Abstract
Background
α-Hydroxy acids (αHA) are reported to reduce signs of aging in the skin and are widely used cosmetic ingredients. Several studies suggest that αHA can increase the sensitivity of skin to ultraviolet radiation. More recently, β-hydroxy acids (βHA), or combinations of αHA and βHA have also been incorporated into antiaging skin care products. Concerns have also arisen about increased sensitivity to ultraviolet radiation following use of skin care products containing β-HA.
Objective
To determine whether topical treatment with glycolic acid, a representative αHA, or with salicylic acid, a βHA, modifies the short-term effects of solar simulated radiation (SSR) in human skin.
Methods
Fourteen subjects participated in this study. Three of the four test sites on the mid-back of each subject were treated daily Monday - Friday, for a total of 3.5 weeks, with glycolic acid (10%), salicylic acid (2%), or vehicle (control). The fourth site received no treatment. After the last treatment, each site was exposed to SSR, and shave biopsies from all 4 sites were obtained. The endpoints evaluated in this study were erythema (assessed visually and instrumentally), DNA damage and sunburn cell formation.
Results
Treatment with glycolic acid resulted in increased sensitivity of human skin to SSR, measured as an increase in erythema, DNA damage and sunburn cell formation. Salicylic acid did not produce significant changes in any of these biomarkers.
Conclusions
Short-term topical application of glycolic acid in a cosmetic formulation increased the sensitivity of human skin to SSR, while a comparable treatment with salicylic acid did not.
Keywords: Hydroxyacids, Glycolic acid, Salicylic acid, UV-Damage, Erythema, DNA Damage, Sunburn Cells, Cosmetics
Introduction
Formulations containing hydroxyacids (HAs) have been used in clinical practice for decades to treat a variety of skin conditions. The most prominent representatives in this class of compounds are glycolic, lactic and salicylic acids. They have been used, typically in concentrations ranging from 2–70%, to treat acne, ichthyosis, keratosis, warts, psoriasis, photoaged skin and other disorders. In the last two decades, αHAs have been widely incorporated into a variety of cosmetic products for daily use over long periods of time [1]. Currently, glycolic acid, lactic acid and salicylic acid (the latter is frequently called a βHA) are commonly used in cosmetics. One of the most cited beneficial effects of HAs is the reported improvement of photoaged skin. These improvements have been measured as decreases in roughness, discoloration, solar keratoses, overall pigmentation, and also as increased density of collagen and improved quality of elastic fibers [2]. The antiaging effects of HAs have become a prominent factor in cosmetic dermatology, leading to proliferation of HA-containing cosmetic products and skin care systems [3].
Questions have been raised about the safety of prolonged use of HA-containing products on sun-exposed skin. A number of clinical studies have reported that topical application of glycolic acid can increase the skin’s sensitivity to solar simulated radiation (SSR) [4,5], however most of these studies used vehicles which differ significantly from those used in cosmetic products. In addition, a single cutaneous marker for UV-induced damage was usually measured. In the few cases in which multiple endpoints were used, they were not evaluated in the same subjects [4]. In 1998, the Cosmetic Ingredient Review Expert Panel evaluated the available studies [6] and concluded that αHA ingredients are not mutagenic or carcinogenic, are not reproductive or developmental toxins, and are not skin sensitizers. To reduce the risk of skin irritation, the Panel recommended limitations on the concentration of αHA (less than 10%) and the pH (at or above 3.5) of cosmetic products containing αHA [7]. In addition, the Panel recommended that αHA-containing products should be formulated to avoid enhancing sun sensitivity and that consumers should be advised to use daily sun protection [6]. Salicylic acid is added to cosmetic products at concentrations usually less than 3% [7]. The Cosmetic Ingredient Review Expert Panel has similarly recommended that effects on the skin’s sensitivity to sunlight be considered in the formulation and use of products containing salicylic acid and salicylates [7]. The doses of glycolic acid and salicylic acid used in this study reflect the doses actually used in cosmetic products.
The present study was devised to address still unanswered questions about the effects of HAs on the SSR-induced sensitivity of human skin. In particular, this study is the first to evaluate a number of prominent biomarkers for SSR-induced damage in the same subject, with each subject serving as his/her own control. This approach makes it possible to examine quantitative correlations among a number of independent biomarkers. In addition, the study was designed to determine the effects of both glycolic acid and salicylic acid on each subject’s sensitivity to UV radiation.
Materials & Methods
Test Products
The test products investigated in this study resembled standard cosmetic formulations containing nonionic emulsifiers (Steareth-21, ceteareth-20 and PEG-100 stearate), glycerin, surfactants, thickeners (xanthan gum), and preservatives (parabens and diazolydinyl urea). These products had minimal absorption of UV and were formulated by and obtained from Cosmetech Laboratories, Inc. (Fairfield, NJ) [8]. One test product contained 10% glycolic acid (pH 3.5). A second test product contained 2% salicylic acid (pH 3.5). The third test product was a vehicle control and was the same as the other test products but lacked glycolic acid or salicylic acid. Prior to use, we confirmed the concentrations of the glycolic acid and salicylic acid and the pHs using a previously published analytical method [9]. All test products were supplied in identical containers labeled A, B, or C for double-blinded application.
Subject Selection Criteria
This study involved 14 healthy Caucasian volunteers of both sexes, ages 24–59 years. All were in good health and without any internal or dermatological diseases. Volunteers were excluded from the study if they had any skin conditions or allergies that might interfere with the study (e.g., atopic eczema or psoriasis). In addition, they were also excluded if they had used sunscreens or any α- or β-hydroxy acid containing preparations in the past 4 weeks that might alter their skin condition. All qualified candidates were of skin types II to III, as determined by the Fitzpatrick classification [10]. All subjects submitted the required questionnaires and signed the Clinical Investigation Consent Document. Prior to the beginning of the study, the attending dermatologist interviewed each subject about their health history and examined their back. The study protocol was approved by the FDA Research Involving Human Subjects Committee.
Product Application
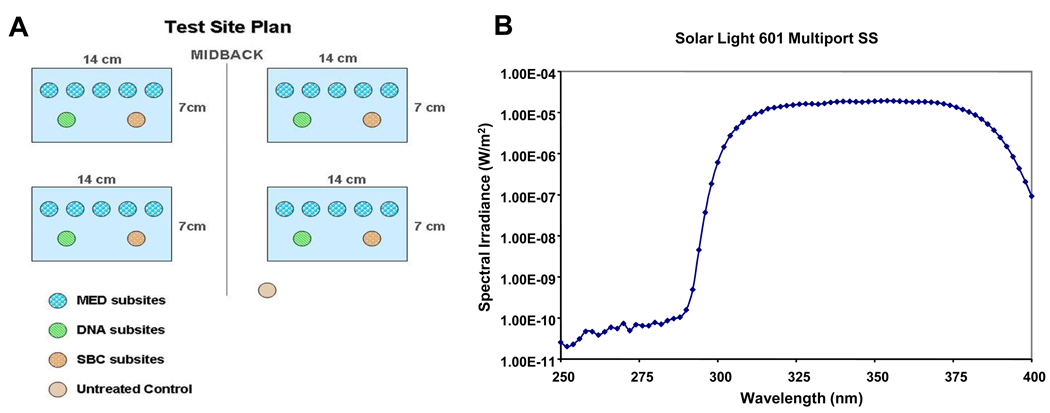
The test sites were located in the mid-back region of each subject. The designated test sites each consisted of a 7×14 cm rectangular area that was marked off by gentian violet pens and ink markers. There were four test sites on each subject, located on two opposite symmetrical areas of the mid-back (Fig. 1A). Three test sites served for test product application, while the fourth site served as a control without topical treatment. Within each test site, five subsites were designated for determination of the minimal erythema dose (MED) and two for obtaining biopsies. One additional subsite was assigned as an untreated, dark (unirradiated) control (Fig. 1A). To minimize potential effects of the site location on the outcome, the topical treatment applied at each test site was established using a randomization procedure for each subject.
Figure 1.
A. The test site plan for the four treatment areas with the respective subsite locations used in this study. B. Emission spectrum of the SSR source. The signal below 300 nm is slightly above the noise level of the spectroradiometer but is very low, 6 orders of magnitude below the peak intensity level (note this is on a semi-log plot).
The formulations were administered once daily (Monday through Friday) for each subject for 3 ½ weeks by an investigator in the study facility. Each test product was dispensed from a 1-ml disposable plastic tuberculin syringe. The topical daily dose was 120 µL to each test site. The investigator uniformly rubbed in each product throughout the designated test site using a finger cot. The subjects were instructed not to shower or bathe for at least 6 hr after the products were applied. During the course of the study, the sites receiving daily product application seemed to become better moisturized than the untreated areas. Subsites did not receive topical application of test products on the days when they were exposed to SSR or thereafter.
SSR Source
A Multiport Solar Simulator (Model 601, Solar Light Co., Glenside, PA) was used in this study. This solar simulator is commonly used in the testing of sunscreens and allows for up to 6 areas on a subject's back to be simultaneously exposed [11]. The solar simulator emitted approximately 12% UVB (290 nm – 320 nm), with a smooth and broad distribution between 290 and 400 nm (Fig. 1B). The output of the solar simulator was characterized by measuring the spectral irradiance (W/cm2/nm) with a double grating spectroradiometer (Optronic Laboratories, Orlando, FL). Daily monitoring of the solar simulator’s output was accomplished using a calibrated erythemally-weighted broadband radiometer (Solar Light PMA 2100, Solar Light Co., Glenside, PA).
Irradiation of Sites for Determination of MED, DNA Damage and Sunburn Cell (SBC) Formation
After application of test products for 3 ½ weeks (17 applications), selected subsites located within each treatment site were irradiated for determination of the MED (Figure 1A). Doses of SSR ranging from 0.7 to 2.8 times the MED predicted for each individual (based on information each subject reported on his/her questionnaire) were administered to the MED subsites in each of the 4 sites on each subject’s back (Fig. 1A). The MED for each test site was determined 24 hr after irradiation. On the day at which the MEDs were determined, subsites to be biopsied for evaluation of DNA damage and SBC formation were irradiated (Figure 1A). These subsites had received an additional application of the appropriate test product the previous day. Due to variations in UV exposures delivered, the dose of SSR delivered to biopsy sites differed between subjects (range: 1 MED to 1.6 MED, average: 1.4 MED, standard deviation: 0.17 MED). The dose of SSR delivered to the biopsy sites was based on the MED that had been determined for each subject on previously untreated skin.
Biopsies
Shave biopsies were taken from control and from treated areas for determination of histological and molecular biomarkers. A total of 9 biopsies per subject were taken by the dermatologist. Each biopsy was approximately 4 mm in diameter. Biopsies were taken 7 min after exposure to SSR for determination of DNA damage and 24 hr post-SSR exposure for SBC determination. Prior to collecting the biopsies, the skin was locally anesthetized with an injection of lidocaine. Skin biopsies were fixed in 10% buffered formalin and were embedded in paraffin using standard techniques.
Biomarkers
DNA damage was measured by immunohistochemistry as previously described [12]. Briefly, a mouse monoclonal antibody (TDM-2, 1:40,000 dilution) that specifically binds TT and CT cyclobutane pyrimidine dimers (CPDs) in a dose-dependent manner was used [13], combined with a biotin-conjugated goat anti-mouse IgG (H+L), F(ab’)2 fragment (Zymed, South San Francisco, CA) incubated with a secondary streptavidin-conjugated fluorescein isothiocyanate (FITC) labeled antibody (Zymed, 1:200 dilution). CPDs were evaluated in biopsies by indirect immunofluorescence and DNA damage is expressed as a ratio of the FITC fluorescence intensity for CPDs to the intensity of propidium iodide for nuclear DNA [12,14]. SBCs are apoptotic cells with dark pyknotic nuclei and eosinophilic cytoplasms that can be counted in 10% formalin-fixed, paraffin-embedded, hematoxylin-eosin stained sections. SBCs were counted in a blinded manner in approximately 16 sections cut at 50 μm intervals from each specimen. The average number of SBCs was calculated from a minimum of 70 high power fields in each biopsy at 400× magnification [4].
Erythemal Responses
Visual observation (Determination of MED)
Subsites irradiated for determination of MED were observed 24 hr after exposure to SSR under fluorescent room lighting. The MED was defined as the minimum dose of SSR that produces pink erythema.
Instrumental observation
Subsites irradiated for biopsy and subsequent determination of SBC formation were measured with a Minolta CM-500 (Minolta Corporation, Ramsey, NJ) diffuse reflectance spectrophotometer 24 hr after exposure to SSR. The Minolta spectrophotometer measures the diffuse reflectance from 400 to 700 nm. Software supplied with the spectrophotometer converts these measurements into numerical indices within the CIE L*a*b* color space system. These values characterize the reflected light according to lightness (L value), red-green component (a* value) and yellow-blue component (b* value) [15]. The a* value is used to define the erythemal response measured as the difference between the respective mean a* of each SSR-exposed area and the mean a* of an unexposed, adjacent skin site. Diffuse reflectance spectrophotometry provides a more objective measurement of the erythemal response than visual assessment.
Statistical Analysis
Response variables of interest were analyzed using a mixed-model analysis of variance with Treatment and Site as fixed effects and Subject as a random effect. Treatment was the effect of interest. Site was included in the model to remove the possible confounding influence of different sites from the test of the treatment effect and to increase the precision of the estimates of treatment effects.
Results
Erythema Assessment by Visual Observation
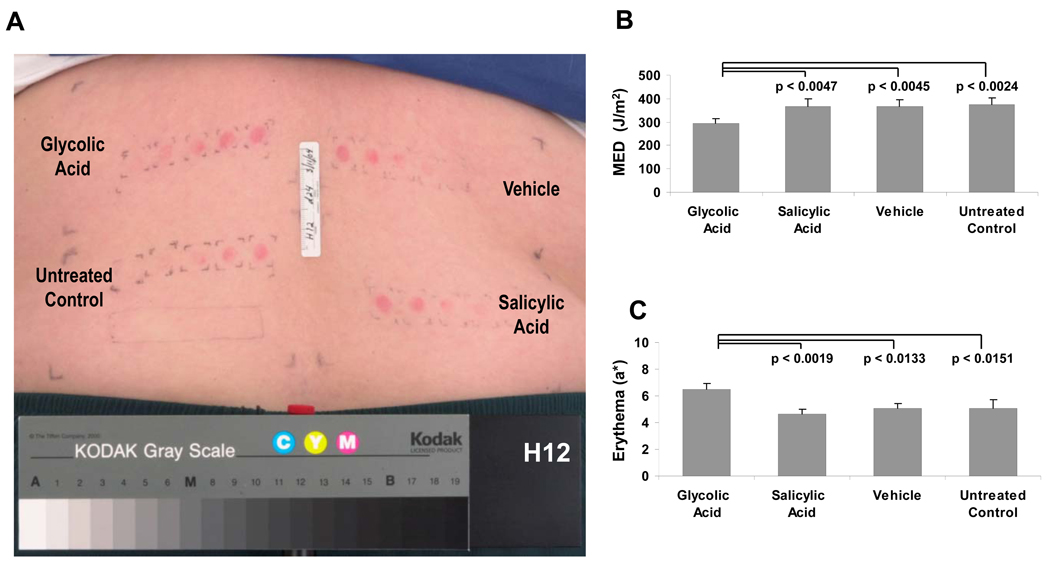
A photograph of the back of subject H12 is shown 24 hr after irradiation with incremental doses of SSR to each of the 4 selected sites to determine the MED (Fig. 2A). A summary of the MED values is presented in Fig. 2B. Topical application of 10% glycolic acid resulted in increased UV sensitivity with statistically significant lower mean MED than salicylic acid, vehicle or no topical treatment. Topical application of salicylic acid resulted in no statistically significant alteration of the MED compared to application of the vehicle or no topical treatment.
Figure 2.
A. A typical example of erythema development within the four test sites 24 hours after SSR exposure on day 24. B. The MED was determined for the 4 treatment sites on each subject. An average of the subjects’ MED at each site was calculated (n=14). C. Changes in erythema after SSR using the Minolta spectrophotometer to calculate the chromametric a* parameter. Sites identified for biopsy and determination of sunburn cells were irradiated as described in Materials and Methods. Data are reported as means ± SEM (n=14); NS = not significant; p values shown are for comparisons to glycolic acid treatment
Erythema Measurements with the Minolta Spectrophotometer at Subsites Exposed for Biopsies
The erythema intensity readings are presented in Fig. 2C. Treatment with glycolic acid resulted in a statistically significant increase in erythema (as measured by the a* parameter) as compared to salicylic acid, vehicle and untreated sites. Treatment with salicylic acid did not result in a statistically significant change in the erythemal response compared to responses at sites receiving no topical treatment or treatment with the vehicle.
Analysis of CPDs
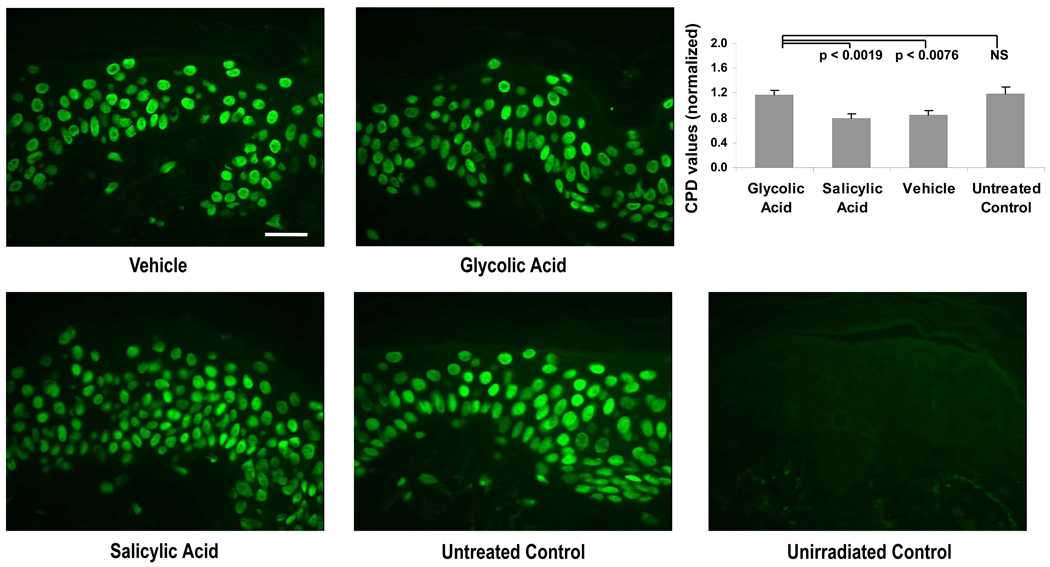
For statistical evaluation, the CPD ratios were normalized to give a mean value of 1.0 for all 4 treatment sites for each subject. As seen in Fig. 3, treatment with glycolic acid resulted in a significant increase in CPDs compared to the vehicle control and salicylic acid. The untreated group had higher CPD values than the vehicle and salicylic acid groups. The average CPD levels for the untreated group were not statistically different from levels found in the group treated with glycolic acid. The average level of CPDs for sites treated with salicylic acid was not statistically different from the average level of CPDs for sites treated with the vehicle.
Figure 3.
Representative images of specimens stained for CPD (green) at the UV-exposed sites and at the control unirradiated site (taken from subject H8). Graph shows levels of CPDs in the epidermis in all the test site areas (n=14). Data are reported as means ± SEM; NS = not significant; p values shown are for comparisons to glycolic acid treatment. All images at the same magnification; bar = 25 µm.
Analysis of SBC Formation
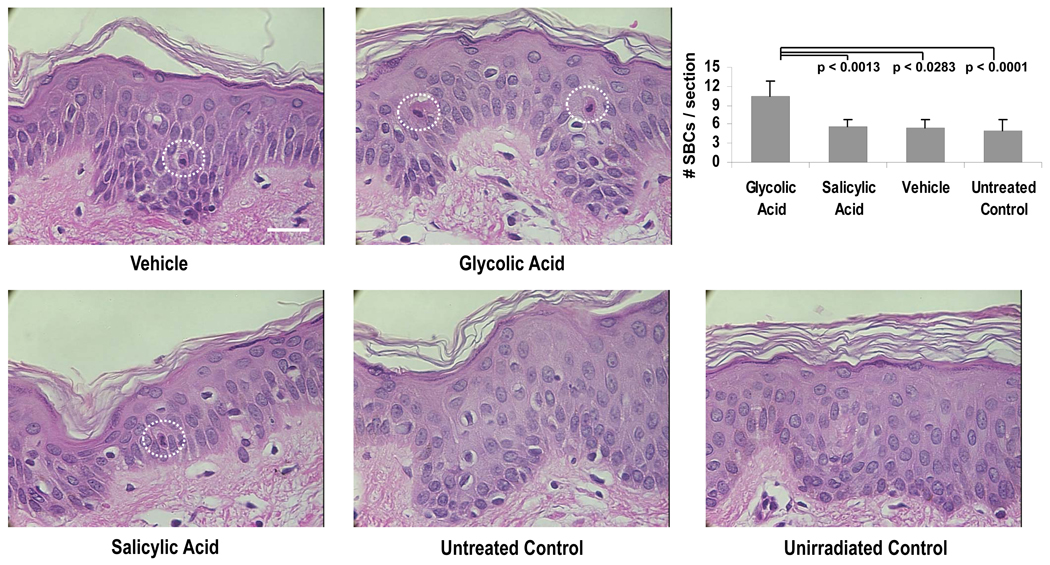
SBC numbers in biopsies obtained from 12 of the subjects are presented in Fig. 4A. Glycolic acid treatment produced significantly higher SBC numbers than salicylic acid, vehicle or no treatment. No statistically significant difference was seen between the average number of SBCs for sites treated with salicylic acid compared to untreated sites or sites treated with the vehicle. A summary of all parameters measured in this study is presented in Table 1.
Figure 4.
Representative images of specimens stained for SBCs (dashed circles) at the UV-exposed sites and at the control unirradiated site (taken from subject H12). Graph shows numbers of SBCs per section in all the test site areas (n=12). Data are reported as means ± SEM; NS = not significant; p values shown are for comparisons to glycolic acid treatment. All images at the same magnification; bar = 25 µm
Table 1.
Summary of data obtained in the study
| Parameter | n | Glycolic Acid | Salicylic Acid | Vehicle | Untreated Control |
|---|---|---|---|---|---|
| Erythema (a*) | 14 | 6.51 ± 0.45 | 4.63 ± 0.40 | 5.06 ± 0.39 | 5.10 ± 0.60 |
| Visual MED (J/m2) | 14 | 295.0 ± 20.2 | 368.6 ± 29.4 | 367.9 ± 27.0 | 375.0 ± 26.6 |
| CPD (normalized) | 14 | 1.18 ± 0.06 | 0.79 ± 0.08 | 0.84 ± 0.08 | 1.19 ± 0.11 |
| SBC (#/section) | 12 | 10.48 ± 2.38 | 5.59 ± 1.23 | 5.47 ± 1.35 | 4.97 ± 1.69 |
Results are reported as means ± SEM
DISCUSSION
Hydroxyacids have transformed skin care and enjoyed huge commercial success since their introduction by Van Scott in the early 1970s [2,16]. Since then, many companies have marketed HA-containing cosmetics and skin care products and their numbers are still growing. In addition, new types of HAs, such as benzilic and mandelic acids, poly-HAs and bionic acids, are being incorporated into cosmetic formulations [3]. At present, there is still an inadequate amount of information with which to assess the safety of topical application of α-HA and β-HA followed by exposure to sunlight or artificial sources of UV radiation. In this study, we confirmed that glycolic acid, commonly used in cosmetic formulations, can increase the sensitivity of human skin to SSR. In contrast, topical application of salicylic acid had no significant effect on the skin’s sensitivity to SSR.
A number of clinical studies have examined the effects of topically applied glycolic acid on the skin’s sensitivity to UV radiation. Perricone [5] reported that repeated topical application of glycolic acid was photoprotective. In contrast, all subsequent studies have found that topically applied glycolic acid increased the sensitivity of human skin to UV radiation. Tsai et al. [17] reported that treatment with 10% glycolic acid for 3 weeks resulted in increased sensitivity to UVB radiation, measured as increased erythema and tanning, and to UVA radiation, measured as tanning. In a previous study using 16 subjects, we reported that 10% glycolic acid, applied 6 days per week for 4 weeks, enhanced the sensitivity to UVB radiation measured as increased SBC formation and lowered the MED [4]. In another cohort group (n=13), CPDs were elevated, but not to a statistically significant level [4].
This study was designed to generate new data on the effects of topically applied α-HA and β-HA on the sensitivity of human skin exposed to SSR. A total of 14 subjects with skin types II to III were involved. Sun exposure produces skin damage resulting in formation of a number of measurable biomarkers, some of the most prominent being: provocation of an inflammatory response (erythema), production of DNA damage (CPDs), and formation of apoptotic keratinocytes (SBCs). The protocol was designed to allow us to measure all of the above biomarkers in a single subject with each subject serving as his/her own control.
Erythema induced by the sun or artificial UV radiation is a widely used, clinically relevant and noninvasive biomarker for assessing UV-induced damage and can be easily quantified in human skin [18,19]. The action spectrum for erythema formation closely resembles the spectra for CPD and SBC formation and for photocarcinogenesis [20]. Several indicators of the erythemal response to SSR were evaluated in this study. Treatment with glycolic acid was consistently associated with a statistically significant increase in the erythemal response and a statistically significant decrease in MED, as compared to salicylic acid, vehicle or the untreated site, measured either by visual grading or by instrumental assessment (Fig. 2B and 2C). Corresponding treatment with salicylic acid did not result in any significant modification of the erythemal response as compared to the vehicle or untreated site (Fig. 2C).
CPDs were selected as biomarkers of special significance, as these lesions are considered to constitute the primary damage responsible for the mutagenic and carcinogenic action of solar radiation. Several lines of evidence support this observation. SSR-induced CPDs result in CC-TT transitions known as “signature mutations” which have been detected in protooncogenes (ras) and tumor suppressor genes (p53) isolated from skin cancers [21,22]. Individuals with impaired DNA repair capacity (e.g. patients with xeroderma pigmentosum, who are deficient in nucleotide excision repair) are much more sensitive to sunburn and develop large numbers of skin tumors on sun-exposed areas [23]. They are at 1000-fold increased risk for developing skin cancer compared with the general population. The formation and repair of these photoproducts may be used to determine individual susceptibility to skin cancer, as well as a basis for performing risk analyses. Application of the formulation containing glycolic acid resulted in statistically significant increases in the formation of CPDs compared to the vehicle control and the salicylic acid group. The untreated group had significantly higher mean CPDs than the vehicle group. The difference between the untreated vs. glycolic acid group was not significant (Fig. 3). We do not have a satisfactory explanation for this finding in our cohort group. The comparisons in this study involved the vehicle-treated site and the other treatment site. The difference between the vehicle-and the glycolic acid-treated groups was always significant in our study. Additionally, we should note that the difference between the untreated and glycolic acid-treated sites was insignificant only for measurements of CPDs. The other biomarkers measured, erythema, MED and SBCs showed significant differences compare to the untreated site.
SBCs were first described more than 4 decades ago [24]. In the last few years, studies on various mechanistic and physiologic aspects of SBCs have significantly intensified. It has been realized that the importance of SBCs goes far beyond that of an alternative biomarker for acute UV injury. SBCs are known to be apoptotic cells, i.e., they undergo controlled individual cell death. Apoptosis is considered a major self-defense mechanism against cell damage. Through apoptosis, UV-damaged keratinocytes that fail to repair their DNA damage will die and be eliminated as SBCs, thereby reducing the risk of further mutations which can lead to malignant transformation. The mechanism of UV-induced apoptosis has been widely studied and involves a cascade of cellular mediators and molecular events that eventually lead to cellular disposal [20,25,26]. The balance between survival and apoptogenic factors determines the cell’s final fate, and growing evidence suggests that the deregulation of this balance by chronic UVB exposure results in the development of skin malignancy.
The relationship between MED, production of CPDs and formation of SBCs has proven to be inconsistent when attempting to describe the sensitivity of an individual to UV [27]. It has been reported that the production of CPD provides for a linear dose response to UV [28–30] while the formation of SBCs affords a non-linear dose response [31]. This accounts for the discrepancy between the different biological endpoints reported in Figure 3 and Figure 4. However, our results remain consistent with previous work, showing enhanced sensitivity to UV with topical application of 10% glycolic acid [4].
The mechanism for the photosensitizing activity of glycolic acid is unknown. As glycolic acid does not absorb in the UVB or UVA spectral regions, the increase in sensitivity to SSR can not result from direct photosensitization. Therefore, alternative mechanisms involving alteration of the skin’s optical or biological properties may be involved. Glycolic acid can, under certain conditions, moisturize and exfoliate the skin [32]. Smoothing the skin through this potential glycolic acid-induced alteration could affect the scattering and absorption of SSR by the skin. This alteration of the physical properties of both epidermis and dermis could result in altered biological responses to SSR. There are also a few published reports that topical application of HAs can modulate keratinization and stimulate biosynthesis of glycosylaminoglycans (especially hyaluronic acid) and collagen fibers, leading to an increased skin thickness [33,34]. We are not aware of any studies that have demonstrated a direct connection between glycosylaminoglycans and sensitivity to UV. In contrast, topical application of salicylic acid (5%) on human forearms has been found to decrease the skin thickness [32].
In addition to clinical studies that have examined the effects of topically applied glycolic acid on the acute responses of the skin to UV radiation, studies on glycolic acid-induced alterations in responses to chronic exposure to UV radiation have been reported. A study, sponsored by the National Toxicology Program, of the photocarcinogenic potential of HAs in SKH-1 mice, was recently completed [8]. The authors analyzed survival data, tumor growth data and pathology results and concluded that glycolic acid did not alter the photocarcinogenesis by SSR. It is noteworthy that the vehicle and test material containing 10% glycolic acid used in this photocarcinogenesis study had the same composition as those used in our clinical study. This allows a straightforward comparison between results obtained in our clinical study on glycolic acid-induced changes in acute responses to UV radiation in humans to changes in chronic responses to UV radiation in an animal model. In addition, Hong et al. have reported that topical application of glycolic acid at 8 mg/cm2, twice a week for 22 weeks, significantly reduced the incidence of UV radiation-induced skin cancer in SKH-1 mice [35]. These studies with animal models suggest that caution must be exercised in using clinical studies of acute sensitivity to UV radiation to predict alterations in the chronic effects of UV radiation, such as photocarcinogenesis.
Several clinical and experimental studies have found that topically applied salicylic acid is photoprotective. Clinical studies have examined the effects of a single application of salicylic acid on UV radiation-induced erythema [36,37]. Salicylic acid, applied shortly before exposure to UV radiation, was reported to decrease the erythemal response of the skin. This experimental protocol is expected to emphasize the sunscreen effect of salicylic acid. It has also been reported that repeated topical application of salicylic acid or sodium salicylate significantly protects SKH-1 mice from skin carcinogenesis induced by UV radiation [8,38]. The mechanisms underlying these photoprotective effects are not well understood. Salicylic acid is known to have a number of dermatological effects. Depending on the concentration, vehicle and experimental procedure, salicylic acid may be an effective sunscreen through direct absorption of UV radiation [36,37]. In addition, salicylic acid may be anti-inflammatory by inhibiting transcription for pro-inflammatory proteins or direct interception of free radical mediators [39]. Because of the relatively high concentrations of salicylic acid used, and because salicylic acid was applied shortly before irradiation, investigators could attribute the photoprotection observed in clinical studies to salicylic acid’s effectiveness as a sunscreen [36,37]. Similarly, mechanistic studies have suggested that sodium salicylate, topically applied to SKH-1 mice, inhibits photocarcinogenesis through a sunscreen effect [38]. In the current study, we observed that repeated application of 2% salicylic acid resulted in no significant change in the response of human skin to SSR. Both our use of a low concentration of salicylic acid (similar to that found in cosmetic formulations) and discontinuation of product application for a day prior to evaluation of the skin’s sensitivity to UV radiation may have reduced salicylic acid’s sunscreen activity.
In this study, we paid special attention to the selection of the vehicle and the choice of the artificial light source. Vehicle effects can significantly alter the results and have been previously reported in several skin studies [40–42]. It has been demonstrated that vehicles can dramatically affect the absorption and deposition of glycolic acid into the skin [6]. Further, the choice of light source has been shown to significantly affect biological responses to UV radiation [43,44]. Therefore, the use of a vehicle (cream) that closely mimics those used in consumer products and an artificial light source that closely duplicates solar radiation (sunlight) are critical factors in designing these types of studies.
The present study confirms previous findings and contributes additional evidence that a commonly used cosmetic formulation can increase the sensitivity of human skin to SSR. Two common cosmetic ingredients, glycolic and salicylic acid, were examined in this study. The effect of both of these ingredients on the sensitivity of human skin to UV-induced damage was examined in each subject. By evaluating results generated from measurement of erythema and two prominent biomarkers, we conclude that glycolic acid (10%) increases the UV-sensitivity of human skin, whereas salicylic acid (2%) does not. The photosensitizing effects of glycolic acid are small but they may be important from the viewpoint of public health, considering that HA-containing products are being used by a large segment of the population for many years, and in many cases, over a lifetime. The limited knowledge available on the interactions between cosmetic formulations containing HAs and solar exposure of human skin should be expanded to make relevant risk assessments possible.
ACKNOWLEDGEMENTS
This research was supported by the U.S. FDA Office of Science and Health Coordination and in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The authors wish to thank K. Korossy, MD and C. Tock, MD for the dermatological support and for obtaining biopsies from all subjects; J. Kniskern, RN for excellent handling of human subjects’ records; W. Wamer for useful input to the manuscript; D. Lowther for her skillful technical and editorial assistance in preparing this manuscript; J. Hubinger for analysis of test products.
Footnotes
DISCLAIMER
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services. The opinions and/or conclusions expressed are solely those of the authors and in no way imply a policy or position of the Food and Drug Administration.
Disclosure Statement
None of the authors has any actual or potential conflict of interest to declare.
REFERENCES
- 1.Jackson EM. AHA-type products proliferate in 1993. Cosmet Dermatol. 1993;6:22–26. [Google Scholar]
- 2.Van Scott EJ, Yu RJ. Control of keratinization with alpha-hydroxy acids and related compounds. I. Topical treatment of ichthyotic disorders. Arch Dermatol. 1974;110:586–590. doi: 10.1001/archderm.110.4.586. [DOI] [PubMed] [Google Scholar]
- 3.Green B. After 30 years … the future of hydroxyacids. J Cosmet Dermatol. 2005;4:44–45. doi: 10.1111/j.1473-2165.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaidbey KH, Sutherland BM, Bennett P, Wamer WG, Barton C, Dennis D, et al. Topical glycolic acid enhances photodamage by ultraviolet light. Photodermatol Photoimmunol Photomed. 2003;19:21–27. doi: 10.1034/j.1600-0781.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 5.Perricone NV, DiNardo JC. Photoprotective and antiinflammatory effects of topical glycolic acid. Dermatol Surg. 1996;22:435–437. doi: 10.1111/j.1524-4725.1996.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 6.Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17 Suppl. 1:1–241. [Google Scholar]
- 7.Andersen FA. Safety assessment of salicylic acid, butyloctyl salicylate, calcium salicylate, C12-15 alkyl salicylate, capryoyl salicylic acid, hexyldodecyl salicylate, isocetyl salicylate, isodecyl salicylate, magnesium salicylate, MEA-salicylate, ethylhexyl salicylate, potassium salicylate, methyl salicylate, myristyl salicylate, sodium salicylate, TEA-salicylate, and tridecyl salicylate. Int J Toxicol. 2003;22 Suppl. 3:1–108. [PubMed] [Google Scholar]
- 8.National Toxicology Program Technical Report. Photocarcinogenic study of glycolic acid and salicylic acid in SKH-1 mice. 2007 TR 524. [PubMed] [Google Scholar]
- 9.Yates RL, Havery DC. Determination of phenol, resorcinol, salicylic acid, and á-hydroxy acids in cosmetic products and salon preparations. J Cosmet Sci. 1999;50:315–325. [Google Scholar]
- 10.Fitzpatrick TB. The validity and practicability of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 11.Sayre RM, Cole C, Billhimer W, Stanfield J, Ley RD. Spectral comparison of solar simulators and sunlight. Photodermatol Photoimmunol Photomed. 1990;7:159–165. [PubMed] [Google Scholar]
- 12.Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003;17:1177–1179. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- 13.Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4)photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem Photobiol. 1991;54:225–232. doi: 10.1111/j.1751-1097.1991.tb02010.x. [DOI] [PubMed] [Google Scholar]
- 14.Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- 15.Fullerton A, Fischer T, Lahti A, Wilhelm K-P, Takiwaki H, Serup J. Guidelines For Measurement Of Skin Colour And Erythema. A Report From The Standardization Group Of The European Society Of Contact Dermatitis. Contact Dermatitis. 1996;35:1–10. doi: 10.1111/j.1600-0536.1996.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Scott EJ, Ditre CM, Yu RJ. Alpha-hydroxyacids in the treatment of signs of photoaging. Clin Dermatol. 1996;14:217–226. doi: 10.1016/0738-081x(95)00157-b. [DOI] [PubMed] [Google Scholar]
- 17.Tsai TF, Bowman PH, Jee SH, Maibach HI. Effects of glycolic acid on light-induced skin pigmentation in Asian and caucasian subjects. J Am Acad Dermatol. 2000;43:238–243. doi: 10.1067/mjd.2000.104894. [DOI] [PubMed] [Google Scholar]
- 18.Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, et al. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A. 2007;104:17500–17505. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coelho SG, Miller SA, Zmudzka BZ, Beer JZ. Quantification of UV-induced erythema and pigmentation using computer-assisted digital image evaluation. Photochem Photobiol. 2006;82:651–655. doi: 10.1562/2005-08-02-TSN-635. [DOI] [PubMed] [Google Scholar]
- 20.Claerhout S, Van A, Agostinis P, Garmyn M. Pathways involved in sunburn cell formation: deregulation in skin cancer. Photochem Photobiol Sci. 2006;5:199–207. doi: 10.1039/b504970a. [DOI] [PubMed] [Google Scholar]
- 21.Daya-Grosjean L, Sarasin A. UV-specific mutations of the human patched gene in basal cell carcinomas from normal individuals and xeroderma pigmentosum patients. Mutat Res. 2000;450:193–199. doi: 10.1016/s0027-5107(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 22.Giglia-Mari G, Sarasin A. TP53 mutations in human skin cancers. Hum Mutat. 2003;21:217–228. doi: 10.1002/humu.10179. [DOI] [PubMed] [Google Scholar]
- 23.van Steeg H, Kraemer KH. Xeroderma pigmentosum and the role of UV-induced DNA damage in skin cancer. Mol Med Today. 1999;5:86–94. doi: 10.1016/s1357-4310(98)01394-x. [DOI] [PubMed] [Google Scholar]
- 24.Daniels F, Jr, Brophy D, Lobitz WC., Jr Histochemical responses of human skin following ultraviolet irradiation. J Invest Dermatol. 1961;37:351–357. [PubMed] [Google Scholar]
- 25.Sheehan JM, Young AR. The Sunburn Cell Revisted: An Update On Mechanistic Aspects. Photochemical and Photobiological Sciences. 2002;1:365–377. doi: 10.1039/b108291d. [DOI] [PubMed] [Google Scholar]
- 26.Van Laethem A, Claerhout, Garmyn, Agostinis The sunburn cell: regulation of death and survival of the keratinocyte. Int J Biochem Cell Biol. 2005;37:1547–1553. doi: 10.1016/j.biocel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Heenen M, Giacomoni PU, Golstein P. Individual variations in the correlation between erythemal threshold, UV-induced DNA damage and sun-burn cell formation. J Photochem Photobiol B. 2001;63:84–87. doi: 10.1016/s1011-1344(01)00205-6. [DOI] [PubMed] [Google Scholar]
- 28.Freeman SE, Gange RW, Sutherland JC, Sutherland BM. Pyrimidine dimmer formation in human skin. Photochem Photobiol. 1987;46:207–212. doi: 10.1111/j.1751-1097.1987.tb04758.x. [DOI] [PubMed] [Google Scholar]
- 29.Young AR, Chadwick CA, Harrison GI, Hawk JL, Nikaido O, Potten CS. The in situ repair kinetics of epidermal thymine dimers and 6-4 photoproducts in human skin types I and II. J Invest Dermatol. 1996;106:1307–1313. doi: 10.1111/1523-1747.ep12349031. [DOI] [PubMed] [Google Scholar]
- 30.Bykov VJ, Jansen CT, Hemminki K. High levels of dipyrimidine dimers are induced in human skin by solar-simulating UV radiation. Cancer Epidemiol Biomarkers Prev. 1998;7:199–202. [PubMed] [Google Scholar]
- 31.Nonaka S, Kaidbey KH, Kligman AM. The influence of UVA and visible radiation on acute damage by short-wave UVR (lambda less than 320 nm) J Invest Dermatol. 1983;81:524–527. doi: 10.1111/1523-1747.ep12522868. [DOI] [PubMed] [Google Scholar]
- 32.Yu RJ, Van Scott EJ. Alpha-hydroxyacids and carboxylic acids. J Cosmet Dermatol. 2004;35:76–87. doi: 10.1111/j.1473-2130.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith WP. Hydroxy acids and skin aging. Cosmet Toilet. 1994;109:41–48. [Google Scholar]
- 34.Bernstein EF, Lee J, Brown DB, Yu R, Van SE. Glycolic acid treatment increases type I collagen mRNA and hyaluronic acid content of human skin. Dermatol Surg. 2001;27:429–433. doi: 10.1046/j.1524-4725.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 35.Hong JT, Kim EJ, Ahn KS, Jung KM, Yun YP, Park YK, et al. Inhibitory effect of glycolic acid on ultraviolet-induced skin tumorigenesis in SKH-1 hairless mice and its mechanism of action. Mol Carcinog. 2001;31:152–160. doi: 10.1002/mc.1050. [DOI] [PubMed] [Google Scholar]
- 36.Weirich EG. Dermatopharmacology of salicylic acid. I. Range of dermatotherapeutic effects of salicylic acid. Dermatologica. 1975;151:268–273. doi: 10.1159/000251346. [DOI] [PubMed] [Google Scholar]
- 37.Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37–40. [PubMed] [Google Scholar]
- 38.Bair WB, III, Hart N, Einspahr J, Liu G, Dong Z, Alberts D, et al. Inhibitory effects of sodium salicylate and acetylsalicylic acid on UVB-induced mouse skin carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2002;11:1645–1652. [PubMed] [Google Scholar]
- 39.Amann R, Peskar BA. Anti-inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol. 2002;447:1–9. doi: 10.1016/s0014-2999(02)01828-9. [DOI] [PubMed] [Google Scholar]
- 40.Leopold CS. The effect of cream and ointment bases on the steady state penetration of permeants through intact skin: the reciprocal of the onset time of a pharmacodynamic effect as parameter of response. Int J Cosmet Sci. 2000;22:133–145. doi: 10.1046/j.1467-2494.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 41.Wright ZM, Basketter PA, Blaikie L, Cooper KJ, Warbrick EV, Dearman RJ, et al. Vehicle effects on skin sensitizing potency of four chemicals: assessment using the local lymph node assay. Int J Cosmet Sci. 2001;23:75–83. doi: 10.1046/j.1467-2494.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 42.Lu YP, Lou YR, Xie JG, Peng Q, Shih WJ, Lin Y, et al. Tumorigenic effect of some commonly used moisturizing creams when applied topically to UVB-pretreated high-risk mice. J Invest Dermatol. 2009;129:468–475. doi: 10.1038/jid.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown DB, Peritz AE, Mitchell DL, Chiarello S, Uitto J, Gasparro FP. Common fluorescent sunlamps are an inappropriate substitute for sunlight. Photochem Photobiol. 2000;72:340–344. [PubMed] [Google Scholar]
- 44.Yarosh DB, Kibitel J, Ullrich SE, Kim TH, Ananthaswamy HN, Krien P, et al. Direct comparison of DNA damage, isomerization of urocanic acid and edema in the mouse produced by three commonly used artificial UV light sources. Photochem Photobiol. 1999;69:571–574. [PubMed] [Google Scholar]