Abstract
Background: We evaluated discordance in expression measurements for estrogen receptor (ER), progesterone receptor (PR), and HER2 between primary and recurrent tumors in patients with recurrent breast cancer and its effect on prognosis.
Methods: A total of 789 patients with recurrent breast cancer were studied. ER, PR, and HER2 status were determined by immunohistochemistry (IHC) and/or FISH. Repeat markers for ER, PR, and HER2 were available in 28.9%, 27.6%, and 70.0%, respectively. Primary and recurrent tumors were classified as triple receptor-negative breast cancer (TNBC) or receptor-positive breast cancer (RPBC, i.e. expressing at least one receptor). Discordance was correlated with clinical/pathological parameters.
Results: Discordance for ER, PR, and HER2 was 18.4%, 40.3%, and 13.6%, respectively. Patients with concordant RPBC had significantly better post-recurrence survival (PRS) than discordant cases; patients with discordant receptor status had similarly unfavorable survival as patients with concordant TNBC. IHC scores for ER and PR showed weak concordance between primary and recurrent tumors. Concordance of HER2–FISH scores was higher.
Conclusions: Concordance of quantitative hormone receptor measurements between primary and recurrent tumors is modest consistent with suboptimal reproducibility of measurement methods, particularly for IHC. Discordant cases have poor survival probably due to inappropriate use of targeted therapies. However, biological change in clinical phenotype cannot be completely excluded.
Keywords: concordance, HER2, hormone receptors, survival, testing
introduction
Expression of both hormone receptors [i.e. estrogen receptor (ER) and progesterone receptor (PR)] and HER2 is frequently used to clinically define the major breast cancer subtypes [1] that show differences in clinical outcome [2]. Breast cancers lacking expression of ER, PR, and HER2 are designated as triple-negative breast cancer (TNBC) and carry a particularly unfavorable prognosis [3, 4]. The latter is understood as a consequence of both worse prognostic features of these tumors (i.e. higher grade) and limited therapeutic options (i.e. lack of benefit from endocrine and trastuzumab therapies).
Given the predictive importance of both hormone receptors and HER2, it is currently recommended that they should be determined as part of diagnostic routine on every primary invasive breast cancer [5]. However, changes in receptor status over the course of disease progression have been described. For instance, concordance rates between primary tumors and recurrence site of 71% and 56% have been reported for ER and PR expression, respectively [6]. For HER2, discrepancy rates for expression among primary tumors compared with matching metastasis may be as high as 20% [7–10]. To date, it is not clear how changes in triple-receptor phenotype affect patient prognosis.
The primary purpose of this analysis was to describe the concordance in triple-receptor expression (i.e. ER, PR, and HER2) between the primary tumor and the recurrent lesion in patients with breast cancer. A secondary purpose was to determine the effect of receptor concordance on disease prognosis. For this purpose, ER, PR, and HER2 expression status of primary tumor and/or metastasis were analyzed among 789 patients with metastatic breast cancer treated at M. D. Anderson Cancer Center (MDACC) for their primary and/or recurrent breast cancer from February 1990 to July 2007.
It is important to realize up front that patients’ expression results did not undergo centralized analysis. To our opinion, this constitutes a major strength of this study, as this represents what is common practice. We decided to distinguish patients with receptor-positive breast cancer (RPBC) expressing at least one receptor and patients with TNBC because while the latter do not represent a therapeutically targetable receptor (such as ER or HER2) patients with RPBC may show benefit from at least one form of targeted therapies (i.e. antiendocrine or anti-HER2).
methods
selection and description of participants
A retrospective review using the Breast Medical Oncology Database was carried out. Data obtained from 789 patients with (locally, regionally, or distantly) recurrent breast cancer was evaluated for this study. Inclusion characteristics comprised diagnosis of primary, unilateral breast cancer with subsequent development of locally, regionally, or distantly recurrent disease with recorded expression status of at least one of ER, PR, and HER2 in both primary tumor and recurrence. Exclusion criteria comprised bilateral breast cancer, male gender, and ductal carcinoma in situ as initial diagnosis. Patients could have any form of surgical, systemic (neoadjuvant and adjuvant) therapy as well as radiotherapy. Clinical and histological characteristics of all patients had been entered prospectively into the above-mentioned database based on information obtained from medical records. While all new patients to M. D. Anderson have a central pathology review by a dedicated breast pathologist, no central pathology rereview was carried out for this analysis. The institutional review board approved this retrospective chart review.
pathology assessment
For patients who had been assessed at MDACC as part of routine clinical management, ER and PR status had been assessed by immunohistochemistry (IHC) [6F11 (Novacastra Laboratories Ltd, Burlingame, CA) for ER and 1A6 (Novacastra Laboratories Ltd) for PR]. The cut-off for ER positivity (ER+) and PR positivity (PR+) was ≥10% tumor cells with nuclear staining. HER2 status had been assessed either by FISH or by IHC (Dako North America, Inc., Carpinteria, CA). HER2 positivity (HER2+) had been defined as either HER2 gene amplification (FISH) or an immunohistochemical score of 3+ (IHC). For patients who had been assessed at different institutions, expression results were obtained either (i) by carrying out de novo if no staining results but unstained patient tissue/slides were available, (ii) by review of slides stained immunohistochemically by the outside institution at the time of initial presentation by a pathologist as part of clinical routine at MDACC (if no unstained slides were available for de novo staining but stained slides were available for review), or (iii) retrieved from the patients referral documents/communication alone if no stained or unstained slides were available. If semiquantitative staining results were given, these were categorized as above; if only positive or negative staining was mentioned, these dichotomized results were employed for the analysis. For each of the three receptors, patients were classified as having concordant receptors if primary tumor and recurrent disease were either both positive or both negative. Other combinations were considered as discordant.
Nuclear grade was assessed using the modified Black's nuclear grading system [11]. Staining location was recorded as either ‘MDACC’ or ‘outside’. Also, biopsy sites corresponding to the stained specimens were recorded. Anatomical biopsy sites were retrieved from the pathology report.
definitions
TNBC was assigned if all three receptors were coded as negative. In contrast, patients were coded as receptor-positive breast cancer (RPBC) if they showed expression of at least one receptor. If data about one or two receptors were missing, but the third was positive, that case was coded as receptor positive. All three receptors had to be known and be negative for a patient to be considered triple-receptor negative.
Triple-receptor concordance was defined as either TNBC or RPBC in both tumor and recurrence, i.e. either concordant TNBC or concordant RPBC.
Triple-receptor discordance was defined as TNBC at one site and RPBC at the other site.
Statistical methods can be found in supplemental File 1 (available at Annals of Oncology online).
results
patient characteristics
Patient characteristics by triple-receptor concordance status are given in Table 1. Additional patient characteristics are given in supplemental File 2 (available at Annals of Oncology online).
Table 1.
Patient characteristics by triple-receptor concordance
| Triple-receptor discordance (n = 55) |
Triple-receptor concordance (n = 176) |
P valuea | |||
| N | % | N | % | ||
| Clinical tumor stage | 0.257 | ||||
| T0/T1 | 6 | 12.8 | 37 | 23.7 | |
| T2 | 21 | 44.7 | 71 | 45.5 | |
| T3 | 5 | 10.6 | 19 | 12.2 | |
| T4 | 15 | 31.9 | 29 | 18.6 | |
| Clinical nodal stage | 0.397 | ||||
| N0 | 18 | 38.3 | 56 | 35.9 | |
| N1 | 16 | 34.0 | 69 | 44.2 | |
| N2 | 7 | 14.9 | 12 | 7.7 | |
| N3 | 6 | 12.8 | 19 | 12.2 | |
| Response to neoadjuvant chemotherapyb | 1.000 | ||||
| RD | 31 | 93.9 | 96 | 94.1 | |
| pCR | 2 | 6.1 | 6 | 5.9 | |
| Interval between staining of primary tumor and staining of recurrence | 0.001* | ||||
| Mean interval (months) | 29.15 | 34.50 | |||
| Range | 6–85 | 0–144 | |||
| Age at diagnosis | 0.757 | ||||
| Mean age (years) | 46.80 | 46.28 | |||
| Range | 27–74 | 24–77 | |||
| Prior taxane | 0.444 | ||||
| No | 16 | 29.1 | 61 | 34.7 | |
| Yes | 39 | 70.9 | 115 | 65.3 | |
| Prior trastuzumab | 0.544 | ||||
| No | 51 | 92.7 | 167 | 94.9 | |
| Yes | 4 | 7.3 | 9 | 5.1 | |
| Prior endocrine therapy | 0.905 | ||||
| No | 33 | 60.0 | 104 | 59.1 | |
| Yes | 22 | 40.0 | 72 | 40.9 | |
| Distant metastasis as first site of recurrence | 0.510 | ||||
| No | 10 | 18.2 | 40 | 22.7 | |
| Yes | 45 | 81.8 | 136 | 77.3 | |
| Location of staining | 0.418 | ||||
| Staining location concordance | 26 | 55.5 | 90 | 63.1 | |
| Staining location discordance | 25 | 45.5 | 65 | 36.9 | |
| Unknown | – | 21 | – | ||
From Pearson's chi square.
Data available for a total of 135 cases.
Statistically significant.
RD, residual disease; pCR, pathologic complete response.
concordance in triple-receptor expression and association with post-recurrence survival
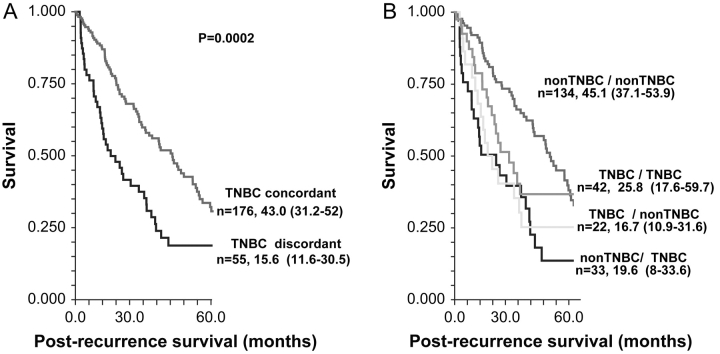
Median follow-up from the date of recurrent breast cancer diagnosis was 16.8 months (range 0–205.4 months). Figure 1 shows Kaplan–Meier survival curves for post-recurrence survival (PRS) by triple-receptor expression concordance. Patients with triple-receptor concordant disease (Figure 1A) had significantly better PRS [median 43.0 months, 95% confidence interval (CI) 31.2–52 months] compared with patients who had discordant receptor results (median 15.6 months, 95% CI 11.6–30.5 months).
Figure 1.
Kaplan–Meier survival curves for post-recurrence survival stratified for (A) triple-negative breast cancer (TNBC) concordance versus discordance and (B) all four combinations of TNBC expression in primary tumor and recurrence.
Patients who had concordant RPBC of both primary and recurrence (Figure 1B) had the most favorable PRS (median 45.1 months, 95% CI 37.1–53.9 months). The other two groups, i.e. concordant TNBC and cases with discordant receptor status, had significantly worse survival. However, the survival experience of these two groups was similar to each other. Most receptor concordant cases were RPBC; therefore, the superior PRS of this group is likely due to the large number of hormone receptor-positive patients in this group.
When survival analysis was repeated for patients with distant metastasis as first site of recurrence, a significantly decreased PRS was observed for patients with discordance compared with concordance (hazard ratio = 1.92, 95% CI 1.19–3.10, P = 0.0014).
association between receptor concordance and staining variables
Of the 211 sets of paired complete triple-receptor measurements, 119 were both carried out at MDACC or outside MDACC. Among these cases, concordance in triple-receptor status was 52.3% compared with 43.2% for cases that had been stained at different institutions (P = 0.418).
Next, cases were grouped according to (i) the date of diagnosis for the primary tumor into four different groups (1982–1990, 1991–1995, 1996–2000, and 2001–2006) and (ii) the date of diagnosis of recurrence into three different groups (1990–1995, 1996–2000, and 2000–2007). The only significant association was found between year of diagnosis of the primary tumor and concordance of HER2 (P = 0.02).
concordance correlation between semiquantitative IHC scores and FISH ratios in primary tumor and recurrence
Dichotomous clinical receptor status is defined by thresholds, thus a patient with widely different receptor measurement may still remain in the same clinical category. For instance, if the primary tumor showed 90% ER+ and the metastatic lesion showed 15% positivity, the case would still be considered concordant for ER status (i.e. ER+).
In Figure 2A–C, percentages of positively stained nuclei for ER and PR and the FISH ratios are plotted for both primary and recurrent tumor. As seen in Figure 2A for ER, correlation coefficients even for the clinically concordant cases show only a weak if any correlation between percent positivity. Figure 2B shows a similarly poor correlation with regard to percent positivity of PR between primary tumor and recurrent site. In contrast, an increased correlation was seen between FISH ratios of the primary and recurrent tumor (Figure 2C). This observation is consistent with the notion that FISH is a technically more robust and reproducible assay.
Figure 2.
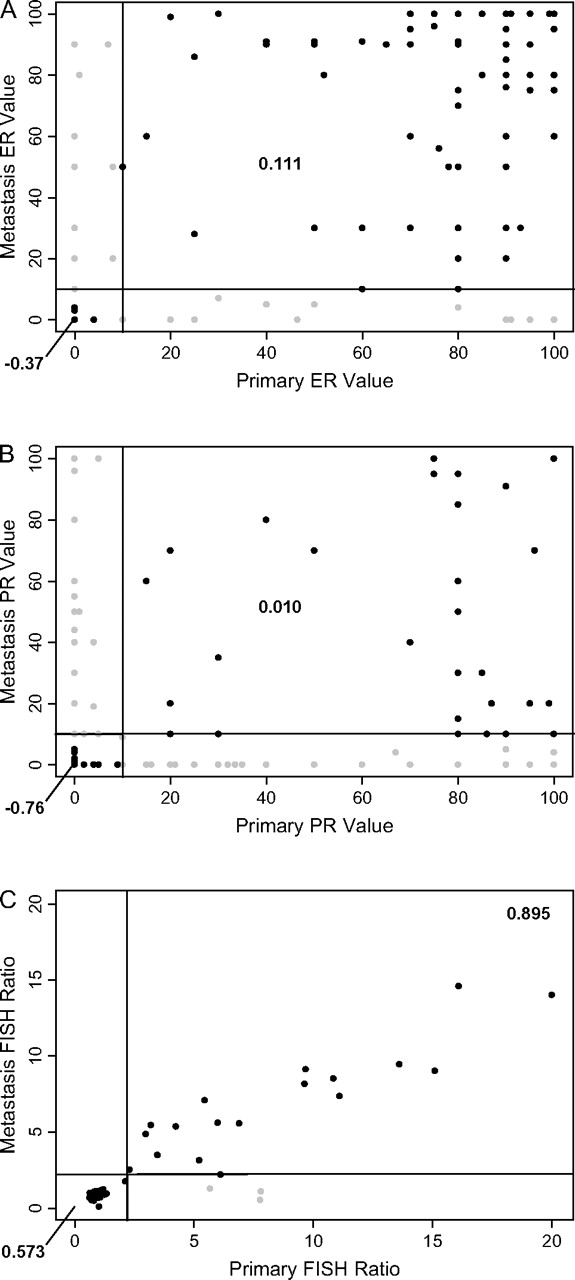
Percentages of positively stained nuclei for (A) estrogen receptor (ER) and (B) progesterone receptor (PR) and (C) FISH scores for both the primary and recurrent tumor are plotted. For each plot, the x-axis refers to the primary tumor and the y-axis to the recurrence site. The black lines represent the thresholds for clinical positivity (i.e. 10% staining for ER and PR immunohistochemistry and a FISH ratio of >2.0 for HER2).
discussion
We report the results of the first systematic evaluation of the association of concordance in triple-receptor expression between the primary tumor and the recurrence. In summary, concordance rates for triple-receptor expression (i.e. triple-receptor negativity versus non-triple-receptor negativity) were 76.2%. Among discordant cases, change from RPBC to TNBC was more common (14.3%) than change from TNBC to RPBC (9.5%).
Several studies have addressed the issue of concordance/discordance in expression of individual receptors between primary tumor and recurrence/metastasis; however, discordance rates varied substantially from study to study [7–10, 12–17].
Of note, even if the same tumor block is tested twice in two different laboratories even with the same method results for ER, PR, and HER2 expression show similarly high discordance rates. For instance, results from recent adjuvant trastuzumab trials indicate that reproducibility of HER2 staining when carried out in different laboratories is given in only ∼85% of cases. Importantly, concordance was higher for HER2–FISH testing compared with HER2–IHC (88.1% and 81.6%, respectively) [18]. A number of technical reasons may result in lack of reproducibility of IHC staining. Further variability may be introduced by the method of tissue sampling. For instance, fine-needle aspiration samples which are frequently being used to diagnose primary metastatic breast cancer may be les reliable with regard to immunostaining for ER determination than core biopsies [19].
Changes in receptor expression may either account for a true biological phenomenon or may result from inconsistent measurement. It has been argued that changes in receptor expression may occur at different time points of disease. For HER2, a number of possible reasons have been indicated, such as (i) change of HER2 status during tumor progression as a consequence of genetic drift or clonal selection [9], (ii) intratumoral heterogeneity [20–22], (iii) cellular exposure to systemic agents such as chemotherapy or hormonal therapy, or (iv) therapeutic selection by exposure to trastuzumab. In general, it is not inconceivable that (i) a tumor may be simultaneously composed of ER− and ER+ (or HER2− and HER2+) cancers with only one component giving rise to metastasis or (ii) some molecular evolution of cancer occurs. However, before such a mechanism is seriously entertained, i.e. a new scientific hypothesis is formulated, it is imperative to rule out receptor measurement errors as a potential source of receptor status discordance, i.e. that observations of discrepant receptor results may simply be due to less than perfect reproducibility of the receptor determination methods. With less than perfect methods, a complete concordance can hardly be expected. Furthermore, a large body of evidence derived from transcriptional profiling suggests that hard-wired characteristics of the individual tumor do not change by chance throughout the course of the disease. Weigelt et al. [23] showed that gene expression profiles of metastases clustered closely to their corresponding primaries indicating that metastatic capability in breast cancer may be an inherent feature of the primary tumor. Also, transcriptional profiling and other genomic data indicate that ER+ and ER− cancers exhibit fundamental differences with regard to molecular machinery and perhaps tissue origin [2]. Therefore, it seems to be as likely that a true ER+ breast cancer would turn into an ER− cancer as it may be for a small-cell lung cancer to recur as non-small-cell cancer. This is not impossible, but certainly infrequent.
Our discordance rates are exactly within the range that we would expect from the modest technical reproducibility of ER and HER testing methods; discordance rates for ER, PR, and HER2 were 18.4%, 40.3%, and 13.6, respectively. We further examined the association between discordance in receptor expression and (i) site of staining, (ii) year of staining, and (iii) concordance between (semi)quantitative staining scores. First, we demonstrated that there was no association between concordance in triple-receptor expression and concordance in staining location indicating that discordant results were seen with similar frequency within-institution and between-institution results. Secondly, based on our results, we assume that the effect between PRS and staining concordance may be due mainly to the modest between-laboratory reproducibility of the staining especially for ER and PR. We observed that there was large variation on semiquantitative receptor IHC results when the analysis was repeated on the recurrent tissue. Concordance coefficients for ER and PR were as low as 0.677 and 0.268 when staining was compared between primary tumor and disease recurrence. In contrast, for HER2–FISH testing, the correlation coefficient was as high as 0.895. This holds true for both within-institution and between-institution staining results. These results indicate that inconsistencies in ER, PR, and to a lesser extent HER2–FISH measurements may result in discordance of receptor expression between primary and recurrent tumor. However, a true change in tumor biology cannot be excluded at least in some instances.
Most importantly, discordance in receptor expression was associated with decreased PRS compared with triple-receptor concordance (median PRS 15.6 months, 95% CI 11.6–30.5 versus 43.0 months, 95% CI 31.2–52, respectively; P = 0.0002). Correlation analysis revealed that this difference was not biased by the number of patients with distant versus local recurrence as first site of disease recurrence. In fact, examination of the individual receptor combinations revealed that this effect was most likely due to the majority of concordant cases being concordant RPBC who had significantly better survival than concordant TNBC (n = 42; 45.1 months, 95% CI 37.1–53.9 versus 25.8 months, 95% CI 17.6–59.7, respectively) and discordant cases [n = 55, 15.6 (11.6–30.5)]. In other words, patients with discordant receptor status had similarly unfavorable outcome as patients with consistently TNBC compared with patients with consistently RPBC. We hypothesize that the decreased survival seen in cases with receptor discordant results may at least partly result from either administration of an ineffective treatment as a result of a false-positive receptor status or omission of an effective treatment as a result of a false-negative result.
conclusions
Discordance in receptor expression between primary tumor and recurrence occurred in 14%–40% of our cases. It is not inconceivable that a tumor is simultaneously composed of ER− and ER+ (or HER2− and HER2+) cancers and one component gives rise to metastasis while the other does not or that some molecular evolution of cancer occurs between the primary and recurrence. However, before such a mechanism is seriously entertained, it is imperative to rule out receptor measurement error as the source of receptor status discordance. Our results are consistent with the lack of reliability of staining methods as a major reason for discordant receptor expression results. Importantly, we show for the first time that patients with discordant receptor status have as unfavorable post-recurrence survival as patients with concordant TNBC. Both of these groups had significantly worse survival than patients with concordant RPBC. Given that the therapeutic effect of targeted therapies depends on correct determination of hormone and HER2 receptor status, mistakes in receptor determination can lead to suboptimal therapy. We hypothesize that the poor survival outcome of patients with discordant receptor results may be due to both false-negative results that could lead to withholding endocrine therapy (or trastuzumab) and false-positive receptor results may also contribute some by leading to an initial period of ineffective therapy with targeted agents in patients who do not benefit. Our results illustrate the need to increase standardization and implementation of guidelines for hormone and HER2 receptor determination.
funding
Deutsche Forschungsgemeinschaft to CL; NIH (K23 CA121994) to AMG.
Supplementary Material
Acknowledgments
This work was presented in part as an oral presentation at the 2008 American Society of Clinical Oncology Annual Meeting in Chicago, IL, 2 June 2008.
References
- 1.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 5.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 6.Li BD, Byskosh A, Molteni A, Duda RB. Estrogen and progesterone receptor concordance between primary and recurrent breast cancer. J Surg Oncol. 1994;57:71–77. doi: 10.1002/jso.2930570202. [DOI] [PubMed] [Google Scholar]
- 7.Vincent-Salomon A, Jouve M, Genin P, et al. HER2 status in patients with breast carcinoma is not modified selectively by preoperative chemotherapy and is stable during the metastatic process. Cancer. 2002;94:2169–2173. doi: 10.1002/cncr.10456. [DOI] [PubMed] [Google Scholar]
- 8.Simon R, Nocito A, Hübscher T, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst. 2001;93:1141–1146. doi: 10.1093/jnci/93.15.1141. [DOI] [PubMed] [Google Scholar]
- 9.Edgerton SM, Moore D, II, Merkel D, Thor AD. erbB-2 (HER-2) and breast cancer progression. Appl Immunohistochem Mol Morphol. 2003;11:214–221. doi: 10.1097/00129039-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gancberg D, Di Leo A, Cardoso F, et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol. 2002;13:1036–1043. doi: 10.1093/annonc/mdf252. [DOI] [PubMed] [Google Scholar]
- 11.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 12.Masood S, Bui MM. Assessment of Her-2/neu overexpression in primary breast cancers and their metastatic lesions: an immunohistochemical study. Ann Clin Lab Sci. 2000;30:259–265. [PubMed] [Google Scholar]
- 13.Niehans GA, Singleton TP, Dykoski D, Kiang DT. Stability of HER-2/neu expression over time and at multiple metastatic sites. J Natl Cancer Inst. 1993;85:1230–1235. doi: 10.1093/jnci/85.15.1230. [DOI] [PubMed] [Google Scholar]
- 14.Symmans WF, Liu J, Knowles DM, Inghirami G. Breast cancer heterogeneity: evaluation of clonality in primary and metastatic lesions. Hum Pathol. 1995;26:210–216. doi: 10.1016/0046-8177(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix H, Iglehart JD, Skinner MA, Kraus MH. Overexpression of erbB-2 or EGF receptor proteins present in early stage mammary carcinoma is detected simultaneously in matched primary tumors and regional metastases. Oncogene. 1989;4:145–151. [PubMed] [Google Scholar]
- 16.Shimizu C, Fukutomi T, Tsuda H, et al. c-erbB-2 protein overexpression and p53 immunoreaction in primary and recurrent breast cancer tissues. J Surg Oncol. 2000;73:17–20. doi: 10.1002/(sici)1096-9098(200001)73:1<17::aid-jso5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso F, Di Leo A, Larsimont D, et al. Evaluation of HER2, p53, bcl-2, topoisomerase II-alpha, heat shock proteins 27 and 70 in primary breast cancer and metastatic ipsilateral axillary lymph nodes. Ann Oncol. 2001;12:615–620. doi: 10.1023/a:1011182524684. [DOI] [PubMed] [Google Scholar]
- 18.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 19.Gong Y, Symmans WF, Krishnamurthy S, et al. Optimal fixation conditions for immunocytochemical analysis of estrogen receptor in cytologic specimens of breast carcinoma. Cancer. 2004;102:34–40. doi: 10.1002/cncr.11906. [DOI] [PubMed] [Google Scholar]
- 20.Thor AD, Berry DA, Budman DR, et al. erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer Inst. 1998;90:1346–1360. doi: 10.1093/jnci/90.18.1346. [DOI] [PubMed] [Google Scholar]
- 21.Pertschuk LP, Axiotis CA, Feldman JG, et al. Marked intratumoral heterogeneity of the proto-oncogene Her-2/neu determined by three different detection systems. Breast J. 1999;5:369–374. doi: 10.1046/j.1524-4741.1999.97088.x. [DOI] [PubMed] [Google Scholar]
- 22.Kerbel RS. Growth dominance of the metastatic cancer cell: cellular and molecular aspects. Adv Cancer Res. 1990;55:87–132. doi: 10.1016/s0065-230x(08)60469-8. [DOI] [PubMed] [Google Scholar]
- 23.Weigelt B, Glas AM, Wessels LF, et al. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci U S A. 2003;100:15901–15905. doi: 10.1073/pnas.2634067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.