterisk. B. Rats also took longer to complete the trial during the Discount test days as opposed to the No Discount test days, as indicated by the asterisk. Data were obtained from three sessions under each condition and represent mean ± S.E.M. n = 9.
Effects of SCH-23390 and Haloperidol Alone
In separate studies, the effects of D1 and D2 receptor blockade on choice behavior were compared to the effects of saline. As shown in Table 1, both the D1 antagonist, SCH-23390 (0.0125 mg/kg), and the D2 antagonist, haloperidol (0.1 mg/kg), decreased the number of trials until a SR arm choice was made across discounting trials in comparison to the effects of saline (paired t(8) = 6.9 & 6.2, p < .0001 & .0003 for respective tests with each drug). Both drugs also significantly increased the time to complete the trials in comparison to saline (paired t(8) = 4.4 & 3.1, p < .002 & .01).
Table 1.
D1 and D2 antagonists decrease the likelihood of high effort choice.
| Drug | Trials until small-reward arm choice | Time to complete trial (in sec.) |
|---|---|---|
| Saline | 3.8 ± 0.4 | 5.8 ± 1.0 |
| SCH-23390 (0.0125 mg/kg) | 0.5 ± 0.4* | 83.6 ± 18.4* |
| Saline | 5.5 ± 0.6 | 7.8 ± 1.7 |
| Haloperidol (0.1 mg/kg) | 1.4 ± 0.2* | 20.6 ± 4.0* |
n = 9 for each experiment. The first measure (Trials) indicates the number of trials until the rat chose the small-reward arm. Data represent mean ± S.E.M. for each group. The asterisks indicate significant differences at p < .01 – .0001 between the effects of saline and SCH-23390 or haloperidol for each measure.
Interactions Between SCH-23390 or Haloperidol and D-Amphetamine
Two additional studies were performed to determine if the effects of D1 and D2 receptor antagonism were sensitive to D-amphetamine, a drug known to increase dopamine release in the brain. The same doses of SCH-23390 and haloperidol used in the studies reported above were used in these studies, and the dose of D-amphetamine was 0.75 mg/kg.
In the SCH-22390/D-amphetamine study, there was a significant effect of pretreatment (SCH-23390 or saline) (F(1, 16) = 11.2, p < .004) and treatment (D-amphetamine or saline) (F(1, 16) = 8.1, p < .01), as well as a significant pre-treatment × treatment interaction (F(1, 16) = 6.2, p < .02) (see Figure 4A). In an individual group comparison, a statistical trend suggested that the rats pretreated with saline and treated with D-amphetamine chose the LR arm more than the rats pretreated and treated with saline (Fisher’s PLSD, p = .10; two-tailed). The rats pretreated with SCH-2390 and treated with saline took significantly fewer trials to choose the SR arm in comparison to all other pretreatment/treatment groups (Fisher’s PLSD, p < .0016 – .0053), including the rats pretreated with SCH-23390 and treated with D-amphetamine.
Figure 4.
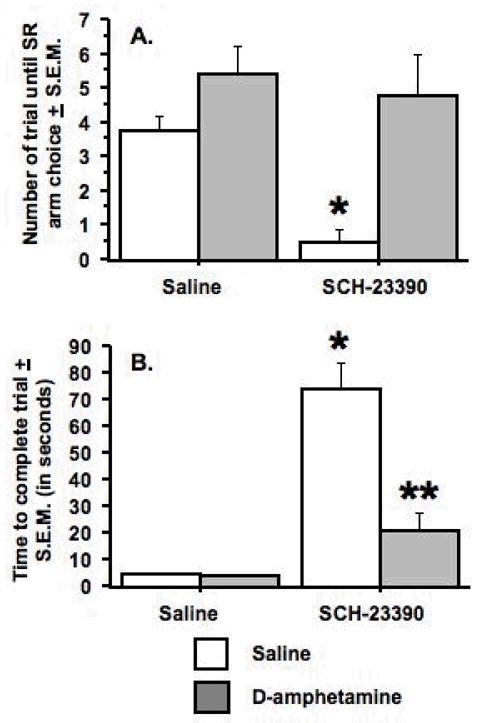
Effects of the D1 antagonist, SCH-23390, and D-amphetamine on choice behavior and time to complete a trial. A. Pretreatment with SCH-23390 (0.0125 mg/kg) and treatment with saline decreased the number of trials to choose the small-reward (SR) arm in comparison to all pretreatment/treatment regimens, as indicated by the asterisk. B. Pretreatment with SCH-23390 and treatment with saline significantly increased the time to complete a trial in comparison to all pretreatment/treatment regimens, as indicated by the single asterisk. Pretreatment with SCH-23390 and treatment with D-amphetamine (0.75 mg/kg) significantly increased the time to complete a trial in comparison to pretreatment with saline, as indicated by the two asterisks. Data represent mean ± S.E.M. n = 9.
Analyses of the time to complete a trial indicated significant pretreatment (F(1, 16) = 58.6, p < .0001) and treatment (F(1, 16) = 20.9, p < .0003) effects, as well as a significant interaction (F(1, 16) = 20.7, p < .0003) (see Figure 4B). The rats pretreated with SCH-23390 and treated with saline took significantly longer to complete the trial when compared to all other pretreatment/treatment groups (Fisher’s PLSD, p < .0002 –.0003). Additionally, statistical trends suggested that the rats pretreated with SCH-23390 and treated with D-amphetamine were slower than the rats pretreated with saline and treated with saline or D-amphetamine (Fisher’s PLSD, p = .07 & .052, respectively).
As can be seen in Figure 5, the pattern of results generated in the haloperidol/D-amphetamine study tended to mirror the results obtained from the SCH-23390/D-amphetamine study. Significant effects of haloperidol pretreatment (F(1, 16) = 22.08, p < .0002) and D-amphetamine treatment (F(1, 16) = 5.8, p < .03) were obtained on the number of trials to choose the SR arm measure (see Figure 5A). The rats pretreated with saline and treated D-amphetamine continued to choose the LR arm after significantly more trials in comparison to the rats pretreated and treated with saline (Fisher’s PLSD, p < .01), and this same difference was observed in the comparison of the two groups pretreated with haloperidol (Fisher’s PLSD, p < .02). In the rats treated with saline, those pretreated with haloperidol chose the SR arm after significantly fewer trials than those pretreated with saline (Fisher’s PLSD, p < .003). Likewise, in the rats treated with D-amphetamine, those pretreated with haloperidol chose the SR arm after fewer trials in comparison to those pretreated with saline (Fisher’s PLSD, p < .03).
Figure 5.
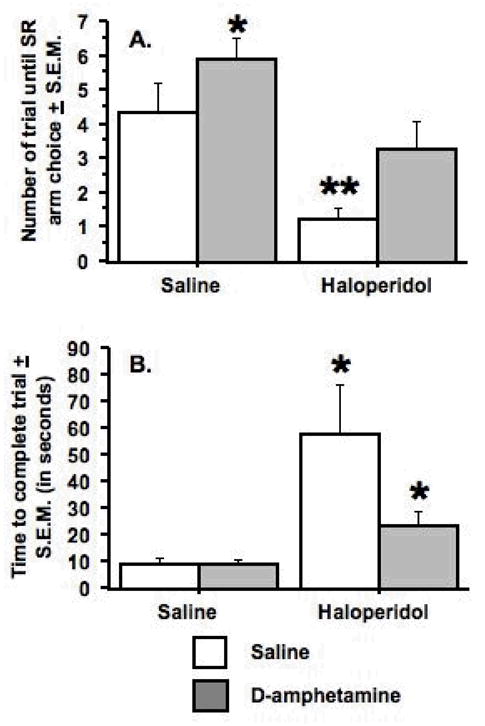
Effects of haloperidol and D-amphetamine on choice behavior and time to complete a trial. A. Pretreatment with saline and treatment with D-amphetamine (0.75 mg/kg) increased the number of trials to choose the SR arm in a comparison with all other regimens, as indicated by the single asterisk. Pretreatment with haloperidol (0.1 mg/kg) and treatment with saline decreased the number of trials to choose the SR arm in comparison to all other pretreatment/treatment regimens as indicated by the two asterisks. B. Pretreatment with haloperidol significantly increased the time to complete each trial in comparison to pretreatment with saline, as indicated by the asterisk. Data represent mean ± S.E.M. n = 9.
There was a significant effect of haloperidol pretreatment on the time to complete the trial measure (F(1, 16)= 10.5, p < .005) (see Figure 5B). Individual comparisons between the two groups of rats treated with saline and between the two groups treated with D-amphetamine revealed that the rats pretreated with haloperidol took significantly more time to complete the trials in comparison to the rats pretreated with saline (Fisher’s PLSD, p < .03 for each comparison).
Effects of a D3 Agonist and a D3 Antagonist on Choice Behavior
The final set of studies determined if choice behavior was influenced by modulation of D3 receptor activity. As can be seen in Figure 6, neither the D3 agonist, 7-OH-DPAT (0.1 and 0.3 mg/kg), nor the D3 antagonist, U99194 (6.25 mg/kg), altered choice behavior. 7-OH-DPAT significantly increased time to complete a trial (F(2, 16) = 7.3, p < .006) (see Table 2), whereas there was no significant effect of U99194 on the same measure. Rats treated with the high dose of 7-OH-DPAT took significantly longer to complete the trial than rats treated with saline. There were statistical trends towards significant between-group differences in the individual comparisons of saline and the low dose of 7-OH-DPAT (Fisher’s PLSD, p = .11) and the low and the high dose of 7-OH-DPAT (Fisher’s PLSD, p = .06).
Figure 6.
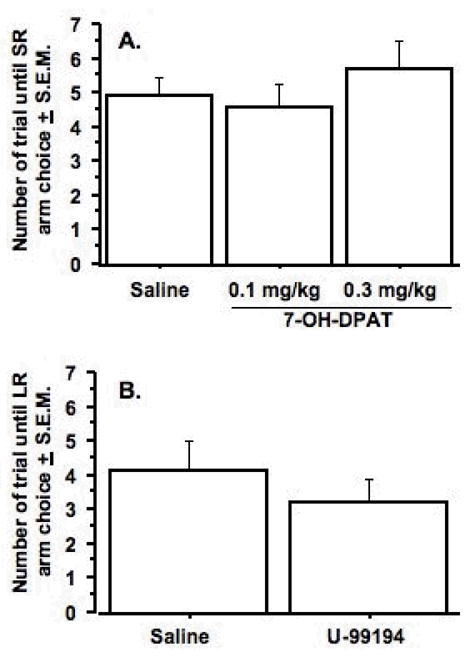
Effects of the D3 agonist, 7-OH-DPAT (A.) and the D3 antagonist, U-99194 (6.25 mg/kg) (B.) on choice behavior. There were no significant treatment effects in either experiment. Data represent mean ± S.E.M. n = 9.
Table 2.
Effects of 7-OH-DPAT and U99194 on the time to complete the trial.
| 7-OH-DPAT | U99194 | ||||
|---|---|---|---|---|---|
| Group | Saline | 0.1 mg/kg | 0.3 mg/kg | Saline | 6.25 mg/kg |
| Time (in sec) | 6.8 ± 1.2 | 9.0 ± 0.9* | 12.3 ± 1.6** | 4.9 ± 1.7 | 5.5 ± 1.8 |
n = 9 for each experiment. Data represent mean time in seconds ± S.E.M. for each group. The single asterisk indicates a trend towards a statistical difference between the 0.1 mg/kg group and the saline and 0.3 mg/kg groups (p = .11 & .06 for respective comparisons). The double asterisk indicates a significant difference between the saline and 0.3 mg/kg groups (p < .008).
Discussion
Many lesion studies have suggested that dopamine modulates effortful behavior motivated by reward. These studies, however, have left a central question unanswered: Are specific dopamine receptors required for such behavior? The present study addressed this question by examining effort-based decision-making behavior in rats after treatment with antagonists for D1, D2, and D3 receptors. The results showed that blockade of D1 or D2 receptors significantly reduced effort-based decision-making, whereas D3 receptor blockade had no such effect. Furthermore, D-amphetamine, a drug known to elevate extracellular dopamine levels, reversed the effects of SCH-23390 and haloperidol, providing further evidence that the effects of the latter two drugs are mediated through dopamine receptors. Overall, the present findings illustrate the role of different dopamine receptors in effort-based decision-making, and are consistent with previous studies that have addressed dopamine’s role in such behavior.
In most studies of effort-based decision-making in the T-maze, rats are given the choice between large- and small-reward arms over a series of trials within a daily test session. The reinforcement magnitude for each arm typically remains the same on each trial. This approach raises the likelihood of more variable choice behavior during later trials since the rats may become satiated after repeated visits to the large-reward arm and would therefore be less motivated to exert effort for a low incentive reward. Accordingly, such a procedure may undermine the sensitivity of the task to different manipulations – an issue raised in previous work using this task (Denk, Walton, Jennings, Sharp, Rushworth, & Bannerman, 2005). One unique feature of the present study was the use of a discounting procedure. When the rats chose the large-reward arm at the beginning of the daily test session, a pellet was removed from that arm on the subsequent trial. This process was repeated until the rat chose the small-reward arm, at which time the daily test session was terminated. This approach was based in part on previous work by Green and colleagues (Green et al., 2004). These authors used an adjustable discounting scheme to determine indifference points between two choices in order to calculate sensitivities to different delays and reward amounts. This approach may enable researchers to determine the neurobiological substrates of effort-based decision-making in the T-maze in a more sensitive manner.
SCH-23390 and haloperidol increased the likelihood that rats would choose the small-reward arm in fewer trials, but also increased the time to complete the task. It was not unexpected that these drugs would exert such an effect, given the substantial neural overlap in dopaminergic control of motor and motivational processes (see Salamone, Correa, Farrar, & Mingote, 2007, for review). Moreover, the drug doses were selected based on their previously reported effects on locomotor activity (see below). Nonetheless, the locomotor slowing raises two questions regarding the alteration in choice behavior produced by SCH-23390 and haloperidol. First, are the drug effects on choice behavior simply a consequence of motor impairment? Second, does the increased time to complete the task serve as a substantial delay of reinforcement?
Several observations reduce the likelihood that the drug-induced alterations in choice behavior can be explained completely by locomotor slowing or reinforcement delay. First, the D3 agonist, 7-OH-DPAT, was found to produce locomotor slowing in a dose-dependent manner, but did not alter choice behavior. Second, D-amphetamine alone increased large-reward arm choice without altering the time to complete the task, suggesting a dissociation between choice behavior and locomotion. Third, work by Denk and colleagues (2005) found that rats treated with 0.2 mg/kg of haloperidol – a higher dose than that used in the present study – chose a large-reward arm more often than a small-reward arm when a barrier was placed in each arm. Likewise, Salamone et al. (1994) found that haloperidol-treated rats, even while slower to choose a goal arm, were still more likely to choose a large-reward arm over a small-reward one if there were no barrier present in the maze. Finally, concerns over the delayed reinforcement induced by drug-induced locomotor slowing may be allayed in part by the observation that 7-OH-DPAT did not alter choice behavior despite causing a significant increase in delay between trial start and food consumption. Along these same lines, Salamone et al. (1994) found that after 6-OHDA lesions of the nucleus accumbens, latencies and choice behavior showed different rates of recovery, with the former recovering faster than the latter. These results taken together clearly suggest that even in the absence of latency differences (i.e., delays in choosing) between the groups, disruption of dopaminergic transmission still decreases effort-based decision-making. It should be emphasized that the procedures reported here cannot rule out locomotor and delay influences on performance. Nonetheless, the pattern of results suggests that some proportion of the altered choice behavior observed after SCH-23390 and haloperidol treatment can be explained by the specific effects of these drugs on effortful behavior.
Our findings provide clear support for the idea that D1 and D2 receptors are critical to effort-based decision-making. Earlier work by others (Salamone et al., 1994; Denk et al., 2005; Walton et al., 2005) demonstrated the disruptive effects of haloperidol on such behavior, but the present results represent, to our knowledge, the first comparison of antagonists at D1, D2, and D3 receptors on effortful decision-making in the T-maze. Although manipulations at D1 and D2 receptors may have divergent effects at the intracellular level (Civelli, 1995), antagonism of either receptor tends to produce a similar behavioral output, especially in studies of locomotor activity (e.g., Bardgett & Henry, 1999). The present results demonstrate that this similarity in drug action extends to effort-based behavior in the T-maze. Moreover, modulation of D3 receptors had no effect on effort-based decision-making in the T-maze.
Agonists at D3 receptors have been observed to inhibit motor function (Pritchard, Logue, Hayes, Welge, Xu, Zhang, Berger, & Richtand, 2003), whereas antagonists exert an opposite effect on locomotor activity (as reviewed in Millan, Seguin, Gobert, Cussac, & Brocco, 2004) or at least augment the effects of other psychostimulants on activity (McNamara, Levant, Taylor, Ahlbrand, Liu, Sullivan, Stanford, & Richtand, 2006). Consistent with this literature, the D3 agonist 7-OH-DPAT was found to increase the time to complete the trial, suggestive of locomotor suppression. However, neither it nor the D3 antagonist U99194 altered choice behavior. These results imply that decision-making based on effort is not amenable to changes in activity at all dopamine receptor subtypes.
The results do not clarify the exact role of D1 and D2 receptors in effort-based decision-making. The similar effects of D1 and D2 receptor antagonism suggest that the contributions of each receptor are simply redundant, or that they impact distinct aspects of dopaminergic function which modulate different behavioral processes (e.g., energy, motivation, incentive, attention, memory, and appetite). Consideration of neuroanatomy may offer some insight into this issue. Schweimer and Hauber (2006) demonstrated that local antagonism of D1 receptors, but not D2 receptors, in the anterior cingulate cortex reduced effort-based decision-making. It is interesting to note that anterior cingulate cortical lesions have been shown to disrupt effort-based decision-making (Walton et al., 2003; Rudebeck et al., 2006), but such lesions do not alter delay-based decision-making (Cardinal, 2001). Moreover, some studies have suggested that delay-based decision-making is less sensitive to systemic antagonism of D1 receptors relative to D2 receptors (Wade et al., 2000), especially when one uses a discounting procedure. Overall, D2 receptors may play a more fundamental role in reward-based behavior through modulation of regions implicated in both delay- and effort-based reinforcement, such as the nucleus accumbens (see Salamone et al., 2007 for review) and amygdala (Floresco & Ghods-Sharifi, 2007). In contrast, D1 receptors may be more specifically involved in effort-based behaviors through the modulation of cortical circuits such as the anterior cingulate cortex. Such speculation supports the notion that different dopamine receptors make distinct contributions to effort-based decision-making.
Two methodological limitations in the present study are noteworthy. First, only single doses of SCH-23390, haloperidol, D-amphetamine, and U99194 were used (two doses of 7-OH-DPAT were studied). The selected dose of each drug was based upon previous research that had shown that approximately the same doses of each drug decreased (SCH-23390, haloperidol, 7-OH-DPAT; Bardgett & Henry 1999; Svensson et al., 1994) or increased (U99194, D-amphetamine; Arnt 1995; Bardgett & Henry 1999; Carr et al., 2002) locomotor activity by 50%. By using this strategy to select drug doses, it was assumed that the dose would be behaviorally active but would not produce the dramatic effects on locomotion or stereotypy observed after treatment with higher doses of these drugs (Arnt 1995; Bardgett, Jackson, Taylor, & Csernansky, 1998). Also, given the repeated-measures design used in the present study, there was a concern that dose-response experiments (i.e., multiple exposures of individual rats to different drug doses) might enhance the likelihood of tolerance and sensitization. Limiting the number of drug exposures was intended to reduce the likelihood of these latter phenomena. By using the selected doses of each drug, it was established that SCH-23390, haloperidol, and D-amphetamine effectively altered performance in the decision-making task. Future studies that incorporate a dose-response approach, especially ones that include lower doses that may alter choice behavior but not response latency, undoubtedly will provide a fuller picture of the role of dopamine receptors in effort-based decision-making.
A second limitation to the study was the use of a repeated-measures design in which individual rats were tested under different drug-treatment regimens. Moreover, treatment was delivered in a non-counterbalanced manner, in which each drug was tested in a separate experiment. Even by limiting the number of doses that the rats received, as noted above, exposure of the same rats to different drugs still could increase the likelihood of sensitization or tolerance. The results of the study, however, do not provide any evidence for such phenomena. Examination of the saline-treated rats in each experiment indicates that baseline choice behavior was relatively consistent across the experiments, as were discounting scores for rats treated with SCH-23390, haloperidol, and D-amphetamine in the separate experiments. Moreover, rats were given a 48-hr period between drug treatments in order to minimize cumulative drug effects, and exposure of the same animals to multiple doses of the same drugs was avoided to circumvent such problems. The results do not completely preclude the possibility of cumulative drug effects, but it appears that such effects played a minimal role in modifying choice behavior across the experiments.
The results of the present study may have some indirect implications for mental disorders associated with decision-making (Paulus, 2007). Attention-deficit hyperactivity disorder (ADHD) is linked to impulsive behavior, but it is possible that some element of this impulsivity is driven by the amount of effort required for specific outcomes. The present data suggest that low dopamine levels may predispose one to favor less effortful choices over more effortful ones, whereas D-amphetamine, a common stimulant treatment for ADHD, corrects this shift in choice behavior. Our data also suggest that antipsychotic drugs, nearly all of which antagonize D2 receptors, would likely exacerbate the impairments in decision-making associated with some forms of schizophrenia (Ludewig, Paulus, & Vollenweider, 2003). Thus, the effort-based decision-making task may have some utility in identifying treatments that may exacerbate or alleviate the decision-making problems observed in some psychiatric disorders.
Acknowledgments
We appreciate the critical reading of this manuscript and feedback provided by Dr. David Hogan.
This research was supported by National Center for Research Resources Grant P20 RR16481 and National Institute of Mental Health Grant R15 MH076788.
Contributor Information
Mark E. Bardgett, Department of Psychology, Northern Kentucky University
Melissa Depenbrock, Department of Psychology, Northern Kentucky University.
Nathan Downs, Department of Psychology, Northern Kentucky University.
Megan Points, Department of Psychology, Northern Kentucky University.
Leonard Green, Department of Psychology, Washington University.
References
- Arnt J. Differential effects of classical and newer antipsychotics on the hypermotility induced by two dose levels of D-amphetamine. European Journal of Pharmacology. 1995;283:55–62. doi: 10.1016/0014-2999(95)00292-s. [DOI] [PubMed] [Google Scholar]
- Audinot V, Newman-Tancredi A, Gobert A, Rivet JM, Brocco M, Lejeune F, Gluck L, Desposte I, Bervoets K, Dekeyne A, Millan MJ. A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103,691 and U 99194. Journal of Pharmacological and Experimental Therapeutics. 1998;287:187–197. [PubMed] [Google Scholar]
- Bardgett ME, Henry JD. Locomotor activity and accumbens Fos expression driven by ventral hippocampal stimulation require D1 and D2 receptors. Neuroscience. 1999;94:59–70. doi: 10.1016/s0306-4522(99)00303-6. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Jackson JL, Taylor BM, Csernansky JG. The effects of kainic acid lesions on locomotor responses to haloperidol and clozapine. Psychopharmacology. 1998;135:270–278. doi: 10.1007/s002130050509. [DOI] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Networks. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Carr KD, Yamamoto N, Omura M, Cabeza de Vaca S, Krahne L. Effects of the D(3) dopamine receptor antagonist, U99194A, on brain stimulation and d-amphetamine reward, motor activity, and c-fos expression in ad libitum fed and food-restricted rats. Psychopharmacology. 2002;163:76–84. doi: 10.1007/s00213-002-1132-0. [DOI] [PubMed] [Google Scholar]
- Civelli O. Molecular biology of dopamine receptor subtypes. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth generation of progress. Philadelphia: Lippincott, Williams, & Wilkins; 1995. pp. 155–161. [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behavioural Brain Research. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cerebral Cortex. 2007;17:251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: is there a magnitude effect? Journal of the Experimental Analysis of Behavior. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman D, Monsma F, Civelli O. Interaction of antipsychotic drugs with dopamine receptor subtypes. In: Csernansky JG, editor. Antipsychotics. Berlin: Springer; 1996. pp. 43–76. [Google Scholar]
- Kozell LB, Machida CA, Neve RL, Neve KA. Chimeric D1/D2 dopamine receptors. Distinct determinants of selective efficacy, potency, and signal transduction. Journal of Biological Chemistry. 1994;269:299–306. [PubMed] [Google Scholar]
- LaHoste GJ, Ruskin DN, Marshall JF. Cerebrocortical Fos expression following dopaminergic stimulation: D1/D2 synergism and its breakdown. Brain Research. 1996;728:97–104. [PubMed] [Google Scholar]
- Lévesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proceedings of the National Academy of Sciences. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig K, Paulus MP, Vollenweider FX. Behavioural dysregulation of decision-making in deficit but not nondeficit schizophrenia patients. Psychiatry Research. 2003;119:293–306. doi: 10.1016/s0165-1781(03)00103-3. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Levant B, Taylor B, Ahlbrand R, Liu Y, Sullivan JR, Stanford K, Richtand NM. C57BL/6J mice exhibit reduced dopamine D3 receptor-mediated locomotor-inhibitory function relative to DBA/2J mice. Neuroscience. 2006;143:141–153. doi: 10.1016/j.neuroscience.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH. Update on dopamine receptors. Annals of Clinical Psychiatry. 1994;6:79–90. doi: 10.3109/10401239409148986. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Seguin L, Gobert A, Cussac D, Brocco M. The role of dopamine D3 compared with D2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats. Psychopharmacology. 2004;174:341–57. doi: 10.1007/s00213-003-1770-x. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007 October 26;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J, Berger SP, Richtand NM. 7-OH-DPAT and PD 128907 selectively activate the D3 dopamine receptor in a novel environment. Neuropsychopharmacology. 2003;28:100–107. doi: 10.1038/sj.npp.1300018. [DOI] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. Journal of the Experimental Analysis of Behavior. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nature Neuroscience. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–82. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behavioural Brain Research. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Hauber W. Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learning & Memory. 2006;13:777–782. doi: 10.1101/lm.409306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J, Saft S, Hauber W. Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behavioral Neuroscience. 2005;119:1687–1692. doi: 10.1037/0735-7044.119.6.1687. [DOI] [PubMed] [Google Scholar]
- Svensson K, Carlsson A, Huff RM, Kling-Petersen T, Waters N. Behavioral and neurochemical data suggest functional differences between dopamine D2 and D3 receptors. European Journal of Pharmacology. 1994;263:235–243. doi: 10.1016/0014-2999(94)90718-8. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. The Journal of Neuroscience. 2003;23:6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. The Journal of Neuroscience. 2002;22:10996–1003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Croxson PL, Rushworth MF, Bannerman DM. The mesocortical dopamine projection to anterior cingulate cortex plays no role in guiding effort-related decisions. Behavioral Neuroscience. 2005;119:323–328. doi: 10.1037/0735-7044.119.1.323. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE. Interactive effects of stimulation of D1 and D2 dopamine receptors on fos-like immunoreactivity in the normosensitive rat striatum. Brain Research Bulletin. 1994;35:85–91. doi: 10.1016/0361-9230(94)90220-8. [DOI] [PubMed] [Google Scholar]


