Abstract
Glucocorticoids contribute to the maintenance of basal and stress-related homeostasis in all higher organisms, influence a large proportion of the expressed human genome and their effects spare almost no organs or tissues. Glucocorticoids regulate many functions of the central nervous system, such as arousal, cognition, mood, and sleep, the activity and direction of intermediary metabolism, the maintenance of a proper cardiovascular tone, the activity and quality of the immune and inflammatory reaction, including the manifestations of the sickness syndrome, as well as growth and reproduction. The numerous actions of glucocorticoids are mediated by a set of at least 16 glucocorticoid receptor (GR) isoforms forming homo- or hetero-dimers. The GRs consist of multifunctional domain proteins operating as ligand-dependent transcription factors that interact with many other cell signaling systems, including large and small G proteins. The presence of multiple GR monomers and homo- or hetero-dimers expressed in a cell-specific fashion at different quantities with quantitatively and qualitatively different transcriptional activities suggest that the glucocorticoid signaling system is highly stochastic. Glucocorticoids are heavily involved in human pathophysiology and influence life expectancy. Common behavioral and/or somatic complex disorders, such as anxiety, depression, insomnia, chronic pain and fatigue syndromes, obesity, the metabolic syndrome, essential hypertension, diabetes type 2, atherosclerosis with its cardiovascular sequelae, and osteoporosis, as well as autoimmune inflammatory and allergic disorders, all appear to have a glucocorticoid-regulated component.
Keywords: Metabolic syndrome, osteoporosis, CDK5, GR phosphorylation, stress system, glucocorticoid resistance, glucocorticoid hypersensitivity
Introduction
Glucocorticoids are amongst the most pervasive hormones in mammalian organisms.1,2 These steroid molecules reach all tissues, including the brain, readily penetrate the cell membrane, and interact with ubiquitous cytoplasmic/nuclear glucocorticoid receptors (GRs), through which they exert markedly diverse actions.1,2 Using DNA microarray technology, we found that about 20 percent of the expressed human leukocyte genome was positively or negatively affected by glucocorticoids.3 This is many-fold higher than the proportion of genes that change in the transformation of a normal cell to a tumor cell and involves a broad array of functions, affecting every aspect of resting and stress-related homeostasis, including a large number of genes expressed by the immune system.3,4 The pervasive nature of glucocorticoids, the rapid advances in our general knowledge of the human and other mammalian genomes, and the massive amount of information that increasingly accumulates, dictate a new model of thinking and testing of hypotheses regarding the actions of these hormones and their involvement in human physiology and pathophysiology.
Glucocorticoid Receptor Gene Polymorphisms and Complex Human Pathophysiology
Wüst et al. reported a convincing association between the hypothalamic-pituitary-adrenal (HPA) axis response to a standardized socio-emotional stimulus (Trier test) and polymorphisms of the GR gene.5 This study followed others that used a similar rationale and examined HPA axis indices and other end-points, such as arterial blood pressure, body mass index (BMI) and markers of the metabolic syndrome, and bone mineral density.6–13 These studies have had some overlap and have produced mostly concordant results, but have also shown inconsistencies. This should have been expected because these studies were performed in a limited number of subjects in different ethnic populations, and because the altered GR would have been expected to function differently in the context of different genetic backgrounds characterized by different panels of genes with differing epistatic effects upon the ability of the GR to exert its actions.14
Glucocorticoid Effects –Physiology, Pathophysiology
Empirically, experimentally, and intuitively, physicians and scientists have made major advances in the general understanding of glucocorticoids and their involvement in human physiology and pathophysiology, and in using these hormones extensively and effectively in the treatment of a wide spectrum of human diseases.1,2 As the end product of the HPA axis, glucocorticoids are literally present in every organ system of the human organism, in almost all physiologic, cellular and molecular networks, and in many crucial modules of these networks.2,3,14 Glucocorticoids, furthermore, participate in a pivotal fashion in the unfolding of vital biological programs employing synchronously or in tandem several networks, including the behavioral and physical response to stress, the inflammatory reaction and the consequent “sickness syndrome”, ie. the collection of “nonspecific symptoms” caused by excessive inflammatory cytokines during infectious or inflammatory illness, as well as the process of sleep, and long- term functions, such as growth and reproduction.4
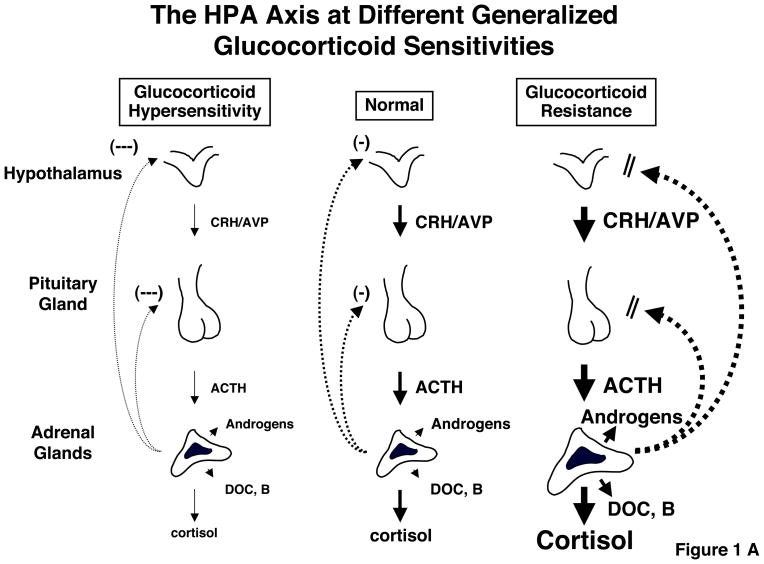
As is true with many other homeostatic systems, too much, as well as too little, HPA axis and/or glucocorticoid activity may be associated with pathology, for instance Cushing syndrome vs. Addison disease, respectively.15–17 Since the responsiveness of the target tissues to glucocorticoids is crucial for the end-effect of these hormones, similar pathology may result from hypersensitivity or resistance of these target tissues to these hormones, respectively (Table 1).16,18 However, because the brain and the pituitary are also the targets of glucocorticoids, and because the organism strives for homeostasis in time-integrated free cortisol exposure, any generalized change in the glucocorticoid signaling system would be expected to be followed by corrective, “compensatory” changes in the activity of the HPA axis. Indeed, in the rare GR-mediated genetic disorder Primary Glucocorticoid Resistance the majority of the clinical manifestations are not Addisonian, but are rather due to the compensatory hyperfunction of the HPA axis leading to adrenal androgen and mineralocorticoid hypersecretion while the opposite would be expected in primary generalized glucocorticoid hypersensitivity (Figure 1A and B).19,20
Table 1.
Expected clinical manifestations in target tissue hypersensitivity or resistance to glucocorticoids
| Target area | Glucocorticoid excess = |
Glucocorticoid deficiency = |
|---|---|---|
| Glucocorticoid hypersensitivity | Glucocorticoid resistance | |
| Central nervous system | Insomnia, anxiety, depression, defective cognition | Fatigue, somnolence, malaise, defective cognition |
| Liver | +Gluconeogenesis, +lipogenesis | Hypoglycemia, resistance to diabetes mellitus |
| Fat | Accumulation of visceral fat (metabolic syndrome) | Loss of weight, resistance to weight gain |
| Blood vessels | Hypertension | Hypotension |
| Bone | Stunted growth, osteoporosis | |
| Inflammation/immunity | Immune suppression, anti-inflammation, vulnerability to certain infection and tumors | +Inflammation, +autoimmunity, +allergy |
Figure 1.
A: Feedback-regulated compensatory changes in the activity of the HPA axis and their effects in peripheral tissues, such as the liver, fat and blood vessels. Note that glucocorticoid sensitivity in the HPA axis and the peripheral tissues can be independently regulated and the former determines the serum free cortisol levels, thus combination of their directions influence net peripheral action of this hormone. ACTH: Adrenocorticotropic hormone; AVP: Arginine vasopressin; CRH: Corticotropin-releasing hormone; DOC: Deoxycorticosterone; B: Corticosterone. Modified from references 68, 73 & 74.
B: Known GR mutations that cause familial/sporadic glucocorticoid resistance syndrome. Localization of GR mutations that cause familial/sporadic glucocorticoid resistance syndrome are shown in the human GR gene (top) and in the human GR protein (bottom). From references 19 & 20.
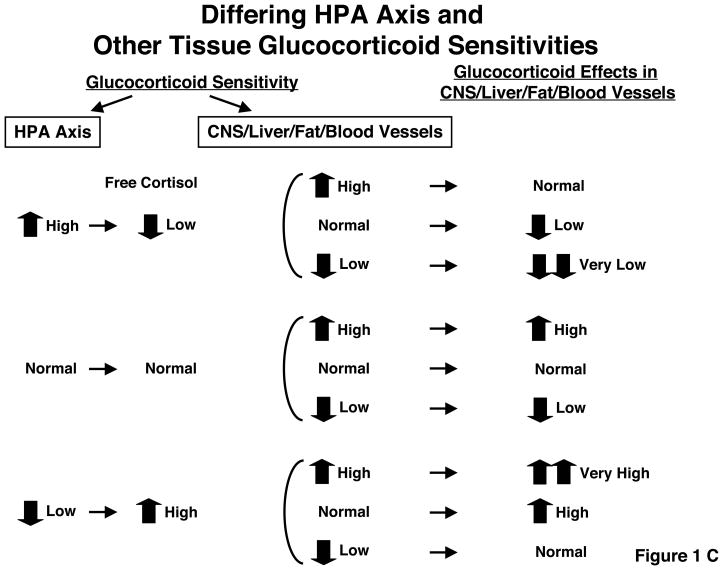
C: Alteration of net glucocorticoid effects in target tissues by activity of the central HPA axis and peripheral tissue sensitivity to glucocorticoids.
Net glucocorticoid action in peripheral tissues, such as the CNS, liver, fat and vasculature, are determined by two components, the central HPA axis and sensitivity of peripheral tissue to glucocorticoids. From reference 68.
However, absence of complete concordance between HPA axis activity and the target tissues outside those responsible for feedback regulation, be it slightly excessive or deficient, could result in a state called allostasis, more accurately termed cacostasis, leading to target tissue pathology, as occurs in chronically stressed or depressed individuals in whom there is frequently mild chronic hypercortisolism.21,22 This has been known for several decades. The key question is whether it is possible to have discordance between the feedback regulation of the HPA axis by glucocorticoids and peripheral target tissue sensitivity to these hormones in totally “normal” individuals.
Indeed it appears to be possible. The glucocorticoid signaling system of the suprahypothalamic, hypothalamic and pituitary glucocorticoid-sensing network is different from the signaling systems of the reward, arousal, associative, cardiovascular, metabolic and immune systems, which are influenced by glucocorticoids. The feedback centers of the HPA axis sense and thus determine the circulating glucocorticoid levels, while other tissues passively accept the actions of circulating glucocorticoids. Indeed, any change in one or more molecules or processes that participate in the glucocorticoid signaling system could potentially have a different impact on the HPA feedback system and other target tissues. Such discrepancies in the glucocorticoid sensing network between the HPA axis and peripheral tissues could, thus, produce peripheral tissue “hypercortisolism” or “hypocorticosolism” depending on their combinations (Figure 1C and Table 1).16 As an example, in a recent study by Alevizaki et al., both high HPA axis reactivity to stress and increased peripheral tissue sensitivity to glucocorticoids were associated with increased severity of coronary artery disease.23 Naturally, GR is not alone in defining the sensitivity of the feedback system and other tissues to glucocorticoids. Numerous GR isoforms with different activities, other molecules or processes with considerable input into the activity of the cellular glucocorticoid signaling system have been described (Table 2).18,24,25
Table 2.
Factors influencing GR functions
| Ligands | Membrane transporters of glucocorticoids 11β-hydroxysteroid dehydrogenases Agonists, antagonists (ex. RU 486) |
| Chemical compounds | Ursodeoxycholic acid, cortivazol, thioredoxin, carnitine |
| Chemical Modifications | Phosphorylation, nitrosylation, acetylation, methylation, sumoylation |
| Chaperones, co-chaperones | Heat shock proteins, RAP46, FK506-binding proteins |
| Receptor isoforms | Glucocorticoid receptor α and β isoforms (16 or more, 256 or more combinations of dimers) |
| Transcriptional co-regulators | Coactivators/corepressors, SWI/SNF, TRAP/DRIP complex, SMAD6 Viral proteins: adenoviral E1A, Human Immunodeficiency virus type-1 Vpr and Tat |
| Transcription factors | Nuclear factor-κB, activator protein-1, CREB-binding protein, p53, chicken ovalbumin upstream promoter-transcription factor-II, GATA-1, SP-1, Nuclear factor-1, PPARα |
| Other proteins | 14-3-3, FLASH, Rho-type guanine nucleotide exchange factors (Brx and c-Lbc), autoimmune regulator gene (AIRE), guanine nucleotide-binding protein β |
| RNAs | SRA, Gas5 |
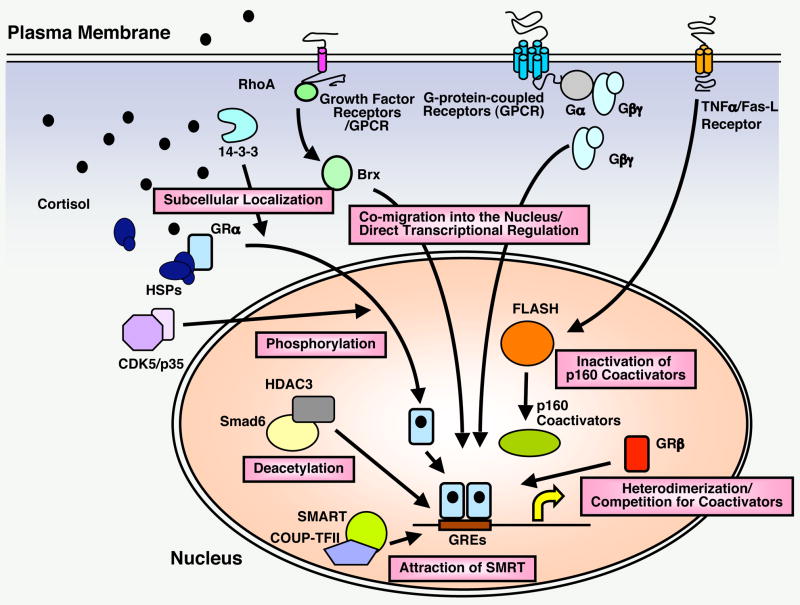
In our studies of the glucocorticoid signaling system, we have identified several molecules from a variety of different signaling systems that interact in many ways with and influence the activity of the GR and vice versa and are depicted in Figure 2 and Table 2.18,24–33 These include the β subunit of the G protein trimeric complex, small G proteins, components of the tumor necrosis factor-α/Fas ligand signaling systems, such as FLASH, the chaperone protein 14-3-3, the HIV accessory proteins Vpr and Tat, the adenoviral protein E1A, and molecular components of brain cyclin-dependent kinase 5 (CDK5).26–31,33 In light of the major physiologic homeostatic influence of glucocorticoids on many brain functions, as well as the major pathologic effects of these hormones on the central nervous system (CNS), we elected to summarize below our work on the interactions of CDK5 and the GR.34
Figure 2. Regulation of tissue GR activity by distinct signaling pathways.
Transcriptional activity of GR in glucocorticoid target tissues is regulated by numerous signaling pathways through distinct mechanisms. From references 18, 24, 26–29, 32, 69, 71 & 72.
Glucocorticoids and the Brain. CNS Cyclin-dependent Kinase 5 Regulates Glucocorticoid Actions in the Brain by Phosphorylating GR
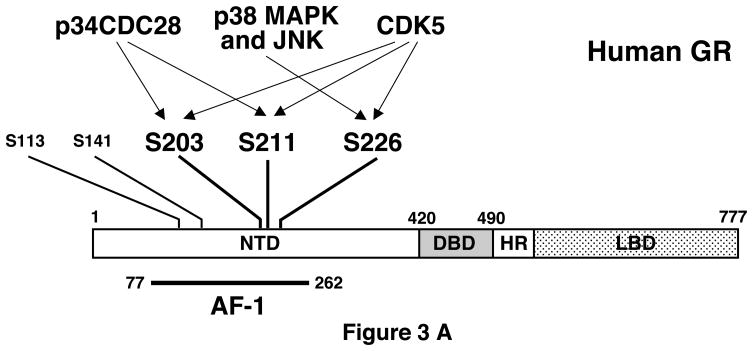
The transcriptional activity of GR is regulated by direct phosphorylation of this receptor by serine/threonine kinases.35 GR has several phosphorylation sites, all of which are located in the AF-1 domain of its NTD,24,36 suggesting that AF-1 acts as an interface for phosphorylation-dependent intracellular molecular signals (Figure 3A). For example, yeast cyclin-dependent kinase p34CDC28 phosphorylates rat GR at serines 224 and 232, which are orthologous to serines 203 and 211 of human GR, with the resultant phosphorylation enhancing rat GR transcriptional activity in the yeast.35 These residues are also phosphorylated after activation of GR with agonists or antagonists and the phosphorylated receptor shows reduced translocation to the nucleus and/or altered subcellular localization in mammalian cells.36,37 The p38 mitogen-activated protein kinase (MAPK) phosphorylates serine 211 of human GR and enhances its transcriptional activity.38 p38 MAPK and JNK also phosphorylate serine 226 of human GR and suppress its transcriptional activity by enhancing nuclear export of the receptor.39 Threonine 171 of rat GR is phosphorylated by p38 MAPK and glycogen synthase kinase-3. Threonine 171-phosphorylated GR demonstrates reduced transcriptional activity in yeast and human cells, however, human GR does not have a threonine residue equivalent to that of the rat GR.40
Figure 3. Regulation of GR transcriptional activity through phosphorylation.
A: Human GR has three major phosphorylation sites at serines 203, 211 and 226 and two minor phosphorylation sites at serines 113 and 141. The former sites are phosphorylated by several kinases indicated. From references 24 & 34–39.
B: Schematic regulation of GR-induced transcriptional activity by CDK5.
CDK5 regulates GR-induced transcriptional activity by changing attraction of transcriptional cofactors to responsive promoters in the central nervous system, possibly through molecules that specifically interact with phosphorylated or nonphosphorylated ligand-bound GR. The direction and size of the effect depends also on the presence of other gene promoter-, tissue- or brain region-specific transcriptional cofactors. Modified from references 33 & 34.
In addition to these kinases that phosphorylate GR and regulate its transcriptional activity, we recently found that CDK5, which is a member of the cyclin-dependent kinase family,41 phosphorylates GR and modulates its transcriptional activity.33 In contrast to other CDKs, which function in the control of the cell cycle, CDK5 has no activity during mitosis but is essential for brain development and neuronal morphogenesis and survival.42,43 CDK5 is expressed ubiquitously in many tissues, but its activity is restricted primarily to the nervous system due to neuron-specific expression of its activator molecules p35 and p39.44,45 In addition to these physiologic roles of CDK5, recent evidence suggests that aberrant CDK5 activation caused by proteolytic conversion of p35 to p25 may play a role in the pathogenesis of neurodegenerative disorders, such as Alzheimer’s disease and amyotrophic lateral sclerosis.43,46 In these conditions, calpain-directed proteolysis of p35 deprives the membrane-associated p35 of its N-terminal myristoylated membrane tether, releasing p25 into the cytoplasm and activating CDK5.47,48
We found that the CDK5/p35 complex physically interacts with the GR LBD and modulates GR-induced transcriptional activity by phosphorylating serines 203, 211 and 226 of human GR.33 The C-terminal part of p35 binds the GR LBD in a ligand-dependent fashion, while both the GR LBD and NTD are necessary for CDK5/p35 to modulate GR transcriptional activity, indicating that these subdomains of GR respectively act as interactor and effector surfaces for CDK5/p35.33 We found that CDK5 positively or negatively regulates the transcriptional activity of GR on endogenous glucocorticoid-responsive genes in rat cortical neuronal cells in microarray analyses, indicating that the effect of CDK5 on GR-induced transcriptional activity is gene promoter-specific.33 Since phosphorylation of GR at serines 203, 211 and 226 alters the attraction of cofactor molecules to the promoter-bound GR,33 it is possible that phosphorylation of the receptor may respectively facilitate or inhibit the attraction of transcription cofactors on promoter-bound GR, through as yet undetermined mechanisms that appear to be gene-specific (Figure 3B). The tumor susceptibility gene 101 (TSG101), which interacts with the co-integulator molecules p300/CBP and inhibits the transcriptional activity of GR by modulating GR-induced attraction of coactivators, preferentially interacts with a nonphosphorylated form of GR.36,40,49 Thus, TSG101 and/or similar molecules that specifically bind nonphosphorylated or phosphorylated forms of GR,40 may mediate phosphorylation-dependent transcriptional modulation by changing accumulation of cofactors on promoter-bound GR.
Glucocorticoids play an essential role in the homeostasis of the CNS;50,51 These hormones indeed regulate cognition, memory, mood, and sleep, and influence the anatomic structure of the brain, causing reduction of hippocampal volume, ventricular enlargement and reversible cortical atrophy.51 Alteration of CDK5 activity in neuronal cells can potentially influence any of these glucocorticoid actions in the CNS. It is also particularly interesting whether the regulatory actions of CDK5 on GR transcriptional activity contribute to the development of neurodegenerative disorders, since glucocorticoids have strong activity on consolidation of memory and neuronal cell survival.51,52 Proteolytically produced cytoplasmic p25, whose excessive production has been associated with Alzheimer’s disease,53,54 demonstrated a much stronger effect than p35 in regulating GR transcriptional activity.33 Thus, it is possible that CDK5 may exert pathologic effects by altering GR transcriptional activity through aberrant conversion of p35 to p25.
Glucocorticoids, the Metabolic Syndrome and Other Somatic Sequelae of Stress
Endogenous or exogenous Cushing syndrome is associated with the full metabolic profile of the metabolic syndrome and with substantially increased cardiovascular morbidity and mortality.55,56 Glucocorticoids directly cause insulin resistance of peripheral target tissues in proportion to their levels and to the particular target tissue’s sensitivity to these hormones. Over time, glucocorticoids also cause progressive accumulation of visceral fat, leading to worsening manifestations of the metabolic syndrome. Thus, when polymorphisms of the GR gene lead to an unfavorable discordance between the activity of the HPA axis and the sensitivity of muscle, fat and/or liver to glucocorticoids, the increased glucocorticoid effect in these tissues could influence the metabolic profile and the longevity of humans in a negative fashion, similar to that which occurs in Cushing syndrome.7,15,57–60
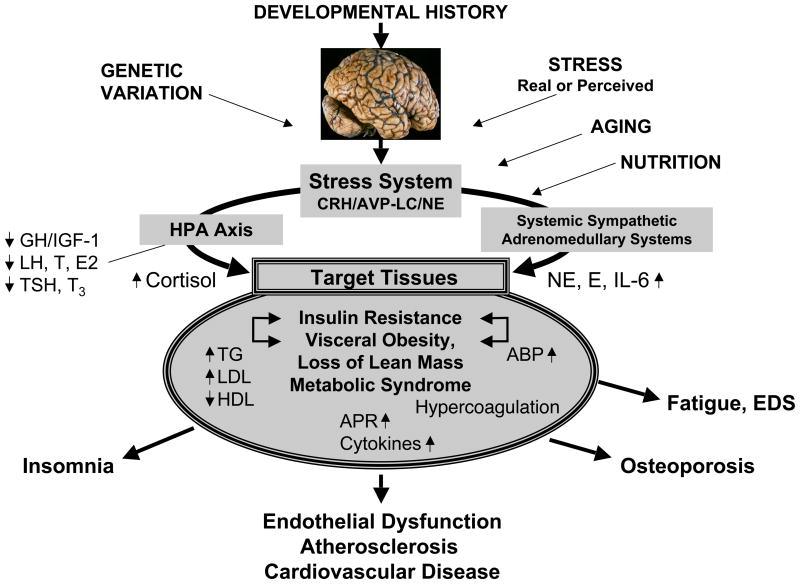
Genetic and developmental factors, nutrition, lifestyle, and cumulative chronic or intermittent stress may lead to development of obesity, primarily of the visceral type with concurrent loss of lean body mass, and, hence, the metabolic syndrome with its components of insulin resistance, dyslipidemia, chronic smoldering inflammation, blood hypercoagulation, arterial hypertension, and/or diabetes mellitus type 2 (Figure 4).61 These changes lead to endothelial inflammation, atherosclerosis, and cardiovascular disease, ultimately resulting in premature cardiovascular morbidity and death.61 Similarly, through decreased bone formation and/or increased bone resorption, osteopenia or osteoporosis may ensue.
Figure 4. Endogenous/exogenous inputs to the stress system and their effects on the metabolic and cardiovascular systems and bone.
ABP: Arterial blood pressure; APR: Acute phase reactants; AVP: Arginine vasopressin; CRH: Corticotropin-releasing hormone; E2: Estradiol; EDS: Excessive daytime sleepiness; GH: Growth hormone; HDL: High-density lipoprotein; HPA Axis: Hypothalamic-pituitary-adrenal axis; IGF-1: Insulin-like growth factor-1; IL-6: Interleukin-6; LC: Locus caeruleus; LDL: Low-density lipoprotein; LH: Luteinizing hormone; NE: Norepinephrine; T: Testosterone; T3: Triiodothyronine; TG: Triglyceride; TSH: Thyroid-stimulating hormone. From reference 68.
Glucocorticoids contribute to the pathogenesis of obesity and the metabolic syndrome not only through unfavorable genetic variations that increase both the activity of the HPA axis and the sensitivity of tissues to glucocorticoids, but also because of fetal programming of the HPA axis by an adverse intrauterine environment, which may lead to a postnatally hyperactive axis, and because of chronic cortisol hypersecretion owing to concurrent real or perceived stress.15,21,62
The opposite result is possible as well. Patients may be protected from obesity, the metabolic syndrome, and premature death because of favorable genetic variations causing a decreased activity of their HPA axis and their tissue sensitivity to glucocorticoids, as well as because of normal or opposite fetal programming and decreased exposure to real or perceived stress.15,21,62 In the studies by van Rossum et al. [see chapter in this volume], Syed et al. and others,58,63–66 favorable genetic variations in the GR gene, in which carriers of a particular polymorphism had peripheral target tissues with decreased sensitivity to glucocorticoids, resulted in increased sensitivity of the same tissues to insulin and hence a healthier metabolic profile.
Beyond Glucocorticoids and the Metabolic Syndrome
Despite their obvious importance, glucocorticoids and their signaling system are only one of several physiologic and molecular networks that participate in the development of obesity and the metabolic syndrome, with a resultant adverse effect on longevity.61 Other major hormones of the stress and other systems and their receptors also participate in these phenomena (Figure 4).4,17
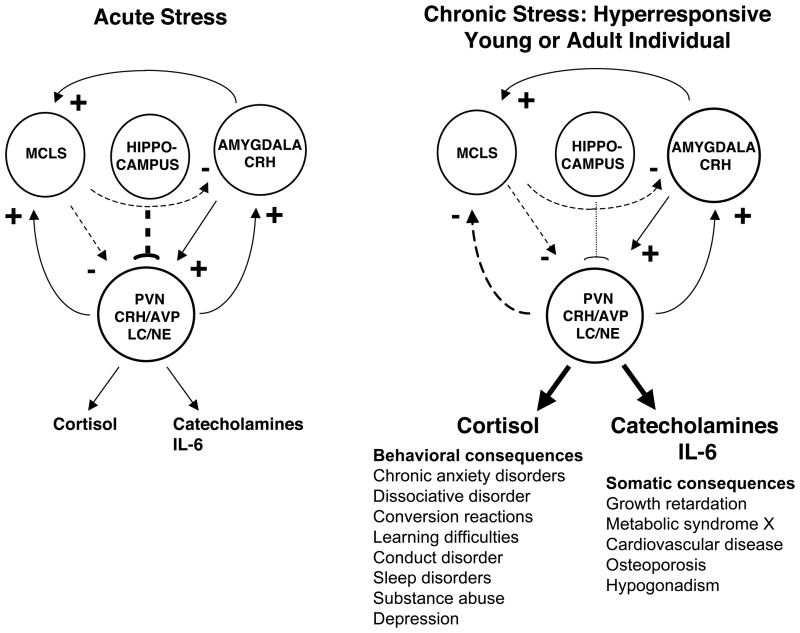
The stress system includes brain nuclei, such as the paraventricular nucleus of the hypothalamus, and the brainstem locus caeruleus norepinephrine/autonomic nervous system nuclei, and two powerful peripheral neuroendocrine limbs, the HPA axis and the systemic sympathetic and adrenomedullary systems.4,50 The stress system normally receives positive regulatory input from the amygdala (fear), negative tonic input from the hippocampus and negative regulation from the mesocorticolimbic dopaminergic system (MCLS, reward), while itself regulates these systems by providing positive influences on all three (Figure 5, left panel).4 Abnormally increased chronic activity and/or reactivity of the stress system can be primary or secondary to excessive input from the amygdala or defective input from the hippocampus and/or MCLS (Figure 5, right panel).4 The normally positive input of the stress system to the MCLS becomes negative in response to chronic hyperactivity of the former, perhaps as a result of the characteristic tolerance of the latter.4
Figure 5.
Central regulation of the stress system in normal (left panel) and chronically stressed and stress hyperresponsive individuals (right panel). The stress system (PVN CRH/AVP and LC/NE) activates the amygdala and the MCLS and receives activating signals from the amygdala and suppressive signals from the MCLS and the hippocampus. Chronic stress has behavioral and somatic consequences summarized in the bottom of right panel. MCLS= Mesocorticolimbic (Reward) System; PVN= Paraventricular nucleus; CRH= Corticotropin-releasing hormone; AVP= Arginine vasopressin. Modified from reference 4.
The main central molecular mediators of the stress system are corticotropin-releasing hormone, arginine vasopressin, and norepinephrine. The key peripheral molecular mediators are corticotropin, cortisol, arginine vasopressin, norepinephrine, epinephrine and, interestingly, interleukin-6 (IL-6).4,15,50 The genes that code for the synthesis, regulation, actions, and metabolism of these mediators and their receptors are major participants in the adaptation to stress.4,15,50 The stress system is activated in a coordinated fashion during acute, time-limited stress, influencing central and peripheral functions that are important for adaptation and survival.4 Chronic activation of the stress system, however, is associated with many negative manifestations and sequelae, beyond obesity/metabolic syndrome, atherosclerosis and loss of bone mineral density, which include a long list of behavioral disorders (Figure 5, right panel, bottom).4
In addition to non-inflammatory stress, even very mild, asymptomatic inflammation stimulates secretion of IL-6 and other inflammatory cytokines, while adipose tissue is a major source of circulating tumor necrosis factor-α and IL-6.14,50,67 Both glucocorticoids and IL-6 synergistically stimulate the acute phase response, including C-reactive protein, fibrinogen, and plasminogen activator inhibitor 1, all of which increase the ability of blood to coagulate and through their pro-atherosclerosis action have a negative effect on longevity.14,50,67 Thus, chronic stress, an indolent infection, an active autoimmune process, and visceral obesity are all associated with mild hypercytokinemia and low-grade inflammation, which ultimately results in blood hypercoagulability, endothelial dysfunction, atherosclerosis, and cardiovascular disease. Finally, it is evident that the metabolic syndrome, regardless of its cause, is a major risk factor for the development of diabetes type 2 and the polycystic ovary syndrome in patients with a genetic propensity to develop these very common disorders.61,68
As a species, we have survived because we were able to adapt to potentially lethal evolutionary stressors during our life on earth. Thus, selective pressures on our genome have allowed adaptive changes that, at this time in our evolutionary history and with our current lifestyle, have become somewhat maladaptive in a large proportion of the population (Table 3).4,22,50,67 Thus, gene networks dedicated to adaptation and survival, with a finite number of members, are probably responsible for much of the contemporary nosology of Western societies presented in Table 3. Even though cancer is not included in this Table, it is likely that modification of the immune system and the inflammatory reaction by stress could increase the susceptibility of the organism to certain neoplasias.
Table 3.
Gene networks subserving functions important for human survival and species preservation, which may produce pathology in contemporary western societies due to changes in lifestyle
| Response to survival threat | Selective advantage | Contemporary diseases |
|---|---|---|
| Combat starvation | Energy conservation | Obesity |
| Combat dehydration | Fluid and electrolyte conservation | Hypertension |
| Combat infectious diseases | Potent immune reaction | Autoimmunity/allergy |
| Anticipate adversaries | Arousal/fear | Anxiety/insomnia |
| Minimize exposure to danger | Withdrawal from danger | Depression |
| Prevent tissue strain and injury | Retain tissue integrity and reserve | Pain and fatigue syndromes |
Conclusions
To understand the roles of polymorphisms of multiple genes related to the HPA axis and the glucocorticoid signaling system in human physiology and pathophysiology, one will have to study large populations of normal subjects, including adequate numbers of representative racial and ethnic subpopulations, as well as populations of patients afflicted by states and diseases that may result from dysfunction of this system, which are summarized in Tables 1 and 3. Once crucial genes and their polymorphisms have been defined, new existing and constantly improving methods could be employed to screen for changes in the entire gene networks of choice, which, in the appropriate context, could predict the relative risk for developing these common disorders. Also, granted that a large subgroup of this gene network plays a major role in regulating immune function, this information could be useful in predicting vulnerability to certain infections and tumors. Finally, this knowledge might help individualize medications and doses for subjects with the above conditions depending on their genetics in a rational way, an effort that is developing into the field of pharmacogenomics.
Acknowledgments
This is a synoptic review of work supported by the University of Athens, Athens Greece, and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD.
References
- 1.Chrousos GP. Glucocorticoid therapy. In: Flig P, Frohman L, editors. Endocrinology and Metabolism. McGraw-Hill; New York, NY: 2001. pp. 609–632. [Google Scholar]
- 2.Franchimont D, et al. Glucocorticoids and inflammation revisited: The state of the art [abstract]. NIH Clinical Staff Conference. Neuroimmunomodulation. 2003;10:247–260. doi: 10.1159/000069969. [DOI] [PubMed] [Google Scholar]
- 3.Galon J, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 5.Wust S, et al. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J Clin Endocrinol Metab. 2004;89:565–573. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
- 6.Buemann B, et al. Abdominal visceral fat is associated with a BclI restriction fragment length polymorphism at the glucocorticoid receptor gene locus. Obes Res. 1997;5:186–192. doi: 10.1002/j.1550-8528.1997.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 7.Dobson MG, et al. The N363S polymorphism of the glucocorticoid receptor: potential contribution to central obesity in men and lack of association with other risk factors for coronary heart disease and diabetes mellitus. J Clin Endocrinol Metab. 2001;86:2270–2274. doi: 10.1210/jcem.86.5.7465. [DOI] [PubMed] [Google Scholar]
- 8.Huizenga NA, et al. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab. 1998;83:144–151. doi: 10.1210/jcem.83.1.4490. [DOI] [PubMed] [Google Scholar]
- 9.Lin RC, Wang WY, Morris BJ. High penetrance, overweight, and glucocorticoid receptor variant: case-control study. BMJ. 1999;319:1337–1338. doi: 10.1136/bmj.319.7221.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panarelli M, et al. Glucocorticoid receptor polymorphism, skin vasoconstriction, and other metabolic intermediate phenotypes in normal human subjects. J Clin Endocrinol Metab. 1998;83:1846–1852. doi: 10.1210/jcem.83.6.4828. [DOI] [PubMed] [Google Scholar]
- 11.Rosmond R, et al. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary-adrenal axis. Obes Res. 2000;8:211–218. doi: 10.1038/oby.2000.24. [DOI] [PubMed] [Google Scholar]
- 12.Ukkola O, et al. Glucocorticoid receptor Bcl I variant is associated with an increased atherogenic profile in response to long-term overfeeding. Atherosclerosis. 2001;157:221–224. doi: 10.1016/s0021-9150(00)00712-7. [DOI] [PubMed] [Google Scholar]
- 13.Weaver JU, Hitman GA, Kopelman PG. An association between a Bc1I restriction fragment length polymorphism of the glucocorticoid receptor locus and hyperinsulinaemia in obese women. J Mol Endocrinol. 1992;9:295–300. doi: 10.1677/jme.0.0090295. [DOI] [PubMed] [Google Scholar]
- 14.Chrousos GP. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann N Y Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- 15.Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24:S50–55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- 16.Chrousos GP, Detera-Wadleigh SD, Karl M. Syndromes of glucocorticoid resistance. Ann Intern Med. 1993;119:1113–1124. doi: 10.7326/0003-4819-119-11-199312010-00009. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 18.Kino T, et al. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. 2003;85:457–467. doi: 10.1016/s0960-0760(03)00218-8. [DOI] [PubMed] [Google Scholar]
- 19.Charmandari E, et al. Generalized glucocorticoid resistance: clinical aspects, molecular mechanisms, and implications of a rare genetic disorder. J Clin Endocrinol Metab. 2008;93:1563–1572. doi: 10.1210/jc.2008-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charmandari E, et al. A novel point mutation in the DNA-binding domain of the human glucocorticoid receptor gene causing generalized glucocorticoid resistance [abstract] Endocrine. 2008;90:280. doi: 10.1210/jc.2006-2830. [DOI] [PubMed] [Google Scholar]
- 21.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 22.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 23.Alevizaki M, et al. High anticipatory stress plasma cortisol levels and sensitivity to glucocorticoids predict severity of coronary artery disease in subjects undergoing coronary angiography. Metabolism. 2007;56:222–226. doi: 10.1016/j.metabol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005;2005:pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- 25.Kino T, Charmandari E, Chrousos GP. Basic and clinical implications of glucocorticoid action -focus on development. National Institutes of Health, Bethesda, Maryland, USA. June 17–18, 2003. Introduction and abstracts. Horm Metab Res. 2003;35:628–648. doi: 10.1055/s-2003-43512-1. [DOI] [PubMed] [Google Scholar]
- 26.Kino T, et al. G protein β interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol. 2005;169:885–896. doi: 10.1083/jcb.200409150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kino T, et al. Rho family guanine nucleotide exchange factor Brx couples extracellular signals to the glucocorticoid signaling system. J Biol Chem. 2006;281:9118–9126. doi: 10.1074/jbc.M509339200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kino T, Chrousos GP. Tumor necrosis factor α receptor- and Fas-associated FLASH inhibit transcriptional activity of the glucocorticoid receptor by binding to and interfering with its interaction with p160 type nuclear receptor coactivators. J Biol Chem. 2003;278:3023–3029. doi: 10.1074/jbc.M209234200. [DOI] [PubMed] [Google Scholar]
- 29.Kino T, et al. Protein 14-3-3σ interacts with and favors cytoplasmic subcellular localization of the glucocorticoid receptor, acting as a negative regulator of the glucocorticoid signaling pathway. J Biol Chem. 2003;278:25651–25656. doi: 10.1074/jbc.M302818200. [DOI] [PubMed] [Google Scholar]
- 30.Kino T, et al. The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. J Exp Med. 1999;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kino T, et al. Human immunodeficiency virus type-1 accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J Virol. 2002;76:9724–9734. doi: 10.1128/JVI.76.19.9724-9734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichijo T, et al. The Smad6-histone deacetylase 3 complex silences the transcriptional activity of the glucocorticoid receptor: potential clinical implications. J Biol Chem. 2005;280:42067–42077. doi: 10.1074/jbc.M509338200. [DOI] [PubMed] [Google Scholar]
- 33.Kino T, et al. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol Endocrinol. 2007;21:1552–1568. doi: 10.1210/me.2006-0345. [DOI] [PubMed] [Google Scholar]
- 34.Kino T. Tissue glucocorticoid sensitivity: beyond stochastic regulation on the diverse actions of glucocorticoids. Horm Metab Res. 2007;39:420–424. doi: 10.1055/s-2007-980193. [DOI] [PubMed] [Google Scholar]
- 35.Krstic MD, et al. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J Biol Chem. 2002;277:26573–26580. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- 38.Miller AL, et al. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19:1569–1583. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- 39.Itoh M, et al. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol. 2002;16:2382–2392. doi: 10.1210/me.2002-0144. [DOI] [PubMed] [Google Scholar]
- 40.Ismaili N, Blind R, Garabedian MJ. Stabilization of the unliganded glucocorticoid receptor by TSG101. J Biol Chem. 2005;280:11120–11126. doi: 10.1074/jbc.M500059200. [DOI] [PubMed] [Google Scholar]
- 41.Kesavapany S, Li BS, Pant HC. Cyclin-dependent kinase 5 in neurofilament function and regulation. Neurosignals. 2003;12:252–264. doi: 10.1159/000074627. [DOI] [PubMed] [Google Scholar]
- 42.Ohshima T, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 44.Tsai LH, et al. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 45.Tang D, Wang JH. Cyclin-dependent kinase 5 (Cdk5) and neuron- specific Cdk5 activators. Prog Cell Cycle Res. 1996;2:205–216. doi: 10.1007/978-1-4615-5873-6_20. [DOI] [PubMed] [Google Scholar]
- 46.Lau LF, et al. Cdk5 as a drug target for the treatment of Alzheimer’s disease. J Mol Neurosci. 2002;19:267–273. doi: 10.1385/JMN:19:3:267. [DOI] [PubMed] [Google Scholar]
- 47.Lee MS, et al. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 48.Kusakawa G, et al. Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem. 2000;275:17166–17172. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- 49.Sun Z, et al. Tumor susceptibility gene 101 protein represses androgen receptor transactivation and interacts with p300. Cancer. 1999;86:689–696. doi: 10.1002/(sici)1097-0142(19990815)86:4<689::aid-cncr19>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 50.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune- mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 51.Kino T, Chrousos GP. Glucocorticoid effects on gene expression. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of stress and the brain. Elsevier B. V; Amsterdam, Netherlands: 2005. pp. 295–311. [Google Scholar]
- 52.Holsboer F, Barden N. Antidepressants and hypothalamic-pituitary- adrenocortical regulation. Endocr Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- 53.Ahlijanian MK, et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci U S A. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patrick GN, et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 55.Friedman TC, et al. Carbohydrate and lipid metabolism in endogenous hypercortisolism: shared features with metabolic syndrome X and NIDDM. Endocr J. 1996;43:645–655. doi: 10.1507/endocrj.43.645. [DOI] [PubMed] [Google Scholar]
- 56.Miller WL, Chrousos GP. The adrenal cortex. In: Felig P, Frohman LA, editors. Endocrinology & Metabolism. McGraw-Hill; New York, NY: 2001. pp. 387–524. [Google Scholar]
- 57.Buemann B, et al. The N363S polymorphism of the glucocorticoid receptor and metabolic syndrome factors in men. Obes Res. 2005;13:862–867. doi: 10.1038/oby.2005.99. [DOI] [PubMed] [Google Scholar]
- 58.DeRijk R, de Kloet ER. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine. 2005;28:263–270. doi: 10.1385/ENDO:28:3:263. [DOI] [PubMed] [Google Scholar]
- 59.Marti A, et al. Meta-analysis on the effect of the N363S polymorphism of the glucocorticoid receptor gene (GRL) on human obesity. BMC Med Genet. 2006;7:50–60. doi: 10.1186/1471-2350-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens A, et al. Glucocorticoid sensitivity is determined by a specific glucocorticoid receptor haplotype. J Clin Endocrinol Metab. 2004;89:892–897. doi: 10.1210/jc.2003-031235. [DOI] [PubMed] [Google Scholar]
- 61.Chrousos GP. The glucocorticoid receptor gene, longevity, and the complex disorders of Western societies. Am J Med. 2004;117:204–207. doi: 10.1016/j.amjmed.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Phillips DI, et al. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab. 1998;83:757–760. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- 63.Syed AA, et al. Association of glucocorticoid receptor polymorphism A3669G in exon 9β with reduced central adiposity in women. Obesity (Silver Spring) 2006;14:759–764. doi: 10.1038/oby.2006.86. [DOI] [PubMed] [Google Scholar]
- 64.van Rossum EF, et al. A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels. Diabetes. 2002;51:3128–3134. doi: 10.2337/diabetes.51.10.3128. [DOI] [PubMed] [Google Scholar]
- 65.van Rossum EF, Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res. 2004;59:333–357. doi: 10.1210/rp.59.1.333. [DOI] [PubMed] [Google Scholar]
- 66.van Rossum EF, et al. The ER22/23EK polymorphism in the glucocorticoid receptor gene is associated with a beneficial body composition and muscle strength in young adults. J Clin Endocrinol Metab. 2004;89:4004–4009. doi: 10.1210/jc.2003-031422. [DOI] [PubMed] [Google Scholar]
- 67.Papanicolaou DA, et al. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 68.Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- 69.Kino T, Ichijo T, Chrousos GP. FLASH interacts with p160 coactivator subtypes and differentially suppresses transcriptional activity of steroid hormone receptors. J Steroid Biochem Mol Biol. 2004;92:357–363. doi: 10.1016/j.jsbmb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Lanz RB, et al. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97:17–27. doi: 10.1016/s0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- 71.Kino T, Chrousos GP. Glucocorticoid and mineralocorticoid receptors and associated diseases. Essays Biochem. 2004;40:137–155. doi: 10.1042/bse0400137. [DOI] [PubMed] [Google Scholar]
- 72.De Martino MU, et al. The glucocorticoid receptor and the orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II interact with and mutually affect each other’s transcriptional activities: implications for intermediary metabolism. Mol Endocrinol. 2004;18:820–833. doi: 10.1210/me.2003-0341. [DOI] [PubMed] [Google Scholar]
- 73.Charmandari E, Kino T, Chrousos GP. Glucocorticoids and their actions: an introduction. Ann N Y Acad Sci. 2004;1024:1–8. doi: 10.1196/annals.1321.001. [DOI] [PubMed] [Google Scholar]
- 74.Kino T, Vottero A, Chrousos GP. Mineralocorticoid and glucocorticoid receptors. In: Burris TP, McCabe ERB, editors. Nuclear Receptor and Genetic Disease. Academic Press; London, England: 2001. pp. 297–307. [Google Scholar]