Abstract
HIV infection is associated with left ventricular (LV) dysfunction and accelerated atherosclerosis. These conditions result in elevation of plasma natriuretic peptide (NP) levels. The present study compares N-terminal-pro-BNP (NT-pro-BNP) levels in HIV-infected and -uninfected women and identifies factors influencingNT-pro-BNP levels in HIV-infected women. A total of 454 HIV-infected and 200 HIV-uninfected participants from the Women's Interagency HIV Study (WIHS) had NT-pro-BNP determination. Elevated NT-pro-BNP level was defined using previously determined age stratified cut-off values of >164 ng/liter (age <60 years) and >225 (age ≥ 60 years). HIV-infected women were older (41.6 ± 8.9 vs. 38.9 ± 10.5 years, p < 0.01) and were more likely to have anemia, hepatitis C virus (HCV) antibodies, and kidney dysfunction than HIV-uninfected women. HIV-infected women had significantly higher NT-pro-BNP levels (142.4 ± 524.8 vs. 73.6 ± 115.1 ng/liter, p = 0.01) and a higher prevalence of elevated NT-pro-BNP (12.1% vs. 7.5%; p = 0.08). In univariate analyses, elevated NT-pro-BNP was significantly associated with age, systolic BP, hypertension, anemia, triglyceride levels, kidney disease, and HCV seropositivity, but not HIV infection. In multivariate analysis, elevated NT-pro-BNP levels were significantly associated with anemia and kidney function, and had a borderline association with the presence of HCV antibodies. Among HIV-infected women, NT-pro-BNP levels were not independently associated with measures of severity of infection or with HAART use. Although HIV-infected women have higher NT-pro-BNP levels than HIV-uninfected women, the differences are due to non-HIV factors such as anemia, kidney disease, and HCV coinfection. These findings suggest that natriuretic peptide levels are a global marker of comorbidity in the setting of HIV infection.
Introduction
HIV infection is associated with left ventricular (LV) dysfunction and congestive heart failure (CHF).1 These conditions result in elevated levels of plasma brain natriuretic peptide (BNP), which is released from the cardiac ventricles in response to increased wall stress.2–4 In recent years, BNP and its precursor, N-terminal pro-BNP (NT-pro-BNP), have been used to aid in the determination of LV dysfunction and the evaluation and management of CHF. BNP is synthesized as a high-molecular-weight precursor, pro-BNP, which is cleaved to release BNP and the amino terminal fragment NT-pro-BNP. Both peptides are released from the cardiac ventricles in response to increased wall stress. Natriuretic peptide levels are also increased in a variety of conditions including myocarditis, LV hypertrophy, and pulmonary hypertension, which are also manifestations of HIV infection.5,6 Although the mechanism is uncertain, BNP levels are similarly increased in the setting of atherosclerosis.7 Cardiovascular (CV) disease is an increasing concern among patients with HIV infection.8,9 Although natriuretic peptide levels have been proposed to be a neurohumoral marker of CV risk,10,11 few prior studies have evaluated such levels in HIV infection.12–15 Accordingly, the objectives of the present study were to identify clinical factors associated with elevated NT-pro-BNP in HIV-infected and -uninfected women and to further investigate the effect of these risk factors on NT-pro-BNP levels in HIV-infected women.
Materials and Methods
Study population
The study was conducted in a sample of participants from the Women's Interagency HIV Study (WIHS), an ongoing, multicenter study of natural history of HIV disease in women. WIHS enrolled women infected with HIV and at risk for HIV acquisition in 1994–1995 (n = 2623) and 2001–2002 (n = 1143) in five cities in the United States: Bronx/Manhattan, NY, San Francisco, CA, Los Angeles, CA, Washington DC, and Chicago, IL. Women eligible for enrollment into the WIHS were 13 years of age or older, gave informed consent, completed an interviewer-assisted questionnaire in English or Spanish, had a physical and gynecological examination, and blood collection. Women attended study visits every 6 months in which they completed an interview-assisted questionnaire, underwent a physical and gynecological examination, and provided biological specimens. The standardized interview-based questionnaire collected information regarding sociodemographics, access to care information, chronic illness, behaviors associated with HIV acquisition, medications, HIV treatment, and disease characteristics. The study's methodology, training, quality assurance activities, baseline characteristics, and updated characteristics of the cohort have been reported elsewhere.16,17 All women from the Brooklyn site attending study visits between October 2004 and September 2005 (n = 437) and 250 unselected women from the Bronx site attending study visits between April 2005 and October 2005 (n = 250) were asked to participate in this nested study of NT-pro-BNP. Cross-sectional data were collected from willing participants (n = 654), 379 of whom were originally enrolled in WIHS during 1993–1994 and 275 of whom were enrolled in 2001–2002. The study was approved by the Institutional Review Boards of both the Brooklyn and Bronx study sites and all subjects provided written informed consent.
Outcome classification
Laboratory values were generated for NT-pro-BNP levels using Roche's NT-pro-BNP Test (Roche Diagnostics Corp., Indianapolis, IN), a fluorescence immunoassay using whole blood and plasma with a lower level of detection of 5 ng/liter. An elevated NT-pro-BNP level was defined using previously determined age-stratified cut-off values of >164 ng/liter among subjects <60 years age and >225 ng/liter among subjects ≥60 years.18
Risk factor classification
The WIHS semiannual visits include medical history and health behavior questionnaires, assessments of medication use, standardized clinical and laboratory measurements, and phlebotomy. Race/ethnicity was self-reported at baseline. Information regarding cigarette smoking, the occurrence of an AIDS-defining illness, and highly active antiretroviral therapy (HAART) is collected routinely at each study visit via self-report. History of an AIDS-defining illness (ADI) was defined as a prior report of conditions consistent with 1993 Centers for Disease Control and Prevention surveillance definition, but excluding immunologic criterion of CD4 <200 cells/μl. These events were ascertained through self-report and some were confirmed through medical record abstraction and cancer and tuberculosis registries. This self-reported measure of clinical AIDS diagnosis has been shown to be associated with greater immunosuppression and higher HIV RNA viral loads in the WIHS.19 HAART was defined as self-reported therapy being taken at the time of data collection. It was defined using the standard WIHS definition, adapted from the Department of Health and Human Services & Kaiser Panel guidelines, as reported use of (1) two or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least one protease inhibitor (PI) or one nonnucleoside RTI (NNRTI), (2) one NRTI in combination with at least one PI and at least one NNRTI, (3) a regimen containing ritonavir and saquinavir in combination with one NRTI and no NNRTIs, or (4) an abacavir or tenofovir-containing regimen of three or more NRTIs in the absence of both PIs and NNRTIs, except for the three-NRTI regimens consisting of abacavir + tenofovir +lamivudine, or didanosine + tenofovir + lamivudine.20
Clinical examination generated data for body mass index (BMI) and blood pressure (BP). Anthropometric measurements of height and weight were performed according to the Third National Health and Examination Survey (NHANES III) procedures.21 BMI was calculated based on measured weight and height and was classified as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). BP was measured in the right arm with a DINAMAP 1846 SX automated oscillometric device (Critikon, Inc., Tampa, FL). Three or more readings were taken after the subject sat for at least 5 min and all readings per participant were averaged. Hypertension was defined as either measured systolic BP >140 mm Hg, or diastolic BP >90 mm Hg, or a self-reported diagnosis of hypertension with use of antihypertensive medications.
Laboratory tests conducted using standard WIHS protocols on blood collected at the study visit were used to generate values for HIV serostatus, fasting glucose, total cholesterol, HDL cholesterol, triglyceride levels, hemoglobin (Hb), creatinine, HCV antibodies, and CD4 count. HIV serostatus was determined using the Food and Drug Administration (FDA)-approved enzyme-linked immunosorbent assay testing and, if reactive, confirmed with the FDA approved Western blot HIV-1 confirmatory assay. Diabetes was defined as either a fasting blood glucose of >126 mg/dl or a self-reported diagnosis of diabetes or treatment of diabetes with medications.22 Serum total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglyceride levels were measured at a central laboratory. Low-density lipoprotein cholesterol (LDL-C) was estimated from the Friedewald equation.23 Hemoglobin was measured using the standard laboratory method and anemia was defined as hemoglobin <12 gm/dl.24 Kidney function was quantified by the estimated glomerular filtration rate (eGFR) calculated by the simplified Modification of Diet in Renal Disease (MDRD) equation, which takes into account a person's age, gender, race, and creatinine level.25 Kidney function was classified as normal function (eGFR ≥90 ml/min/1.73 m2), mild kidney disease (eGFR 60–89.9 ml/min/1.73 m2), and moderate to severe kidney disease (eGFR <60 ml/min/1.73 m2). Hepatitis C (HCV) serostatus was determined using second-generation enzyme immunoassay (HCV EIA 2.0; Abbott Laboratories, Abbott Park, IL). CD4 count was measured within 24 h of specimen collection by a standard flow cytometry technique at laboratories certified by the National Institutes of Health Division of AIDS flow cytometry testing program.
Statistical methods
Continuous variables were reported as mean ± standard deviation and Student's t tests were used to test statistically significant differences in means. The Pearson's χ2 test statistic was used to assess statistically significance differences in proportions for dichotomous and categorical variables; if the numbers within a cell were small (i.e., n <10) then Fischer's exact χ2 test statistic was used. Univariate and multivariate logistic regression models of elevated NT-pro-BNP levels were used to estimate odds ratios (OR) and 95% confidence intervals (95% CI) for clinical risk factors. In addition to HIV status, other variables that were investigated included age, race, BMI category, systolic BP, smoking, hypertension, diabetes, anemia, hepatitis C antibody status, kidney function, and lipid fractions. The prevalence of active or chronic hepatitis B infection among the subjects studied was very low (only eight cases), and therefore was not evaluated. To further investigate NT-pro-BNP levels specifically in HIV+ women, univariate and multivariate linear regression models of continuous NT-pro-BNP were used to measure the percent change in NT-pro-BNP. The distribution of NT-pro-BNP was positively skewed, so the natural log transformation of NT-pro-BNP was used as the dependent variable in the linear regression models. Natural log transformation was also used for normalizing distribution of lipid fractions. A quadratic term for systolic BP was used in the linear regression model to better model the observed J-shaped relationship of systolic BP and natural log transformed NT-pro-BNP. In addition to the clinical risk factors used in the logistic regression model, HIV-specific variables including current CD4 count, history of nadir CD4 <200 cells/μl, history of ADI, and current use of HAART were also investigated in the linear regression models. A p-value <0.05 was used to guide interpretation. All analyses were performed using STATA version 9.2 (College Station, TX).
Results
Of the 654 women enrolled in this study, 454 were HIV infected and 200 were HIV uninfected. Characteristics of the participants are shown in Table 1. Compared to HIV-uninfected women, HIV-infected women were older and were more likely to have anemia, HCV antibodies, and kidney dysfunction. The use of medications for treating hypertension and diabetes was similar between the HIV-infected and -uninfected groups (hypertension treatment 22.6 vs. 23.0%, p = 0.49; diabetes treatment 5.5 vs. 7.1%, p = 0.28).
Table 1.
Characteristics of HIV-Infected and -Uninfected Participants, the Women's Interagency HIV Study (WIHS)
| Characteristicsa | HIV-infected women (N = 454) | HIV-uninfected women (N = 200) | p-valueb |
|---|---|---|---|
| Mean age (years) | 41.6 ± 8.7 | 38.9 ± 10.5 | <0.01 |
| Race/ethnicity | |||
| Black, non-Hispanic | 293 (65%) | 134 (67%) | 0.22 |
| Hispanic | 121 (27%) | 55 (28%) | |
| Other | 32 (7%) | 7 (4%) | |
| BMIc | |||
| Underweight | 8 (2%) | 5 (3%) | 0.36 |
| Normal BMI | 137 (30%) | 49 (25%) | |
| Overweight | 143 (32%) | 58 (29%) | |
| Obese | 159 (35%) | 80 (40%) | |
| Current smoker | 217 (48%) | 103 (52%) | 0.38 |
| Mean systolic BPd (mm Hg) | 119 ± 18 | 121 ± 19 | 0.77 |
| Hypertension | 122 (27%) | 56 (28%) | 0.37 |
| Anemia | 160 (35%) | 20 (10%) | <0.01 |
| Kidney functione | |||
| Normal | 292 (64%) | 145 (73%) | 0.02 |
| Mild kidney disease | 128 (28%) | 49 (25%) | |
| Moderate to severe kidney disease | 30 (7%) | 4 (2%) | |
| Hepatitis C antibody | 118 (26%) | 34 (17%) | 0.01 |
| Diabetes | 26 (6%) | 16 (8%) | 0.27 |
| Total cholesterol (mg/dl) | 174 ± 39 | 177 ± 36 | 0.25 |
| HDL cholesterol (mg/dl) | 47 ± 17 | 53 ± 15 | <0.01 |
| Triglycerides (mg/dl) | 139 ± 93 | 110 ± 125 | <0.01 |
| LDL cholesterol (mg/dl) | 101 ± 32 | 103 ± 33 | 0.33 |
| CD4 count | |||
| ≥500 cells/μl | 193 (43%) | — | |
| 200–499 cells/μl | 200 (44%) | — | — |
| <200 cells/μl | 58 (13%) | — | |
| History of ADIf | 162 (36%) | — | — |
| History of nadir CD4 <200/μl | 177 (40%) | — | — |
| Current HAART | 273 (60%) | — | — |
| Mean plasma NT-pro-BNP (ng/liter) | 142.44 ± 524.80 | 73.60 ± 115.09 | 0.01 |
| Elevated NT-pro-BNPg | 55 (12%) | 15 (8%) | 0.08 |
Means are presented ± standard deviation; percentages may not add up to 100% due to missing data.
p-values for differences in means were calculated using t test statistics. p-values for differences in proportions were calculated using Pearson's χ2 test statistic unless the cells have n <10; then Fisher's exact χ2 test was used.
BMI, body mass index; underweight is <18.5, normal is 18.5–24.9, overweight is 25.0–29.9, and obese is ≥30.0.
BP, blood pressure.
Kidney function defined as normal is EGFR ≥ 90 mL/min/1.73 m2, mild kidney disease is EGFR = 60–89.9 mL/min/1.73 m2; moderate to severe kidney disease is EGFR < 60 mL/min/1.73 m2.
AIDS defining illness is a prior report of conditions consistent with the 1993 CDC surveillance definition, excluding the criterion of CD4 < 200 cells/mL.
Elevated NT-pro-BNP level is defined as values >164 ng/liter among subjects <60 years age and >225 ng/liter among subjects ≥60 years.
HIV-infected individuals had a significantly higher mean NT-pro-BNP level compared to HIV-uninfected individuals (142.4 ± 524.8 vs. 73.6 ± 115.1 ng/liter, p-value = 0.01) (Table 1). When NT-pro-BNP was defined as a binary variable, HIV-infected women had a higher prevalence of elevated NT-pro-BNP compared to HIV-uninfected women; however, the difference was not significant at traditional p-value criteria (12% vs. 8%; p = 0.08, univariate OR = 1.70, 95% CI 0.94, 3.09). In the univariate logistic models, elevated NT-pro-BNP was significantly associated with age, hypertension, anemia, kidney dysfunction, serum triglyceride levels, and the presence of HCV antibodies (Table 2). After adjusting for relevant covariates in multivariate analyses, elevated NT-pro-BNP was associated with anemia and kidney dysfunction but not with HIV status (Table 2). Mild, moderate, or severe kidney disease was prevalent in 60% (9/15) of HIV-uninfected women and 55% (30/55) of HIV-infected women with elevated NT-pro-BNP. Multivariate analyses also suggested an association of HCV with higher NT-pro-BNP and obesity with lower NT-pro-BNP, although these findings were of borderline significance (Table 2).
Table 2.
Correlates of Elevated NT-Pro-BNP in HIV-Infected and -Uninfected Women in the Women's Interagency HIV Study (WIHS)
| Risk factor | OR (95% CI) | AdjustedaOR (95% CI) |
|---|---|---|
| HIV infection | 1.70 (0.94–3.09) | 0.79 (0.34–1.85) |
| Age, per year | 1.07 (1.04–1.10) | 1.01 (0.97–1.06) |
| BMIb | ||
| Normal | 1.00 | 1.00 |
| Underweight | 1.06 (0.22–5.03) | 0.84 (0.09–8.00) |
| Overweight | 0.61 (0.33–1.15) | 0.60 (0.28–1.30) |
| Obese | 0.54 (0.29–0.99) | 0.37 (0.16–0.83) |
| Current smoker | 1.67 (1.00–2.81) | 1.56 (0.79–3.08) |
| Hypertension | 3.40 (2.05–5.64) | 1.69 (0.73–3.91) |
| Anemia | 3.11 (1.83–5.27) | 3.75 (1.88–7.47) |
| Kidney functionc | ||
| Normal | 1.00 | 1.00 |
| Mild kidney disease | 2.23 (1.32–4.09) | 1.64 (0.61–4.43) |
| Moderate to severe kidney disease | 13.11 (6.01–28.60) | 4.19 (1.56–11.27) |
| Hepatitis C antibodies | 3.80 (2.28–6.34) | 2.01 (0.99–4.10) |
| Diabetes | 0.64 (0.19–2.13) | 0.31 (0.07–1.37) |
| Total cholesterold | 0.29 (0.02–3.57) | 2.15 (0.05–89.54) |
| HDL cholesterold | 0.39 (0.10–1.47) | 0.86 (0.12–6.21) |
| Triglyceridesd | 2.93 (1.11–7.69) | 1.36 (0.27–6.77) |
Adjusted for race and systolic blood pressure.
BMI, body mass index; underweight is <18.5, normal is 18.5–24.9, overweight is 25.0–29.9, and obese is ≥30.0.
Kidney function defined as normal is eGFR ≥ 90 ml/min/1.73 m2, mild kidney disease is eGFR 60–89.9 ml/min/1.73 m2, and moderate to severe kidney disease is eGFR <60 ml/min/1.73 m2.
Lipid subfractions entered after log10 transformation to correct for skewed distribution.
Of the 654 women, 23 (5%) HIV-infected and 12 (6%) -uninfected women were on beta blockers (p = 0.63), 52 (12%) HIV-infected and 27 (14%) -uninfected women were on ACE inhibitor or ARB therapy (p = 0.46), 17 (4%) HIV-infected and 10 (5%) -uninfected women were on calcium channel blockers (0.61), and 28 (6%) HIV-infected and 13 (7%) -uninfected women were on diuretic therapy (p = 0.87). There were no statistically significant class-specific antihypertensive relationships with plasma NT-pro-BNP.
In univariate analyses limited to HIV-infected women, age, smoking, hypertension, anemia, kidney disease, HCV antibodies, low CD4 count (both current and history of a nadir <200 cells/μl), and history of a prior ADI were associated with significantly higher NT-pro-BNP levels, while being overweight or obese was associated with lower levels (Table 3). In the adjusted model, anemia, kidney disease, and HCV seropositivity were significantly associated with higher NT-pro-BNP levels, the highest levels seen with moderate to severe kidney disease (112.5%, p-value ≤0.01). Obesity remained associated with lower NT-pro-BNP levels in the adjusted model (– 34.5%, p-value = 0.01). There was an observed J-shaped relationship between systolic BP and natural log-transformed NT-pro-BNP levels. This relationship was modeled using a quadratic term and was statistically significant in both the univariate and multivariate linear models. Low current and nadir CD4+ count and history of ADI were not significantly associated with higher NT-pro-BNP after adjustment for comorbidities and other variables (Table 3).
Table 3.
Correlates of NT-Pro-BNP among HIV-Infected Women in the Women's Interagency HIV Study (WIHS)
| Unadjusted expected % change in plasma NT-pro-BNP, ng/liter (p-value) | Adjusteda expected % change in plasma NT-pro-BNP, ng/liter (p-value) | |
|---|---|---|
| Age, per year | 3.5% (<0.01) | 0.7% (0.33) |
| BMIb (vs. normal) | ||
| Underweight | −6.5% (0.87) | −34.9% (0.40) |
| Overweight | −30.0% (0.01) | −20.0% (0.12) |
| Obese | −35.8% (<0.01) | −34.4% (0.01) |
| Current smoker | 24.7% (0.04) | 19.6% (0.07) |
| Hypertension | 71.8% (<0.01) | 21.9% (0.14) |
| Anemia | 37.3% (<0.01) | 23.4% (0.04) |
| Kidney functionc (vs. normal) | ||
| Mild kidney disease | 25.5% (0.04) | −2.1% (0.91) |
| Moderate to severe kidney disease | 412.6% (<0.01) | 112.5% (<0.01) |
| Hepatitis C antibodies | 95.6% (<0.01) | 27.1% (0.04) |
| Diabetes | −15.2% (0.47) | −36.2% (0.10) |
| Total cholesterold | −52.1% (0.33) | −29.9% (0.63) |
| HDL cholesterold | −26.8% (0.37) | −4.2% (0.91) |
| Triglyceridesd | 28.3% (0.21) | −17.2% (0.51) |
| Current CD4 count (vs. >500 cells/μl) | ||
| 200–500 cells/μl | 15.1% (0.22) | −1.2% (0.92) |
| <200 cells/μl | 88.6% (<0.01) | 19.9% (0.29) |
| History of nadir CD4 <200 | 34.2% (0.01) | 15.7% (0.20) |
| History of AIDS-defining illnesse | 70.4% (<0.01) | 18.9% (0.11) |
| Current use of HAART | 5.9% (0.61) | −0.5% (0.97) |
Adjusted for race and systolic blood pressure.
BMI, body mass index; underweight is <18.5, normal is 18.5–24.9, overweight is 25.0–29.9, and obese is ≥30.0.
Kidney function defined as normal is eGFR ≥90 ml/min/1.73 m2, mild kidney disease is eGFR 60–89.9 ml/min/1.73 m2, and moderate to severe kidney disease is eGFR <60 ml/min/1.73 m2.
Lipid subfractions entered after log10 transformation to correct for skewed distribution.
AIDS-defining illness defined a prior report of conditions consistent with 1993 Centers for Disease Control and Prevention surveillance definition, but excluding immunologic criterion of CD4+ <200 cells/μl.
Among the HIV-infected women, 174 were on PI drugs, 288 were on ≥1 NRTI drugs (maximum 4), and 95 were on NNRTI drugs (maximum 1). On univariate analyses, there was a trend toward higher plasma NT-pro-BNP levels among subjects who were on PIs (n = 174) compared to those who were not on PIs (n = 273) (log transformed NT-pro-BNP 4.1 ± 1.2 vs. 3.9 ± 1.1, p = 0.07). There was no statistically significant relationship between the use of NRTIs or NNRTIs and plasma NT-pro-BNP levels. On multivariate analysis, there was no independent relationship between plasma NT-pro-BNP levels and any specific class of antiretroviral drugs.
Discussion
We found that HIV-infected women have higher mean NT-pro-BNP than HIV-uninfected women. Similarly, markers of advanced HIV disease including CD4+ count below 200 cells/μl and ADI were associated with higher NT-pro-BNP, although multivariate analyses suggested that these associations were explained by comorbidities including anemia, HCV, and impaired kidney function, which were more prevalent in the HIV-infected group. Our findings suggest that NT-pro-BNP levels among HIV-infected patients are a global marker of non-HIV-related comorbidities known to be associated with higher natriuretic peptide levels.
Concern regarding CV disease in HIV-infected individuals has led to multiple studies examining the associations of HIV infection with atherosclerosis, left ventricular dysfunction, and congestive heart failure.26 Natriuretic peptides including NT-pro-BNP are markers of hemodynamic stress, and are used clinically to aid in the detection of LV dysfunction and the evaluation and management of congestive heart failure.27 Recently, however, the measurement of natriuretic peptides has been shown to provide prognostic information in patients with acute and chronic coronary artery disease.7,28 Accordingly, measurement of plasma BNP level has been suggested to be a cost-effective means of screening for CV disease.29,30
Few prior studies have assessed natriuretic peptide levels and cardiac function in HIV-infected populations12–15 and none hasspecifically examined NT-Pro-BNP levels. Carillo-Jimenez found serum levels inversely related to LV function in 10 HIV-infected patients assessed by echocardiography.12 In contrast, a Danish study of 95 HIV-infected patients found no correlation between NT-pro-BNP levels and LV or right ventricular ejection fraction measured by radionuclide ventriculography.13 That finding could be explained by unmeasured factors other than cardiac function contributing to elevated NT-pro-BNP levels. A prior German study performed cardiac magnetic resonance imaging on 12 HIV-infected subjects with elevated BNP levels and found multiple abnormalities including left ventricular hypertrophy, myocarditis, dilated cardiomyopathy, and right ventricular failure.15 These findings support the interpretation that natriuretic peptide levels are a global measure of CV disease.30 Our findings indicate that the prevalence of elevated NT-pro-BNP in HIV-infected individuals is almost twice that of HIV-uninfected individuals (12.1% vs. 7.5%, respectively, p = 0.08).
In our study, elevated NT-pro-BNP levels were significantly associated with HCV infection, anemia, and kidney dysfunction. These findings are consistent with prior studies showing higher natriuretic peptide levels associated with the same factors in HIV-uninfected populations.31 HCV has been previously reported to be associated with myocarditis, cardiomyopathy, and elevated NT-pro-BNP in HIV-uninfected populations.32,33 Among 1355 patients with heart failure, elevated NT-pro-BNP levels were found in 42 of 42 patients (100%) who had HCV antibodies.31 Though the exact mechanism of cardiac involvement in HCV infection is unclear, it is believed that HCV may promote the development of cardiomyopathy by inducing myocarditis, wherein an inflammatory response induces growth and cell death in cardiac cells.34 The strong relationship between NT-pro-BNP and advanced renal disease has also been reported in prior studies of HIV-uninfected individuals.35–37 Elevated NT-pro-BNP levels among patients with renal disease may reflect a chronic fluid overload state or may be related to other comorbidities that may increase LV wall tension.
We found that HIV infection was not associated with NT-pro-BNP after adjustment for comorbidities and other variables in the linear regression models. However, HIV is associated with an increased occurrence of myocarditis, cardiomyopathy, as well as atherosclerosis.1,7,38,39 It is also associated with comorbid conditions including anemia, renal insufficiency, and hepatitis, which were independently associated with elevated NT-pro-BNP. These findings suggest natriuretic peptide levels are nonspecific markers of general medical (non-HIV-specific) comorbidities in patients with HIV infection.
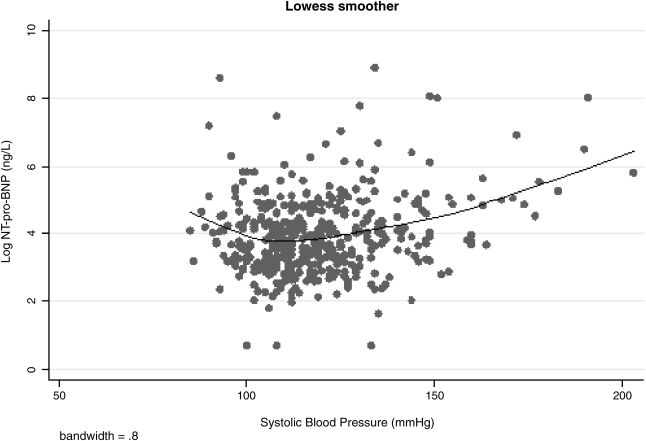
In the present study, there was a strong inverse relationship between obesity and plasma NT-pro-BNP levels. This finding is consistent with prior studies of natriuretic peptides in non-HIV-infected populations. Although the explanation for this finding is currently debated, higher testosterone levels and lower adiponectin levels seen in overweight and obese subjects have been implicated.40,41 Another interesting finding in this study was the J-shaped relationship between systolic BP and natural log-transformed NT-pro-BNP levels (Fig. 1). Although a J-shaped curve has been previously reported for BP and CV outcomes,42 natriuretic peptide levels are linearly related to CV outcomes.43 High systolic BP may directly increase LV workload and tension. Although the exact mechanism for the association between low systolic BP and higher NT-pro-BNP level is unclear, clinically stable hypotension is a frequent finding in advanced congestive cardiomyopathy.44 High LV wall stress or expanded plasma volume may contribute to higher NT-pro-BNP in such patients.
FIG. 1.
The J-shaped relationship between systolic blood pressure and naturally log-transformed NT-pro-BNP (log-NT-pro-BNP) among HIV-infected women, the Women's Interagency HIV Study (WIHS).
This is the largest study of NT-pro-BNP in HIV-infected individuals in the United States. However, this study has limitations, including a cross-sectional design that makes it difficult to attribute causality between the investigated factors and NT-pro-BNP levels. LV function and atherosclerosis are known to be associated with NT-pro-BNP; however, they were not investigated in this study. Finally, although the women in the WIHS reflect the demographics of the HIV epidemic among women in the United States, generalizability of our findings to HIV-infected men is limited as women have been found to have higher levels of natriuretic peptide. Moreover, women in this study had relatively well-controlled HIV infection, and had been enrolled in a prospective study for many years at the time of NT-pro-BNP assessment.
In summary, we found HIV-infected women had higher mean NT-pro-BNP levels compared to HIV-uninfected women. However, HIV infection was not associated with higher levels of NT-pro-BNP after controlling for relevant covariates. Among HIV-infected women, higher NT-pro-BNP levels were related to non-HIV-specific comorbidities including anemia, hepatitis C, and kidney disease. Increased life expectancy of HIV-infected individuals has led to the need for new biomarkers of comorbidities in this population. Longitudinal research is needed to determine the prognostic value of high natriuretic peptide levels in HIV-infected individuals for cardiac and noncardiac clinical endpoints.
Acknowledgments
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff ); Washington, DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Barbaro G. Di Lorenzo G. Grisorio B. Barbarini G. Cardiac involvement in the acquired immunodeficiency syndrome: A multicenter clinical-pathological study. Gruppo Italiano per lo Studio Cardiologico dei pazienti affetti da AIDS Investigators. AIDS Res Hum Retroviruses. 1998;14:1071–1077. doi: 10.1089/aid.1998.14.1071. [DOI] [PubMed] [Google Scholar]
- 2.Iwanaga Y. Nishi I. Furuichi S. Noguchi T. Sase K. Kihara Y, et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: Comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–748. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Bettencourt P. Ferreira A. Dias P. Castro A. Martins L. Cerqueira-Gomes M. Evaluation of brain natriuretic peptide in the diagnosis of heart failure. Cardiology. 2000;93:19–25. doi: 10.1159/000006997. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Rumayor A. Richards AM. Burnett JC. Januzzi JL., Jr Biology of natriuretic peptides. Am J Cardiol. 2008;101:3–8. doi: 10.1016/j.amjcard.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Silver MA. Maisel A. Yancy CW, et al. BNP Consensus Panel 2004: A clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest Heart Fail. 2004;10:1–30. doi: 10.1111/j.1527-5299.2004.03271.x. [DOI] [PubMed] [Google Scholar]
- 6.Fijalkowska A. Kurzyna M. Torbicki A. Szewczyk G. Florczyk M. PruZsczyk M, et al. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129:1313–1321. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 7.Ashley KE. Galla JM. Nicholls SJ. Brain natriuretic peptides as biomarkers for atherosclerosis. Prev Cardiol. 2008;11:172–176. doi: 10.1111/j.1751-7141.2008.08578.x. [DOI] [PubMed] [Google Scholar]
- 8.Levy WS. Simon GL. Rios JC. Ross AM. Prevalence of cardiac abnormalities in human immunodeficiency virus infection. Am J Cardiol. 1989;63:86–89. doi: 10.1016/0002-9149(89)91081-3. [DOI] [PubMed] [Google Scholar]
- 9.Friis-Møller N. Weber R. Reiss P. Thiébaut R. Kirk O. d'Arminio Monforte A, et al. Cardiovascular risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD Study. AIDS. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 10.Uusimaa P. Tokola H. Ylitalo A. Vuolteenaho O. Ruskoaho H. Risteli J, et al. Plasma B-type natriuretic peptide reflects left ventricular hypertrophy and diastolic function in hypertension. Int J Cardiol. 2004;97:251–256. doi: 10.1016/j.ijcard.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ. Larson MG. Levy D. Benjamin EJ. Leip EP. Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 12.Carrillo-Jimenez R. Treadwell TL. Goldfine H. Buenano A. Lamas GA. Hennekens CH. Brain natriuretic peptide and HIV-related cardiomyopathy. AIDS Reader. 2002;12:501–503. , 508. [PubMed] [Google Scholar]
- 13.Lebech AM. Gerstoft J. Hesse B. Petersen CL. Kjaer A. Right and left ventricular cardiac function in a developed world population with human immunodeficiency virus studied with radionuclide ventriculography. Am Heart J. 2004;147:482–488. doi: 10.1016/j.ahj.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Breuckmann F. Nassenstein K. Kondratieva J. Esser S. Erbel R. Potthoff A, et al. German Heart Failure Network; Competence Network HIV/AIDS: MR characterization of cardiac abnormalities in HIV+ individuals with increased BNP levels. Eur J Med Res. 2007;29:185–190. [PubMed] [Google Scholar]
- 15.Kristoffersen US. Lebech AM. Gerstoft J. Hesse B. Petersen CL. Gutte H, et al. Right and left cardiac function in HIV-infected patients investigated using radionuclide ventriculography and brain natriuretic peptide: A 5-year follow-up study. HIV Med. 2008;9:180–186. doi: 10.1111/j.1468-1293.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 16.Barkan SE. Melnick SL. Preston-Martin S. Weber K. Kalish LA. Miotti P, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 17.Bacon MC. von Wyl V. Alden C. Sharp G. Robison E. Hessol N, et al. The Women's Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Lemos JA. Hildebrandt P. Amino-terminal pro-B-type natriuretic peptides: Testing in general populations. Am J Cardiol. 2008;101:16–20. doi: 10.1016/j.amjcard.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Hessol NA. Anastos K. Levine AM. Ameli N. Cohen M. Young M, et al. Factors associated with incident self-reported AIDS among women enrolled in the women's interagency HIV study (WIHS). WIHS Collaboratorive Study Group. AIDS Res Hum Retroviruses. 2000;16:1105–1111. doi: 10.1089/088922200414947. [DOI] [PubMed] [Google Scholar]
- 20.Panel on Clinical Practices for Treatment of HIV Infection. Department of Health and Human Services (DHHS); Washington, DC: 2006. Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents. October 10. [Google Scholar]
- 21.National Center for Health Statistics. The Third National Health and Examination Survey Reference Manuals and Reports. National Center for Health Statistics; Hyattsville, MD: 1996. CD-ROM, 2002. [Google Scholar]
- 22.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997:20–1183. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT. Levy RI. Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Petersdorf RG. Adams RD. Braunwald E, et al. Principles of Internal Medicine. McGraw-Hill; New York: 1983. Harrison's laboratory values of clinical importance. [Google Scholar]
- 25.Levey AS. Greene T. Kusek JW. Beck GL. A simplified equation to predict glomerular filtration rate from serum creatinine (abstract) J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 26.Jacob AJ. Sutherland GR. Bird AG. Brettle RP. Ludlam CA. McMillan A, et al. Myocardial dysfunction in patients infected with HIV: Prevalence and risk factors. Br Heart J. 1992;68:549–553. doi: 10.1136/hrt.68.12.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costello-Boerrigter LC. Boerrigter G. Redfield MM. Rodeheffer RJ. Urban LH. Mahoney DW. Jacobsen SJ. Heublein DM. Burnett JC., Jr Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: Determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bibbins-Domingo K. Gupta R. Na B. Wu AH. Schiller NB. Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–176. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Struthers A. Lang C. The potential to improve primary prevention in the future by using BNP/N-BNP as an indicator of silent 'pancardiac' target organ damage: BNP/N-BNP could become for the heart what microalbuminuria is for the kidney. Eur Heart J. 2007;28:1678–1682. doi: 10.1093/eurheartj/ehm234. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa K. Oida A. Sugimura H, et al. Clinical significance of blood brain natriuretic peptide level measurement in the detection of heart disease in untreated outpatients: Comparison of electrocardiography, chest radiography and echocardiography. Circ J. 2002;66:122–126. doi: 10.1253/circj.66.122. [DOI] [PubMed] [Google Scholar]
- 31.März W. Tiran B. Seelhorst U. Wellnitz B. Bauersachs J. Winkelmann BR, et al. LURIC Study Team: N-terminal pro-B-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: The Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem. 2007;53:1075–1083. doi: 10.1373/clinchem.2006.075929. [DOI] [PubMed] [Google Scholar]
- 32.Matsumori A. Shimada T. Chapman NM. Tracy SM. Mason JW. Myocarditis and heart failure associated with hepatitis C infection. J Card Fail. 2006;12:293–298. doi: 10.1016/j.cardfail.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Okabe M. Fukuda K. Arakawa K, et al. Chronic variant of myocarditis associated with hepatitis C virus infection. Circulation. 1997;96:22–24. doi: 10.1161/01.cir.96.1.22. [DOI] [PubMed] [Google Scholar]
- 34.Matsumori A. Matoba Y. Nishio R. Shioi T. Ono K. Sasayama S. Detection of hepatitis C virus RNA from the heart of patients with hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 1996;222:678–682. doi: 10.1006/bbrc.1996.0803. [DOI] [PubMed] [Google Scholar]
- 35.Buckley MG. Sethi D. Markandu ND. Sagnella GA. Singer DRJ. MacGregor GA. Plasma concentrations and comparisons of brain natriuretic peptide and atrial natriuretic peptide in normal subjects, cardiac transplant recipients and patients with dialysis-independent or dialysis-dependent chronic renal failure. Clin Sci. 1992;83:437–444. doi: 10.1042/cs0830437. [DOI] [PubMed] [Google Scholar]
- 36.Khan IA. Fink J. Nass C. Chen H. Christenson R. deFilippi CR. N-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide for identifying coronary artery disease and left ventricular hypertrophy in ambulatory chronic kidney disease patients. Am J Cardiol. 2006;97:1530–1534. doi: 10.1016/j.amjcard.2005.11.090. [DOI] [PubMed] [Google Scholar]
- 37.Vickery S. Price CP. John RI. Abbas NA. Webb MC. Kempson ME, et al. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: Relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. 2005;46:610–620. doi: 10.1053/j.ajkd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 38.De Castro S. Migliau G. Silvestri A. D'Amati G. Giannantoni P. Cartoni D, et al. Heart involvement in AIDS: A prospective study during various stages of the disease. Eur Heart J. 1992;13:1452–1459. doi: 10.1093/oxfordjournals.eurheartj.a060085. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan RC. Kingsley LA. Gange SJ. Benning L. Jacobson LP. Lazar J, et al. Low CD4+T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang AY. Abdullah SM. Jain T. Stanek HG. Das SR. McGuire DK. Auchus RJ. Lemos JA. Associations among androgens, estrogens, and natriuretic peptides in young women: Observations from the Dallas Heart Study. J Am Coll Cardiol. 2007;49:109–116. doi: 10.1016/j.jacc.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 41.Dieplinger B. Poelz W. Haltmayer M. Mueller T. Association of adiponectin and amino terminal proBNP in peripheral arterial disease. Clin Chim Acta. 2007;377:192–197. doi: 10.1016/j.cca.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Boutitie F. Gueyffier F. Pocock S. Fagard R. Boissel JP. INDANA Project Steering Committee: Individual data analysis of antihypertensive intervention. J-shaped relationship between blood pressure and mortality in hypertensive patients: New insights from a meta-analysis of individual-patient data. Ann Intern Med. 2002;136:438–448. doi: 10.7326/0003-4819-136-6-200203190-00007. [DOI] [PubMed] [Google Scholar]
- 43.Grewal J. McKelvie RS. Persson H. Tait P. Carlsson J. Swedberg K, et al. Usefulness of N-terminal pro-brain natriuretic peptide and brain natriuretic peptide to predict cardiovascular outcomes in patients with heart failure and preserved left ventricular ejection fraction. Am J Cardiol. 2008;102:733–737. doi: 10.1016/j.amjcard.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 44.Sugrue DD. Rodeheffer RJ. Codd MB. Ballard DJ. Fuster V. Gersh BJ. The clinical course of idiopathic dilated cardiomyopathy: A population-based study. Ann Intern Med. 1992;117:117–123. doi: 10.7326/0003-4819-117-2-117. [DOI] [PubMed] [Google Scholar]