Abstract
We evaluated reported contraindications to early beta-blocker use and associated mortality within and across patient age groups. Contraindications to early beta-blocker use were evaluated in CRUSADE patients with non-ST elevation acute coronary syndrome from 2/2003–12/2006. Prevalence, reasons, and trends in contraindications were evaluated by age (≤65, 66–74, ≥75). Associations between reported contraindications and in-hospital mortality were determined within and compared across age groups using logistic generalized estimating equations method adjusting for baseline patient characteristics. Of 112,448 patients, 11,711 (10.4%) had a reported contraindication to early beta-blocker. Prevalence varied by age (≤65, 7.9%; 66–74, 10.6%; ≥75, 13.4%; P<0.0001); there were no significant changes over time except for a small increase in patients ≤65 (P=0.001). Among hospitals with >40 patients in the registry, the median hospital-level rate of reported contraindications was 9.9% (IQR: 6.7–14.3%). The distribution of rates was more widespread among the hospitals’ older versus younger patients. In all age groups, there was a statistically significant greater association with in-hospital mortality in those with reported contraindications versus those without contraindications who received a beta-blocker (adjusted OR [95% CI]: 2.81 [2.28–3.46]; 2.50 [2.07–3.03]; 2.11 [1.88–2.37], for ages ≤65, 66–74, and ≥ 75); the strength of the association was similar across age groups (interaction P=0.19). Reported contraindications to early beta-blocker use were common and increased with age. Contraindications were independently associated with higher in-hospital mortality, underscoring the importance of accurately identifying contraindications. In conclusion, the results did not indicate any disparity in reporting contraindications based upon patient age.
Keywords: beta-blockers, contraindications, non-ST elevation acute coronary syndrome, age, mortality
This study evaluated reported contraindications to the use of beta-blockers within 24 hours of symptom onset in patients with non-ST elevation (NSTE) ACS to determine frequency, trends, and types of reported contraindications in the overall analysis cohort and by patient age. In addition, the association between reported contraindications and in-hospital mortality was determined and compared across age groups to explore the possibility of age-related disparities.
Methods
The study cohort was obtained from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines) Quality Improvement Initiative, which contains data from patients hospitalized for NSTE ACS at participating U.S. hospitals. Collected data elements, inclusion criteria, and registry processes have been previously presented.1 Patients who were transferred out of the admitting hospital or died within 24 hours of admission, or had missing information on the use of beta-blocker within the first 24 hours of hospitalization or age were excluded from the study. The institutional review board of each participating hospital approved its organization’s participation in CRUSADE. Data were collected anonymously without patient informed consent.
Administration of an intravenous or oral beta-blocker within 24 hours of hospital admission was considered early beta-blocker use. If no early beta-blocker was administered, presence of a contraindication, as recorded in the medical record, was collected. Only 1 contraindication for each patient was recorded. If more than 1 contraindication to early beta-blocker use was present, the most substantial one was selected by the chart abstracter.
Choices on the data collection form for contraindications included: allergy/intolerance/hypersensitivity; hypotension; bradycardia, heart block >first-degree heart block; cardiogenic shock; chronic obstructive pulmonary disease (COPD)/asthma/bronchospasm; pulmonary edema; severe comorbid illness; do not resuscitate orders; enrollment in a clinical trial; and other. In an early version of the data collection form, active bleeding was included as a choice, but it was removed in later versions.
The outcome measure for this study was in-hospital death. Patients who were transferred out of the admitting hospital after 24 hours were excluded from the analyses.
The prevalence of reported contraindications to early beta-blocker and the specific reason for the contraindication were reported in the overall analysis cohort and by patient age group. For this study, the population was divided by age: ≤65 years; 66–74 years; ≥75 years.. Baseline patient and hospital characteristics were compared between those with and without contraindications. Medians (interquartile ranges [IQR]) were reported for continuous variables, and percentages were reported for categorical variables. To test for differences in baseline patient characteristics, hospital characteristics, and clinical presentation between those patients with and without reported contraindications to early beta-blocker therapy, continuous and ordinal categorical variables were compared using stratum-adjusted Wilcoxon rank-sum tests, while nominal categorical variables were compared using stratum-adjusted chi-square tests where stratification was by hospital. To determine if recorded vital sign measurements confirmed the reason for the recorded contraindication, the median (IQR) systolic blood pressure recorded on admission for patients who received early beta-blocker, did not receive early beta-blocker but had no contraindications, had hypotension listed as a contraindication, and had a reason other than hypotension listed as a contraindication were compared across age groups. A similar analysis was performed on recorded heart rate on admission to examine the report of bradycardia as a contraindication. In addition, variations in reported contraindications at the hospital level were explored for the overall analysis population and by age group by calculating the proportion of reported contraindications for each hospital.
To investigate the association between reported contraindications to early beta-blocker use and in-hospital mortality by age groups, logistic generalized estimating equations (GEE) method with exchangeable working correlation matrix was used to account for within-hospital clustering, where patients at the same hospital were more likely to have similar responses relative to patients in other hospitals (i.e., within-center correlation for response).2 In these models, early beta-blocker use status was defined as: 1) reported contraindication to early beta-blocker; 2) no reported contraindication but no early beta-blocker use; or 3) early intravenous or oral beta-blocker use (as the reference group). Three models were performed, each including age groups (≤65, 66–74, ≥75 years), early beta-blocker use status (as defined above), and interactions between age groups and early beta-blocker use status. The second and third models included different sets of covariate adjustment. In the fully adjusted model (third model including all factors), the following factors were included: sex (male vs. female), continuous body mass index, race (white vs. non-white), family history of coronary artery disease, history of hypertension, diabetes mellitus, current or recent smoking history, hypercholesterolemia, prior MI, prior percutaneous coronary intervention, prior coronary artery bypass grafting, prior stroke, renal insufficiency (defined as creatinine >2 mg/dL, estimated creatinine clearance <50 mL/min, or need for dialysis), ST depression, transient ST elevation, positive cardiac markers, systolic blood pressure at admission, heart rate at admission, prior heart failure, and signs of heart failure on admission. These factors, along with age, have been previously shown to be associated with mortality risk in NSTEMI patients.3
Because hypotension, bradycardia, and heart failure are potential contraindications to the use of early beta-blockers, the second model (excluding factors that are contraindications to beta-blocker use) was constructed to exclude potentially correlated variables (systolic blood pressure, heart rate, prior heart failure, and signs of heart failure on admission). Within each model, the odds ratios were compared and an interaction P value was calculated to determine the differences in associations between reported contraindications and in-hospital death across age groups. The average percentage of missing data was less than 2% for the baseline covariates. Missing values were imputed to the lower-risk groups for discrete variables and replaced with sex-specific medians for continuous variables.
Lastly, in determining association between contraindications to early beta-blocker and patient year of hospital presentation stratified by age group, patient year of hospital presentation was entered as an ordinal independent variable in the logistic GEE models to test for a linear trend. A P value <0.05 was considered statistically significant for all tests and all tests were 2-tailed. No adjustments were made for multiple comparisons. All analyses were performed using SAS software (version 9.1, SAS Institute, Cary, NC).
Results
Overall, 118,252 patients with a hospital admission for NSTE ACS from February 15, 2003 through December 2006 were identified from the CRUSADE registry. A total of 5804 (4.9%) patients were excluded for the following reasons (in sequential order of review): transferred to another hospital within 24 hours of admission (4380); death within 24 hours of admission (672); missing data on early beta-blocker use (580); and missing age (172). The final study cohort of 112,448 was derived from a total of 489 hospitals. Of these patients, 50,237 (45%) were ≤65 years of age, 22,312 (20%) were aged 66–74 years, and 39,899 (35%) were ≥75 years. Baseline patient and hospital characteristics for the overall population and with and without a reported contraindication to early beta-blocker are presented in Table 1.
Table 1.
Patients with and without reported contraindications to early beta-blocker during ACS hospitalization.
| Variable | Total population (n=112,448) | Reported Contraindication to Beta-blocker | P value | |
|---|---|---|---|---|
| No (n=100,737) | Yes (n=11,711) | |||
| Age (years) | 68 (56, 79) | 67 (56, 78) | 73 (61, 82) | <0.0001 |
| BMI (kg/m2) | 27.9 (24.4, 32.2) | 28.0 (24.6, 32.3) | 26.7 (23.2, 30.8) | <0.0001 |
| Male | 59.9% | 60.2% | 56.9% | <0.0001 |
| Race | ||||
| White | 80.2% | 80.0% | 82.1% | |
| Black | 10.5% | 10.5% | 9.7% | <0.0001 |
| Hispanic | 4.1% | 4.2% | 3.9% | |
| Asian | 1.2% | 1.1% | 1.3% | |
| Other | 2.4% | 2.4% | 1.6% | |
| Missing | 1.7% | 1.7% | 1.4% | |
| Insurance status | ||||
| HMO/Private | 43.8% | 44.4% | 38.3% | |
| Medicare | 44.1% | 43.3% | 51.8% | |
| Military/VAMC | 1.3% | 1.4% | 1.2% | <0.0001 |
| Medicaid | 3.9% | 4.0% | 3.4% | |
| Self/None | 6.1% | 6.3% | 4.6% | |
| Missing | 0.7% | 0.8% | 0.7% | |
| History of hypertension | 71.3% | 71.7% | 67.9% | <0.0001 |
| Diabetes mellitus | 33.5% | 33.7% | 31.7% | <0.0001 |
| Peripheral artery disease | 11.6% | 11.3% | 14.1% | <0.0001 |
| Renal insufficiency | 13.8% | 13.2% | 18.3% | <0.0001 |
| Hyperlipidemia | 52.5% | 53.1% | 47.1% | <0.0001 |
| Prior myocardial infarction | 29.0% | 28.9% | 29.4% | 0.28 |
| Prior PCI | 21.6% | 22.0% | 18.4% | <0.0001 |
| Prior CABG | 19.2% | 19.4% | 18.2% | 0.001 |
| Prior stroke | 10.2% | 10.0% | 12.4% | <0.0001 |
| Prior heart failure | 17.3% | 16.5% | 24.4% | <0.0001 |
| Current/recent smoker | 27.1% | 27.2% | 25.9% | 0.009 |
| Heart failure symptoms on admission | 23.6% | 22.3% | 35.7% | <0.0001 |
| Heart rate on admission (bpm) | 83 (70, 99) | 83 (71, 98) | 81 (64, 102) | <0.0001 |
| Systolic BP on admission (mm Hg) | 144 (124, 165) | 145 (126, 166) | 132 (109, 154) | <0.0001 |
| Teaching hospital | 31.7% | 32.0% | 28.8% | <0.0001 |
| Hospital region | ||||
| West | 11.1% | 10.7% | 15.2% | |
| Northeast | 22.8% | 23.0% | 21.4% | |
| Midwest | 35.9% | 36.1% | 34.2% | <0.0001 |
| South | 30.1% | 30.2% | 29.2% | |
| Missing | 0.1% | 0.1% | 0.1% | |
Continuous variables are presented as median (25th, 75th). BMI = body mass index; bpm = beats per minute; CABG = coronary artery bypass grafting; HMO = health maintenance organization; PCI = percutaneous coronary intervention; BP = blood pressure; VAMC = Veterans Administration Medical Center.
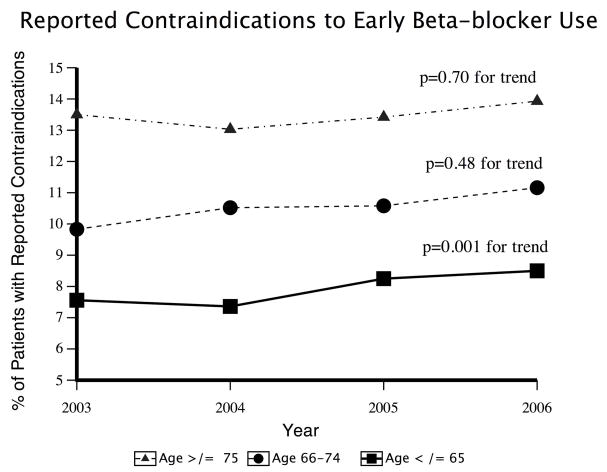
A total of 11,711 (10.4%) patients had a contraindication to early beta-blocker recorded. There was a statistically significant difference in the proportion of patients with a reported contraindication by age group (7.9% of those ≤65, 10.6% of those 66–74, and 13.4% of those ≥75; P<0.0001). The proportions of patients with reported contraindications by year of hospital presentation stratified by age group are presented in Figure 1.
Figure 1.
Reported contraindications to early beta-blocker use.
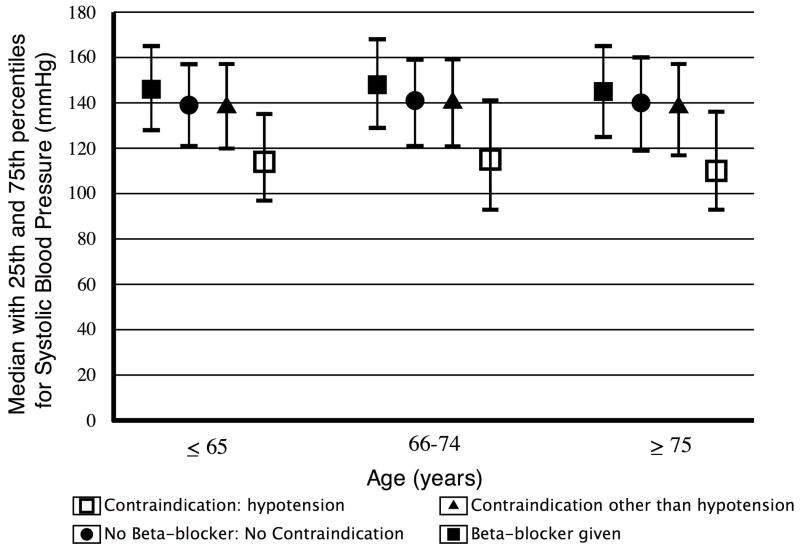
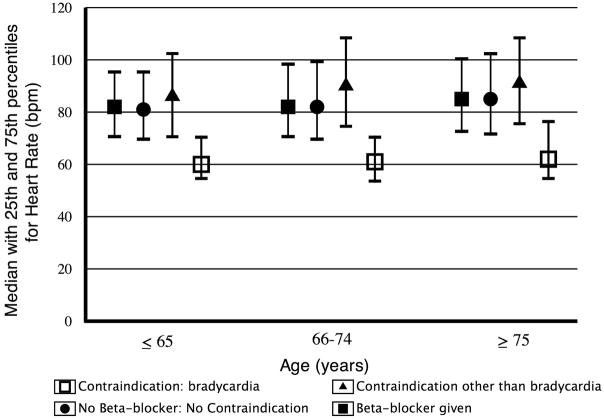
The proportions of patients with each cited reason for the recorded contraindication within each age group are presented in Table 2. Patients with reported hypotension were found to have a lower admission systolic blood pressure within all age groups (Figure 2) than patients who did not have hypotension reported as a contraindication. Likewise, patients with reported bradycardia also had a lower admission heart rate within all age groups (Figure 3).
Table 2.
Rates of specific contraindications by age group.
| Age (years) | ||||
|---|---|---|---|---|
| Reported reason | ≤65 (n=50,237) | 66–74 (n=22,312) | ≥75 (n=39,899) | P value |
| Hypotension | 2.40% | 2.99% | 4.14% | <0.001 |
| Bradycardia | 2.59% | 2.81% | 2.78% | 0.005 |
| Respiratory | 1.10% | 2.16% | 2.10% | <0.001 |
| Pulmonary edema | 0.32% | 0.62% | 1.05% | <0.001 |
| Heart block | 0.16% | 0.25% | 0.45% | <0.001 |
| Shock | 0.14% | 0.25% | 0.24% | 0.068 |
| Severe comorbidities | 0.12% | 0.23% | 0.36% | <0.001 |
| Allergy | 0.16% | 0.30% | 0.33% | <0.001 |
| Active bleeding | 0.04% | 0.13% | 0.16% | <0.001 |
| Do not resuscitate status | 0.03% | 0.12% | 0.78% | <0.001 |
| Other | 0.79% | 0.67% | 0.89% | <0.001 |
| Reason not stated | 0.09% | 0.09% | 0.13% | 0.009 |
Figure 2.
Admission systolic blood pressure by age group and early beta-blocker use status.
Figure 3.
Admission heart rate by age group and early beta-blocker use status. Bpm = beats per minute.
Of 489 hospitals, 98 had ≤40 patients within the registry during the study period and were excluded from the analysis exploring hospital-level variations in reported contraindications to early beta-blocker. The median hospital-level rate of reported contraindications was 9.9% (IQR: 6.7–14.3%) and was higher among older versus younger patients (7.7% for age ≤65; 10.3% for age 66–74; and 12.6% for age ≥75; P< 0.0001). In addition, the distribution of median hospital-level rates of reported contraindications was more widespread among the hospitals’ older versus younger patients (Figure 4).
Figure 4.
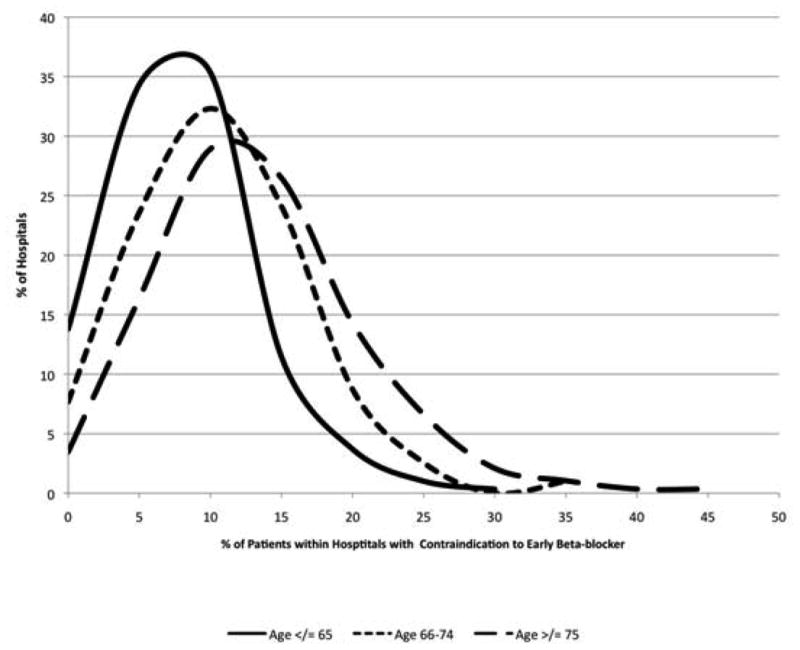
Distribution of reported hospital-level rates of contraindications to early beta-blockers stratified by age group.
A total of 102,870 patients were included in the mortality analyses. A total of 3557 (3.5%) patients died during hospitalization. The rate of in-hospital mortality was higher among those with a reported contraindication to early beta-blocker than without (9.2% vs. 2.8%; P<0.0001). In-hospital mortality was statistically significantly greater in those with reported contraindications to early beta-blocker than in those with early beta-blocker use in both the unadjusted and adjusted models for all age groups (Table 3). The odds of death associated with reported contraindications to early beta-blocker versus use of early beta-blocker was lower in the oldest age group as compared with the younger age groups, but the interaction P values did not reach statistical significance. These results were similar in all 3 models (Table 3).
Table 3.
Association between early beta-blocker use status and mortality among age groups.
| Variable | OR (95% CI) | P value (interaction) |
|---|---|---|
| Unadjusted model* | ||
| Contraindication | ||
| Among age ≤65 years | 3.94 (3.21, 4.85) | |
| Among age 66–74 years | 3.43 (2.85, 4.13) | 0.14 |
| Among age ≥75 years | 2.93 (2.61, 3.28) | |
| No early beta-blocker/no contraindication | ||
| Among age ≤65 years | 1.56 (1.19, 2.06) | |
| Among age 66–74 years | 1.54 (1.17, 2.02) | |
| Among age ≥75 years | 1.30 (1.09, 1.54) | |
| Adjusted model (excluding factors that are contraindications to beta-blocker use)† | ||
| Contraindication | ||
| Among age ≤65 years | 3.74 (3.05, 4.58) | |
| Among age 66–74 years | 3.25 (2.70, 3.91) | 0.11 |
| Among age ≥75 years | 2.73 (2.44, 3.07) | |
| No early beta-blocker/no contraindication | ||
| Among age ≤65 years | 1.58 (1.20, 2.08) | |
| Among age 66–74 years | 1.59 (1.20, 2.10) | |
| Among age ≥75 years | 1.34 (1.13, 1.59) | |
| Adjusted model (all factors)‡ | ||
| Contraindication | ||
| Among age ≤65 years | 2.81 (2.28, 3.46) | |
| Among age 66–74 years | 2.50 (2.07, 3.03) | 0.19 |
| Among age ≥75 years | 2.11 (1.88, 2.37) | |
| No early beta-blocker/no contraindication | ||
| Among age ≤65 years | 1.42 (1.07, 1.87) | |
| Among age 66–74 years | 1.42 (1.07, 1.88) | |
| Among age ≥75 years | 1.24 (1.05, 1.47) | |
Reference group is use of early beta-blocker.
Includes interaction between age group and early beta-blocker use status.
Includes interaction between age group and reported contraindications with early beta-blocker; male sex, BMI, white race, family history of CAD, hypertension, diabetes, current/recent smoker, hypercholesterolemia, prior MI, prior PCI, prior CABG, renal insufficiency, ST depression, transient ST elevation, positive cardiac markers.
Includes all of the above variables plus systolic blood pressure, heart rate, prior heart failure, and signs of heart failure. CI = confidence interval; OR = odds ratio.
Discussion
In this study we found a statistically significant greater odds of in-hospital mortality associated with reported contraindications to early beta-blocker when compared with early beta-blocker use in all age groups and that the association did not significantly vary across age groups. Whether the greater odds of in-hospital mortality were due to higher risk of death because no early beta-blocker was administered and/or the reported contraindication was a marker for a potentially life-threatening comorbidity, these findings highlight the need for a better understanding of how to accurately and equitably identify contraindications to early beta-blocker use in clinical practice.4–12
Because older patients have more comorbidities and more advanced disease than younger patients, challenges associated with determining if an absolute contraindication is present disproportionately affect older patients. In an observational study, 59% of Medicare beneficiaries with acute MI were eliminated from the analysis because of a possible contraindication to beta-blocker.13 The authors concluded that additional research was critical for determining the future role of beta-blockers in those patients considered to have contraindications because contraindications were reported in such a large number of patients.
Issues related to accurate identification of contraindications to early beta-blockers were highlighted by revisions in guideline recommendations and the removal of the clinical practice performance measures for use of early beta-blocker following ACS.14–16 One of the key studies that led to these changes was COMMIT.17 While there was no statistically significant difference in the composite endpoint of death, reinfarction, ventricular fibrillation, or other arrest between those receiving early beta-blocker versus placebo, there was a statistically significant higher risk of death due to cardiogenic shock in the group that received early beta-blocker.10 Clinical practice guidelines were subsequently revised to include greater consideration of shock risk factors derived from COMMIT (age ≥70 years, systolic blood pressure <120 mm Hg, heart rate >110 beats per minute [bpm], heart rate <60 bpm, or increased time between onset of symptoms) as a contraindication to early beta-blocker use.14, 15 In addition, the ACC/AHA performance measures for STEMI/NSTEMI were revised to remove the use of early beta-blockers from the performance measures list.16 Despite these changes, considerable differences in the patient population and dosing in COMMIT as compared with the patient population and dosing in clinical practice in the U.S. have led to additional questions and the need for additional research.
In clinical practice, it is difficult to determine if a medication contraindication exists, and it is even more challenging to assess the validity of a condition that was documented in the medical record to be a contraindication. Because of these challenges, assessments of adherence to guidelines exclude all patients with recorded contraindications regardless of the stated reason and without substantiation of the recorded reason.16 The results of this study might also be used to explore the extent to which criteria used in clinical practice to determine if a contraindication exists are uniformly applied across age groups.18–21 Except for medication allergies, the presence of a true contraindication indicates the presence of comorbidity and is expected to be associated with worse clinical outcomes. Factors previously identified to be associated with in-hospital mortality in patients with NSTEMI have been determined.3 Therefore, by adding early beta-blocker use status to the model, we were able to assess the relative association of a reported contraindication to in-hospital mortality across age groups. If a lower threshold for reporting a contraindication to early beta-blocker existed in older versus younger patients, we would expect to see a statistically significant smaller association between the presence of a reported contraindication and in-hospital mortality in older versus younger patients. In this study, there was no statistically significant difference in the association between reported contraindications and in-hospital mortality across age groups, which may indicate that there was no differential reporting of contraindications based solely on patient age. To our knowledge, this method has not previously been used to assess a threshold effect and requires additional research.
There are several limitations to this study. First, reported contraindications were retrospectively collected and not adjudicated. Because only a single contraindication was recorded for each patient, it is unknown what role the presence of multiple comorbidities played in the decision to label the patient as having a contraindication. Use of intravenous versus oral early beta-blockers was not collected in the registry.
Acknowledgments
CRUSADE is funded by the Schering-Plough Corporation. Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership provides additional funding support. Millennium Pharmaceuticals, Inc. also funded this work. This work was also supported in part by a grant from the National Institute on Aging (5R01 AG025312, PI Peterson). The authors are solely responsible for the design and conduct of this study, all study analyses, and the drafting and editing of the paper and its final contents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoekstra JW, Pollack CV, Jr, Roe MT, Peterson ED, Brindis R, Harrington RA, Christenson RH, Smith SC, Ohman EM, Gibler WB. Improving the care of patients with non–ST-elevation acute coronary syndromes in the emergency department: the CRUSADE initiative. Acad Emerg Med. 2002;9:1146–1155. doi: 10.1111/j.1553-2712.2002.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 2.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 3.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 4.Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–807. doi: 10.1056/NEJM198104023041401. [DOI] [PubMed] [Google Scholar]
- 5.Hjalmarson A, Elmfeldt D, Herlitz J, Holmberg S, Málek I, Nyberg G, Rydén L, Swedberg K, Vedin A, Waagstein F, Waldenström A, Waldenström J, Wedel H, Wilhelmsen L, Wilhelmsson C. Effect on mortality of metoprolol in acute myocardial infarction. A double-blind randomised trial. Lancet. 1981;2:823–827. doi: 10.1016/s0140-6736(81)91101-6. [DOI] [PubMed] [Google Scholar]
- 6.Beta-blocker Heart Attack Trial Research Group. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247:1707–1714. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 7.MIAMI Trial Research Group. Metoprolol in acute myocardial infarction (MIAMI). A randomised placebo-controlled international trial. Eur Heart J. 1985;6:199–211. [PubMed] [Google Scholar]
- 8.TIMI Study Group. Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) phase II trial. N Engl J Med. 1989;320:618–627. doi: 10.1056/NEJM198903093201002. [DOI] [PubMed] [Google Scholar]
- 9.Roberts R, Rogers WJ, Mueller HS, Lambrew CT, Diver DJ, Smith HC, Willerson JT, Knatterud GL, Forman S, Passamani E. Immediate versus deferred beta-blockade following thrombolytic therapy in patients with acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) II-B Study. Circulation. 1991;83:422–437. doi: 10.1161/01.cir.83.2.422. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Goldstein S. Beta-blockers in the post-myocardial infarction patient. Circulation. 2002;106:394–398. doi: 10.1161/01.cir.0000019582.39797.ef. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med. 1998;339:489–497. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RJ, Spencer FA, Steg PG, Flather M, Montalescot G, Gurfinkel EP, Kennelly BM, Goodman SG, Dedrick R, Gore JM. Increasing use of single and combination medical therapy in patients hospitalized for acute myocardial infarction in the 21st century: a multinational perspective. Arch Intern Med. 2007;167:1766–1773. doi: 10.1001/archinte.167.16.1766. [DOI] [PubMed] [Google Scholar]
- 13.Krumholz HM, Radford MJ, Wang Y, Chen J, Marciniak TA. Early beta-blocker therapy for acute myocardial infarction in elderly patients. Ann Intern Med. 1999;131:648–654. doi: 10.7326/0003-4819-131-9-199911020-00003. [DOI] [PubMed] [Google Scholar]
- 14.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert SS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the ACC/AHA Task Force on Practice Guidelines. J Am Coll Cardiol. 2004;44:e1–e211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Adams CD, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the ACC/AHA Task Force on Practice Guidelines. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, Ho PM, Kosiborod MN, Masoudi FA, Nallamothu BK. ACC/AHA 2008 performance measures for adults with ST-elevation and non–ST-elevation myocardial infarction: a report of the ACC/AHA Task Force on Performance Measures. J Am Coll Cardiol. 2008;52:2046–2099. doi: 10.1016/j.jacc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, Xie JX, Liu LS. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1622–1632. doi: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 18.Setoguchi S, Glynn RJ, Avorn J, Levin R, Winkelmayer WC. Ten-year trends of cardiovascular drug use after myocardial infarction among community-dwelling persons > or =65 years of age. Am J Cardiol. 2007;100:1061–1067. doi: 10.1016/j.amjcard.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 19.Vega DD, Dolan KL, Pollack ML. Beta-blocker use in elderly ED patients with acute myocardial infarction. Am J Emerg Med. 2006;24:435–439. doi: 10.1016/j.ajem.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Alexander KP, Newby LK, Armstrong PW, Cannon CP, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the AHA Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. [DOI] [PubMed] [Google Scholar]
- 21.Skolnick AH, Alexander KP, Chen AY, Roe MT, Pollack CV, Jr, Ohman EM, Rumsfeld JS, Gibler WB, Peterson ED, Cohen DJ. Characteristics, management, and outcomes of 5,557 patients age > or =90 years with acute coronary syndromes: results from the CRUSADE Initiative. J Am Coll Cardiol. 2007;49:1790–1797. doi: 10.1016/j.jacc.2007.01.066. [DOI] [PubMed] [Google Scholar]