Summary
Evidence suggests that p190RhoGAP (p190), a GTPase activating protein (GAP) specific for Rho, plays a role in cytokinesis. First, ectopic expression of p190 induces a multinucleated cellular phenotype. Second, endogenous p190 localizes to the cleavage furrow of dividing cells. Lastly, its levels are reduced in late mitosis by ubiquitin-mediated proteasomal degradation, consistent with the idea that low levels of p190 and high levels of active Rho are required for completion of cytokinesis. As with p190, RhoA and the RhoGEF, ECT2, have been localized to the cleavage furrow. These findings raise the question of whether p190 and ECT2 cooperate antagonistically to regulate the activity of Rho and contraction of the actomyosin ring during cytokinesis. Here we demonstrate ECT2 can, in a dose-dependent manner, reduce multinucleation induced by p190. Furthermore, endogenous p190 and ECT2 colocalize at the cleavage furrow of dividing cells and stably associate with one another in co-immunoprecipitation assays. Functional and physical interactions between p190 and ECT2 are reflected in the levels of Rho activity, as assessed by Rho pull-down assays. Together, these results suggest that co-regulation of Rho activity by p190RhoGAP and ECT2 in the cleavage furrow determines whether cells properly complete cytokinesis.
Keywords: p190RhoGAP, Ect2 RhoGEF, Rho, cytokinesis, mitosis, cell cycle
Introduction
Cytokinesis is the last step in mitosis in which cells physically divide to form two daughter cells. This process is initiated by specification of the cleavage plane and continues with assembly of the contractile ring components, furrow ingression, formation of the midbody, and cell separation (Eggert et al., 2006; Glotzer, 2004; Glotzer, 2005). Defects in cytokinesis may lead to apoptosis and/or endoduplication, the latter frequently resulting in a multinucleated phenotype. Although cytokinesis is known to be a highly dynamic process involving the actomyosin network, how this network and the overall process are regulated has not yet been fully elucidated.
Small GTPases are known to play important roles in cell adhesion, motility, and contraction through regulation of the actin/microtubule cytoskeleton (Etienne-Manneville and Hall, 2002). Within the family of small GTPases, Rho has been most extensively studied in mitosis, such that misregulation of Rho and its downstream effectors have been shown to induce multinucleation (Kishi et al., 1993; Mabuchi et al., 1993). Specifically, a dominant negative mutant of Rho or silencing of Rho by siRNA leads to abrogation of furrow ingression and results in multinucleation (Drechsel et al., 1997; Jantsch-Plunger et al., 2000; O'Connell et al., 1999). SiRNA-mediated silencing of formin proteins, effectors of Rho that regulate actin filament polymerization as well as microtubule nucleation and elongation, results in multinucleation, as does knock-down of ROCK which phosphorylates myosin light chains and/or myosin phosphatases to regulate actomyosin contractility (Kimura et al., 1998; Tominaga et al., 2000). These studies indicate that Rho signaling pathways play substantial roles in mitosis.
Not only Rho and Rho effectors, but also upstream regulators of Rho, such as Rho guanine nucleotide exchange factors (RhoGEFs), Rho GTPase activating proteins (RhoGAPs), and Rho guanine nucleotide dissociation inhibitors (RhoGDIs), (Garcia-Mata and Burridge, 2007; Tcherkezian and Lamarche-Vane, 2007; Dovas and Couchman, 2005) have been implicated in the regulation of mitosis.
One of the well-characterized RhoGEFs, ECT2, has been shown to function in both early and late stages of mitosis. ECT2 was originally discovered as a proto-oncogene consisting of two BRCT domain repeats, a DBL homology (DH) domain, and a pleckstrin homology (PH) domain, the latter two required for GEF activity towards Rho (Kimura et al., 2000). Overexpression of the N-terminal half of ECT2 (dominant negative), microinjection of ECT2 antibody, or silencing of ECT2 by siRNA induces multinucleation, indicative of cytokinesis failure (Kim et al., 2005; Tatsumoto et al., 1999; Yuce et al., 2005). In late metaphase (when the cleavage plane is determined), inactivation of ECT2 leads to downregulation of RhoA activity, accumulation of contractile ring components at the furrow initiation site, and failure of furrow ingression (Kimura et al., 2000). These events are regulated not only by ECT2 but also other components of the centraspindlin complex, which include MgcRacGAP and MKLP1 (Yuce et al., 2005). Depletion of MLKP1 does not affect accumulation of RhoA, F-actin, and myosin on the cell equator; however, it prevents concentration of ECT2 at the site. MgcRacGAP is required for ECT2 activation so it in turn can recruit and activate RhoA at the cleavage furrow.
Recently, p190RhoGAP-A (p190), a member of the large RhoGAP family (Tcherkezian and Lamarche-Vane, 2007), was identified as a regulator of cytokinesis (Su et al., 2003). P190 is a multi-domain protein (Settleman et al., 1992) that contains a GTP Binding Domain (GBD) at the N-terminus, a large protein-protein interaction domain in the middle of the molecule, and a GTPase Activating Domain (GAP) at the C-terminus . In intact cells, p190 displays specificity for RhoA as compared to Cdc42 and Rac (Haskell et al., 2001; Ridley et al., 1993), and inactivation of RhoA by p190 leads to actin cytoskeleton disassembly (Chang et al., 1995; Fincham et al., 1999). As a consequence of its ability to regulate actin cytoskeleton dynamics, p190 has been implicated in many cellular processes such as migration, invasion, tumor suppression, neuronal morphogenesis (Arthur and Burridge, 2001; Brouns et al., 2000; Brouns et al., 2001; Nakahara et al., 1998; Tikoo et al., 2000; Wang et al., 1997; Zrihan-Licht et al., 2000), and most recently, cytokinesis (Su et al., 2003). Specifically, ectopic expression of p190 induces multinucleation, and its RhoGAP domain is essential for this phenomenon. Endogenous p190 localizes to sites of cleavage furrow initiation in late metaphase/early anaphase, and to the ingressing furrow, structures that also contain Rho. In late-stage mitosis, p190 levels are reduced by ubiquitin-mediated proteosome degradation, suggesting that low p190 activity and high RhoGTP are essential for proper completion of cytokinesis.
The present study was undertaken to determine whether the antagonistic actions of ECT2 and p190 on Rho can co-regulate cytokinesis. We demonstrate that ECT2 can in a dose-dependent fashion reverse multinucleation induced by overexpressed p190 that endogenous ECT2 and p190 co-localize and co-immunoprecipitate during mitosis, and that RhoGTP levels correlate with the extent of multinucleation. Together, these findings indicate that a balance between RhoGEF and RhoGAP activities in general, and ECT2 6 and p190 in particular, is necessary for Rho to orchestrate accurate completion of cell division.
Results
Coexpression of ECT2 reduces multinucleation in p190-overexpressing cells
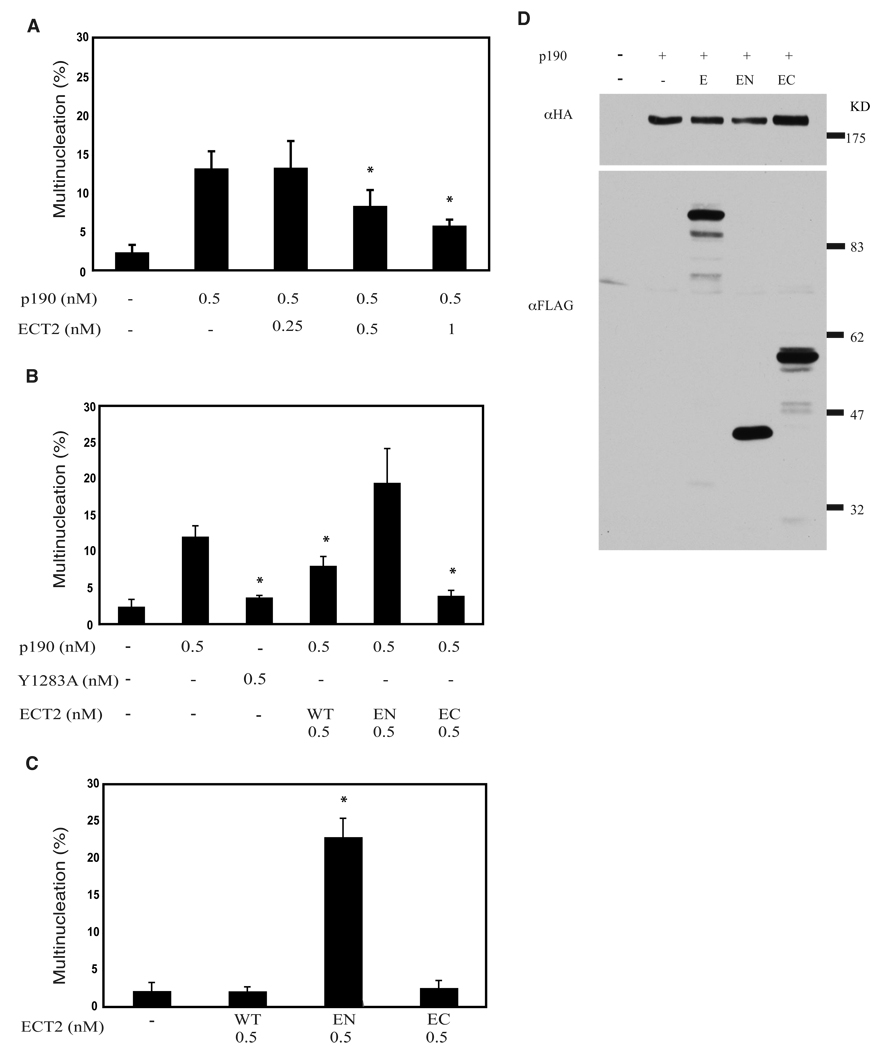
Induction of a multinucleated phenotype by ectopically expressed p190 is dependent on its RhoGAP domain, suggesting that the phenotype occurs as a result of Rho inactivation (Su et al., 2003). Similarly, expression of dominant negative ECT2 or silencing of its expression induces multinucleation through downregulation of Rho activity (Kimura et al., 2000). P190 localizes to the cleavage furrow, as do Rho (Narumiya and Yasuda, 2006; O'Connell et al., 1999) and ECT2 (Tatsumoto et al., 1999), suggesting that proper completion of cytokinesis requires the dynamic action of RhoGEFs and RhoGAPs to appropriately regulate RhoGTP levels. Imbalance between these two activities is postulated to result in aborted cell division. To test this hypothesis, varying amounts of ECT2 plasmid were transiently transfected with a constant amount of p190 into HeLa cells to determine whether ECT2 could reduce the number of multinucleated cells induced by p190. Fig. 1A shows that co-expression of ECT2 in p190-overexpressing cells reduced the number of multinucleated cells in a dose-dependent manner, indicating that ECT2 could counteract or balance the action of p190.
Fig. 1. Coexpression of ECT2 RhoGEF reduces p190-induced multinucleation.
(A) Coexpression of ECT2 reduces p190-induced multinucleation in a dose-dependent manner. Varied amounts of wild type, FLAG-tagged ECT2 plasmid (as indicated) were co-transfected with a constant amount of HA-tagged p190 plasmid into Hela cells as described in Methods. Forty-eight hrs later, cells were analyzed by immunofluorescence for the presence of HA-p190- and Flag-ECT2- expressing cells that displayed a multinucleated phenotype. Data are presented as the Mean ± SEM for n≥3. * represents p<0.05.
(B) Full length and constitutively active FLAG-ECT2 reduce multinucleation in HA-p190-overexpressing cells. Equal moles of plasmids encoding wild type or mutant FLAG-ECT2 were co-expressed with HA-p190 in HeLa cells, and transfected cells were analyzed as in Panel A. Data are presented as Mean ± SEM for n≥3. * represents p<0.05.
(C) Dominant negative ECT2 induces multinucleation. Equal moles of plasmids encoding wild type or mutant Flag ECT2 were transfected into Hela cells, and transfected cells were analyzed as in Panel A. Data are presented as the Mean ± SEM for n≥3. * represents p<0.05.
(D) Western blotting of whole cell lysates from HA-p190 and FLAG-ECT2 co-expressing cells analyzed in Panel B. Thirty µg whole cell lysates were immunoblotted with anti-FLAG antibody to detect levels of E (ECT2), EN (ECT2N), and EC (ECT2C), and with anti-HA antibody to detect p190.
ECT2 contains two BRCT domains in the N-terminal half of the molecule and the RhoGEF catalytic DH and PH domains in the C-terminal half. Tatsumoto et al. (Tatsumoto et al., 1999) showed that the N-terminal half of ECT2 (ECT2N) functions as a dominant negative and induces multinucleation in transfected cells. In contrast, the constitutively active C-terminal half (ECT2C) is an oncogene that transforms NIH3T3 cells and increases proliferation through enhanced Rho activity (Solski et al., 2004). Equimolar (0.5 nM) amounts of ECT2N (EN), ECT2C (EC), or wild type (wt) ECT2 plasmid DNA were co-transfected with p190 in HeLa cells to determine whether the GEF activity of ECT2 mediates reduction of the number of multinucleated cells induced by p190. Fig. 1B shows that ECT2C significantly reduced the number of multinucleated cells (p-value<0.05) to an even greater extent than wt ECT2, while ECT2N increased the number. These results demonstrate that the C-terminal half of ECT2, which contains the RhoGEF activity, is sufficient to counteract the action of p190. Also consistent with this notion is the finding that GAP inactive p190 (Y1283A) (Haskell et al., 2001) failed to induce multinucleation. Fig. 1D shows levels of p190 (triple HA-tagged) and the various forms of ECT2 (FLAG-tagged) expressed in transfected cells.
Wild type ECT2, ECT2N, and ECT2C were also singly transfected in Hela cells to verify their action on completion of mitosis. Consistent with previous studies, Fig. 1C shows that both wild type and ECT2C have no effect on multinucleated cells, but ECT2N induced multinucleation (Kimura et al., 2000; Tatsumoto et al., 1999).
P190 and ECT2 co-associate during mitosis
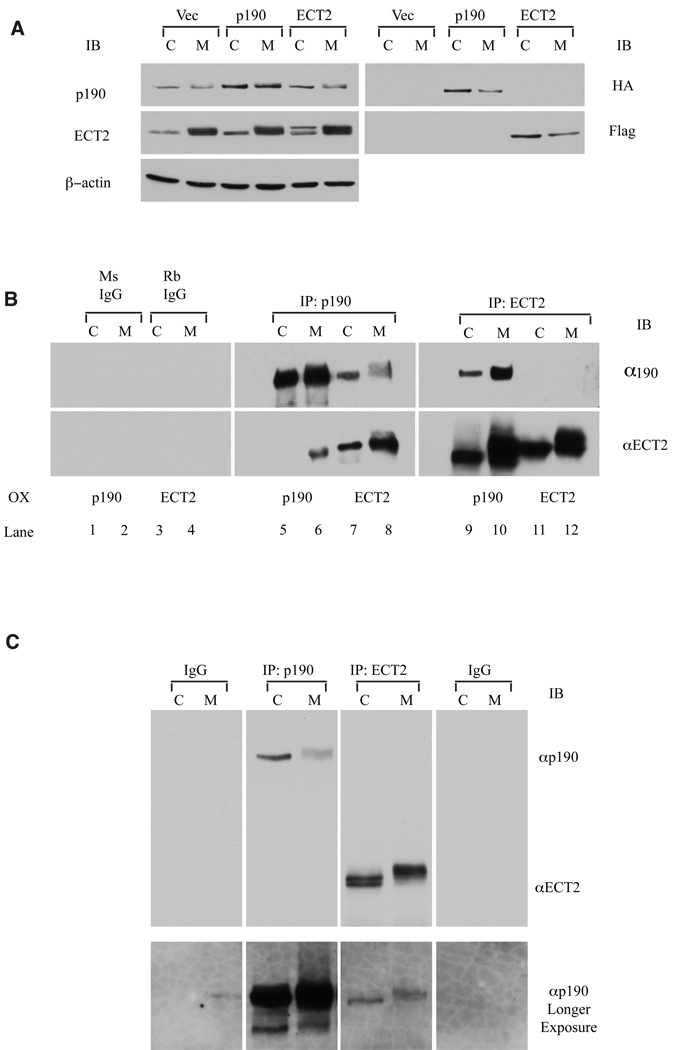
The ability of RhoGEFs to reverse the effect of p190-overexpression on cytokinesis suggested that ECT2 might associate with p190 during mitosis. In an initial test of this possibility, HA-tagged p190 or FLAG-tagged ECT2 was singly overexpressed in Hela cells, the cells were left to cycle (C) or arrest in mitosis (M), as described in Materials and Methods, and each protein (overexpressed plus endogenous) was immunoprecipitated. Immunoprecipitates were then immunoblotted for both proteins. Fig. 2A depicts a direct immunoblot of whole cell lysates showing levels of ectopically expressed HA-p190 and FLAG-ECT2 relative to endogenous proteins. Fig. 2B demonstrates that in cells expressing only HA-p190, ECT2 reciprocally co-precipitated with p190 (lanes 5 & 6, 9 & 10), and more co-association was observed in mitotic cells than in cycling cells. In FLAG-ECT2 expressing cells, ECT2 was found in the p190 precipitates (lanes 7 & 8), and again, more complex was detected in mitotic cells. However, the reciprocal co-immunoprecipitation was not detected in FLAG-ECT2 expressing cells (lanes 11 & 12), in contrast to the result obtained from cells ectopically expressing p190. Fig. 2A suggests that these differences in co-precipitation were not due to alterations in gene expression brought about by overexpressing one of the pair. Rather, the reduced level of precipitable ECT2 seen in Fig. 2B (compare lane 10 with lane 12 and Fig. 2B with 2C) suggests that the FLAG tag or another associated protein may be interfering with the binding of the ECT2 antibody, particularly in mitotic cells.
Fig. 2. p190 and ECT2 form stable complexes.
(A) Western blotting of endogenous or ectopically expressed p190 and ECT2 in whole cell lysates. HeLa cells were left untransfected or transfected with 0.5 nM HA-p190 or transfected with FLAG-ECT2 plasmids alone, and synchronized in mitosis (M) as described in Methods or left as cycling (C) populations. Thirty µg whole cell lysates were immunoblotted with anti-FLAG or anti-ECT2 antibody to detect levels of ECT2 or with anti-HA or anti-p190 antibody to detect p190.
(B) Reciprocal co-immunoprecipitation of p190 and ECT2 in singly transfected cells. HA-p190 or Flag-ECT2 plasmids were individually transfected into Hela cells, as indicated. C (cycling ~95% in interphase) and M (mitotic after 2 hr release from prometaphase) cells were collected, and 1mg whole cell lysate was immunoprecipitated with mouse anti-p190 (UBI) or rabbit anti-ECT2 (Santa Cruz), and immunoblotted with anti-ECT2 (Santa Cruz) or anti-p190 (Becton Dickinson) antibodies, as described in Methods. ECT2 and p190 preferentially and reciprocally co-immunoprecipitated from mitotic cells.
(C) Co-immunoprecipitation of endogenous p190 with endogenous ECT2. Untransfected HeLa cells were synchronized in mitosis (M) or left as a cycling population and immunoprecipitated as in Panel B. P190 specifically co-immunoprecipitated with ECT2 in both cycling and mitotic cells. However, reciprocal co-precipitation was not observed in this experiment (see text).
To determine whether endogenous p190 and RhoGEFs physically associated, p190 and ECT2 were individually immunoprecipitated from cycling and mitotic HeLa cells. Fig. 2C shows that immunoprecipitates of endogenous ECT2 co-precipitated endogenous p190 from cycling and mitotic cells. However, reciprocal interactions were not consistently observed (See also Supplementary material. Fig. 1S). The abundance of the endogenous p190/ECT2 complex was estimated to be 1–2% of total p190 or ECT2, with a greater amount (2–4%) found in mitotic cells overexpressing one of the pair.
The ability of ECT2 and p190 to form complexes in cycling cells was unexpected, since p190 is cytoplasmic/perinuclear and ECT2 is predominantly nuclear in interphase cells (~95% of cycling cells are in interphase). Only during mitosis, following nuclear envelope breakdown, are the proteins in the same subcellular compartment. We therefore tested the idea of whether the interaction occurred spontaneously upon cell lysis and detergent-mediated nuclear envelope disruption. Cycling (interphase) cells were hypotonically disrupted, dounce homogenized, and separated into cytoplasmic and nuclear fractions. The fractions were then individually lysed, and ECT2 or p190 was immunoprecipitated from each fraction or from a combination of the two fractions. Precipitates were then immunoblotted for both proteins. ECT2 and p190 were found to associate preferentially from combinations of the two fractions in p190 immunoprecipitates (Supplementary material Fig. 1S), suggesting that the interaction between them can occur spontaneously and is prevented by subcellular partitioning during interphase. These studies were also performed in p190 and ECT2 co-expressing cells with the same results. Interestingly, preparing nuclear and cytosolic fractions before lysis allowed the detection of the p190/ECT2 complex in p190 but not ECT2 immunoprecipitates, the opposite of results depicted in Fig. 2C. The reasons for this are not clear, but together, Fig. 2C and supplemental Fig. 1S demonstrate that the complex can be detected in a reciprocal fashion.
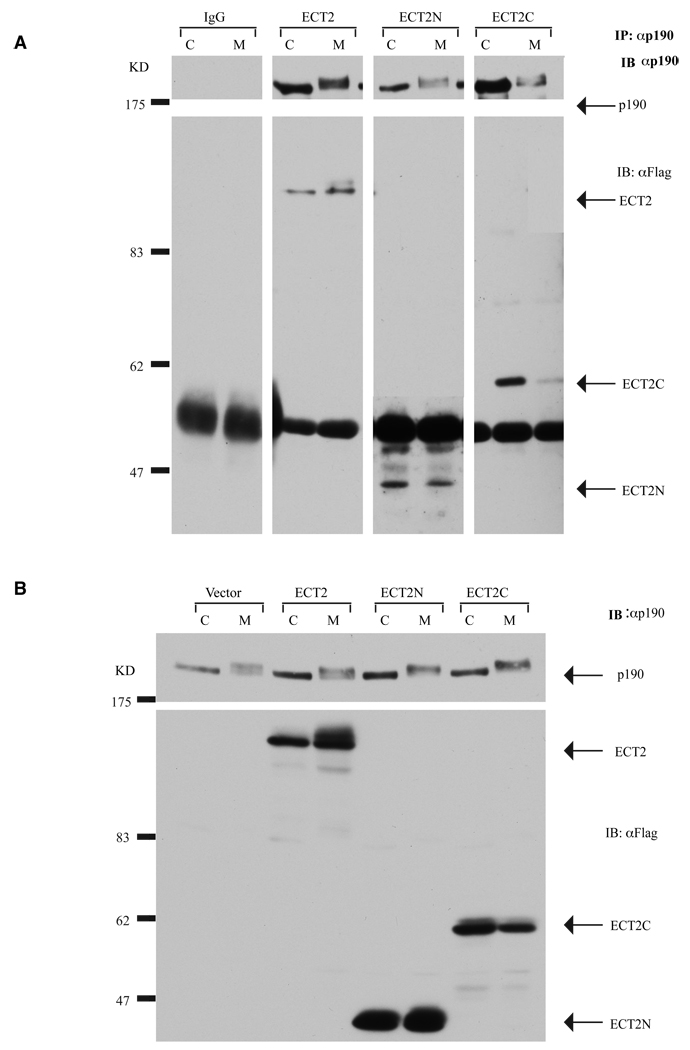
To determine which region of ECT2 bound to p190, ECT2N and ECT2C were tested for their ability to associate with endogenous p190 from cycling and mitotic cells. Plasmids encoding FLAG-tagged wild type ECT2, ECT2N or ECT2C were transfected into HeLa cells, and immunoprecipitates of endogenous p190 were immunoblotted with p190 and FLAG (ECT2) antibodies. Fig. 3A shows that wild type as well as ECT2N and ECT2C interacted with p190. However, based on a comparison of the amount of each protein co-immunoprecipitated by anti-p190 antibodies vs. total protein expressed, ECT2C (IP/Total=1.5) appeared to preferentially interact with p190 as compared to ECT2N (IP/WL=0.2) (Fig. 3B).
Fig. 3. The C-terminal half of ECT2 preferentially interacts with p190.
(A) Co-immunoprecipitation of endogenous p190 with ECT2 variants. HeLa cells were transfected with wild type FLAG-ECT2, FLAG-ECT2N, or FLAG-ECT2C and synchronized at mitosis (M) or left as a cycling (C) population. One mg protein lysate was immunoprecipitated with mouse anti-p190 antibody (UBI). Immunoprecipitates were immunoblotted with mouse anti-p190 antibody (Becton-Dickinson) or anti-FLAG antibody. FLAG-ECT2, FLAG-ECT2N, and FLAG-ECT2C co-immunoprecipitated with p190 from both cycling and mitotic cell extracts, with preferential association observed between p190 and ECT2C.
(B) Western blotting of endogenous p190 or FLAG-tagged, wild type ECT2, ECT2N, and ECT2C overexpressing cells. Thirty µg whole cell lysates from cells in Panel A were immunoblotted with anti-FLAG antibody to detect levels of ECT2 or with anti-p190 (Becton Dickinson) to detect p190.
P190 and RhoGEFs colocalize at the cleavage furrow during mitosis
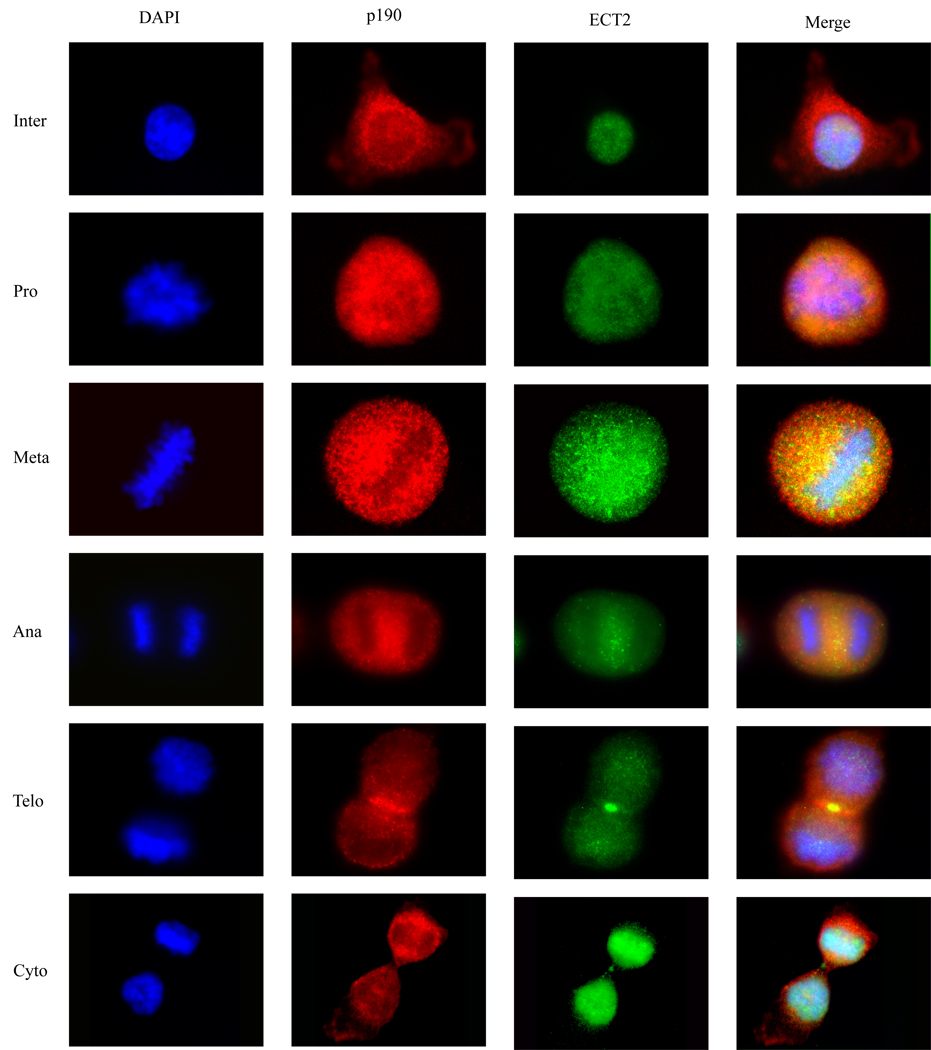
The ability of ECT2 to physically associate with p190 during mitosis and to reduce the frequency of multinucleation induced by overexpressed p190 suggested that ECT2 might co-localize with p190 during cytokinesis. To test this possibility, HeLa cells were blocked in G1/S phase, released from the block for various lengths of time, and examined by immunofluorescence microscopy for the subcellular localization of endogenous ECT2 and p190 in different stages of mitosis. Fig. 4 shows that p190 was predominantly cytoplasmic and perinuclear in interphase cells, dispersed throughout the cell in prophase and metaphase, and concentrated at the cleavage furrow during early anaphase, where it remained throughout anaphase and telophase. Consistent with our previous study, endogenous p190 was not observed in the midbody (Su et al., 2003). ECT2, on the other hand, resided in the nucleus during interphase and after nuclear membrane breakdown in prometaphase, was found dispersed throughout the cell. As with p190, ECT2 translocated to the cleavage furrow and midbody during anaphase and telophase, respectively. These findings indicate that p190 and ECT2 are appropriately localized spatially and temporally to reciprocally regulate Rho activity during cytokinesis.
Fig. 4. Colocalization of endogenous p190 and ECT2 during mitosis.
Hela cells were synchronized in mitosis, released for various lengths of time, and collected at each mitotic phase: inter- (interphase), pro- (prophase), meta- (metaphase), ana-(anaphase), telo- (telophase), and cytokinesis. Endogenous p190 and ECT2 were immunostained with mouse anti-p190 and rabbit-ECT2 antibody, respectively, and analyzed by immunofluorescence microscopy as described in Methods. Co-localization of p190 and ECT2 was observed in the cleavage furrow.
Rho activity reciprocally correlates with multinucleation
We have demonstrated that p190 and ECT2 co-localize and physically interact with each other during mitosis and that these molecules reciprocally regulate proper passage through mitosis. Both p190 and ECT2 are well-known Rho regulators. We therefore reasoned that the interaction between p190 and ECT2 may modulate Rho activity to mediate completion of mitosis. To test this hypothesis, wild type and mutant constructs of p190 and ECT2 were co-transfected into HeLa cells, and RhoGTP pull-down assays using the GST-Rho binding domain of rhotekin were performed. Fig. 5A depicts Rho activity in cells transfected with p190 alone or in combination with wild type or mutant forms of ECT2. Ectopic expression of p190 resulted in reduced Rho activity compared to vector control (p<0.05). This low Rho activity correlated with a high multinucleation rate (See Fig. 1A). Co-transfection of wild type ECT2 with p190 restored RhoGTP to near normal levels, concomitant with a reduction in multinucleation (Fig. 1A). Co-transfection of ECT2N maintained Rho in a low activity state, which correlated again with high numbers of multinucleated cells, while constitutively active ECT2C generated the highest RhoGTP levels and low frequency of multinucleation (Fig. 1B). These results suggest that p190 and ECT2 act counterpoint to one another in regulating cytokinesis.
Fig. 5. Rho activity inversely correlates with incidence of multinucleation.
HeLa cells were nucleofected with vector or designated plasmids, synchronized, and released for 1.5 hrs. At this time, cell lysates were prepared and used for Rho-pull-down assays, as described in Methods.
(A) Representative Western immunoblot of GTP-bound RhoA pulled down with GST-RBD agarose beads (upper panel) and total Rho in 30 µg whole cell lysate (lower panel).
(B) Western immunoblots in Panel A and two additional experiments were quantified by the Alpha EC program. Data are presented as the Mean ratio of RhoGTP/Total RhoA ± SEM, n=3 in each cell population. *represents a statistically significant difference from vector control (p-value<0.05).
Discussion
The small GTPase, Rho, is a major regulator of actin and microtubule dynamics, with misregulation leading to multiple cellular defects, including aberrant mitotic progression (Bement et al., 2006; Kishi et al., 1993; Narumiya and Yasuda, 2006). Specifically, inactivation of Rho by C3 exotoxin or siRNA silencing leads to cytokinesis failure and multinucleation (Drechsel et al., 1997; Jantsch-Plunger et al., 2000; Kishi et al., 1993). Moreover, silencing of the RhoGEF, ECT2, or introduction of a dominant negative form of ECT2 induces a multinucleated phenotype that correlates with lowered Rho activity (Chalamalasetty et al., 2006; Kimura et al., 2000; Tatsumoto et al., 1999). Overexpression of p190RhoGAP, a negative regulator of Rho, also leads to mitotic defects, multinucleation, and reduced RhoGTP levels in the cleavage furrow (Su et al., 2003) (Su et al., submitted). Together, these studies suggest that imbalances in the appropriate ratio of RhoGTP to RhoGDP impede cell division.
This concept raises the question of whether such an imbalance induced by alterations in level or activity of one Rho regulator could be corrected by altering the level or activity of an opposing Rho regulator, thus modulating Rho activity and rescuing the abnormal phenotype. We reasoned that the ability to rescue would be enhanced if the regulators were spatially and temporally juxtaposed and thus able to affect the same pool of Rho. In this report, we asked whether multinucleation induced by ectopic expression of p190 RhoGAP could be rescued by the RhoGEF, ECT2, and if so, whether the activity of their mutual downstream target, Rho, would be altered accordingly.
We found that p190-induced multinucleation was rescued in a dose-dependent manner by introduction of wild type ECT2 or a constitutively active mutant of ECT2 but not by dominant negative ECT2. Furthermore, RhoGTP levels were correlated with successful cytokinesis, such that reduced RhoGTP, brought about by either ectopic expression of p190 or dominant active ECT2, prevented completion of cell division. Co-coordinated regulation of Rho by p190 and ECT2 was further substantiated by the co-localization of p190 and ECT2 to the cleavage furrow during cytokinesis (where Rho is known to localize) and their preferential association in mitosis. Together, these data support the notion that Rho levels must be carefully modulated for cells to successfully complete mitosis and that this modulation involves in large part a balance between the actions of a RhoGAP (p190) and a RhoGEF (ECT2).
An alternative approach utilized by our laboratory to investigate the role of p190 in cytokinesis was gene silencing. Unexpectedly, knock-down of p190 resulted in failure of cells to enter mitosis. Cells with reduced p190 exhibited a highly flattened morphology and were unable to release from the substratum and round up, thereby preventing initiation of mitosis. While uninformative with regard to cytokinesis, these results suggested that p190 plays multiple roles in the cell cycle, one at the G2/M border (early prophase, as revealed by gene silencing) and another in cytokinesis (as revealed by overexpression). In fact, Burridge and coworkers have suggested a role for p190 in early prophase that involves contraction of the actinomyosin network at the cell cortex, a process involved in cell rounding (Maddox and Burridge, 2003).
The original design of these experiments involved reversing an already established multinucleated phenotype. Specifically multinucleated cells that had been pre-transfected with p190 were secondarily transfected with ECT2, and cells were scored for multinucleation. However, p190 overexpressing cells failed to express ECT2 in any form. This failure could be due to a variety of reasons, including disruption of protein synthesis, endoduplication, cell cycle arrest at late mitosis, and/or apoptosis. As a result, the co-overexpression approach was taken.
Fig. 4 demonstrates co-localization of p190 and ECT2 at the cleavage furrow during anaphase and telophase. These are established sites of Rho localization, and related studies in our laboratory and others have shown the presence of active Rho in these structures (Bement et al., 2006; Yuce et al., 2005; Yoshizaki et al., 2004) (Su et al., submitted). Studies depicted in Fig. 2B, C of this report suggest that p190 and ECT2 exist in complexes that are more abundant in mitotic than in cycling cells, particularly when one of the pair is overexpressed. In such complexes, it is proposed that the two antagonistic regulators of Rho are poised to rapidly catalyze cycling of Rho between the active and inactive states. Based on this hypothesis, p190/ECT2 complexes were also probed for Rho, but we failed to detect its presence, even when complexes extracted from cells synchronized in various stages of mitosis were examined. The reasons for this are unclear, but the result suggests that Rho does not exist in a stable complex with its regulators (excluding GDI). Rather, once activated, it may preferentially form associations with its downstream effectors, and when inactivated for more protracted lengths of time, it is likely to form stable complexes with RhoGDI. This reasoning implies that association of Rho with the p190/ECT2 complexes is short-lived. A similar complex has been reported between ECT2 and MgcRacGAP in early mitosis (Yuce et al., 2005).
Both the N- and C-terminal halves of ECT2 interacted with p190, with a significant preference for the C-terminal half. This was a somewhat unexpected result, as ECT2N has been shown to function by multiple mechanisms as a dominant negative and would be expected to competitively bind ECT2 interactive proteins. First, ECT2N prevents endogenous ECT2 from activating Rho (possibly by intramolecular binding of its own C-terminus) (Kimura et al., 2000; Kim et al., 2005). Second, through its BRCT domains, ECT2N competes with endogenous ECT2 in early mitosis for binding of proteins that are necessary for completion of cytokinesis (Kim et al., 2005). Third, ECT2N accumulates in ring-like structures together with MgcRacGAP and MKLP1, a process that leads to failed abscission (Chalamalasetty et al., 2006). We speculate that p190 may bind to ECT2 in such a way as to disrupt the intramolecular interaction of ECT2 and thereby modulate ECT2 activity. The BRCT domains in the N-terminal half of ECT2 also interact with MgcRacGAP within the centralspindlin complex (Kim et al., 2005), suggesting that MgcRacGAP may compete with p190 for binding to ECT2N.
The Rho downstream effectors, such as ROCK, mDia, and citron kinase have all been implicated in regulation of mitosis as well as Rho (Eda et al., 2001; Kamijo et al., 2006; Madaule et al., 2000; Suetsugu et al., 1999; Tominaga et al., 2000; Yoshizaki et al., 2004). Inactivation by small molecule inhibitors, introduction of dominant negative forms of the molecules, or silencing of individual effectors leads to multinucleation. It has been postulated that inactivation or silencing of ROCK inhibits actin polymerization/contractility and this defect leads to incomplete cleavage and cytokinetic failure (Yoshizaki et al., 2004). Silencing of mDia destabilizes actin and microtubule networks, which leads to loss of actin contractility and defective abscission in late cytokinesis (Suetsugu et al., 1999; Tominaga et al., 2000). Expression of dominant negative citron kinase or silencing of its gene has also been shown to induce multinucleation; however, neither the substrates of this kinase nor the signaling cascades in which it functions have been well-established (Eda et al., 2001; Madaule et al., 2000). Our long-term goal is to fully define the p190 signaling pathway(s) that function through Rho and its effectors to regulate specific events in cytokinesis.
Experimental Procedures
Antibodies and Reagents
Mouse anti-p190 RhoGAP antibody used for Western blotting was obtained from Beckton Dickinson (Mountain View, CA), while mouse anti-p190 RhoGAP used for immunoprecipitation was purchased from UBI (Lake Placid, NY). Mouse and rabbit anti-Flag antibodies and 4',6-Diamidino-2-phenyindole (DAPI) were obtained from Sigma (St. Louis, MO). Rabbit ECT2 and mouse anti-RhoA antibodies were acquired from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Sheep HRP-conjugated anti-mouse and donkey HRP-conjugated anti-rabbit antibodies were from Amersham (Little Chalfont, Buckinghamshire, HP7 9NA, United Kingdom), and goat anti-mouse and anti-rabbit antibodies conjugated with Alexa 488 and 594 were purchased from Molecular Probes (Eugene, OR). Chrompure mouse and rabbit whole molecular IgG were obtained from Jackson ImmunoResearch (West Grove, PA).
Plasmids
pCEV29F3-ECT2 (Tatsumoto et al., 1999) was obtained from T. Miki (National Institute of Health). N- and C-terminal truncated ECT2 were amplified by PCR using full length ECT2 as a template and subcloned between the BamHI and EcoRI sites of pCEV29F3 as previously described (Lorenzi et al., 1999; Tatsumoto et al., 1999). The following primer sets were used to generate ECT2N (amino acids, 1–334) and ECT2C (amino acids, 414–883): ECT2N; 5’-GCG GAT CCA TGG CTG AAA ATA GTG TAT TA-3’ and 5’-CGG AAT TCT CAC ACT GAT TTC TTG AGC TCA GG-3’; ECT2C; 5’-GCG GAT CCG TTC CTT CAA AGC AGT CAG CA-3’ and 5’-ACT GAA TTC GGT AAC GCT TCA TAT CAA ATG-3’.
pKH3p190 was obtained from I. Macara (University of Virginia) (Tatsis et al., 1998) and used to generate pKH3p190Y1283A using QuickChangeII Site-Directed Mutagenesis Kits (Stratagene, Cedar Creek, TX) as previously described (Haskell et al., 2001). The following oligonucleotides were used in PCR to introduce this mutation: 5’-GCA CAG AAG GCA TCG CCC GGG TCA GCG GAA AC-3’ and 5’-GTT TCC GCT GAC CCG GGC GAT GCC TTC TGT GC-3’. All constructs were confirmed by DNA sequencing.
Cell culture and Transfection
Hela cells were purchased from the American Type Culture Collection (Manassas, VA), cultured in Dulbecco’s modified Eagle’s medium (DMEM) GIBCO (Grand Island, NY) containing 10% fetal bovine serum, 100 µg/ml penicillin, and 100 U/ml streptomycin, and maintained in a 37°C humidified, 5% CO2 environment. For multinucleation studies, transfection was performed according to the manufacturer’s protocol with Polyfect (QIAGEN, Valencia, CA) in 6 well plates seeded with 1× 105 cells/well. The amounts of plasmid transfected per well are indicated in the Figures or Figure Legends.
For Rho pull-down assays, p190 and ECT2 plasmids were nucleofected using the AMAXA Nucleofection kit R (AMAXA, Gaithersburg, MD) according to the manufacturer’s protocol. Briefly, 4×106 cells were plated in 150 mm plates, 200 mM thymidine was added 6 hr later, and cells were incubated for 14 additional hrs at 37°C in a 5% CO2 atmosphere. Equimolar ratios of p190 and ECT2 plasmids (40 nM each) were then added to the cells in 100 µl nucleofection solution as follows: Cells were harvested with Trypsin/EDTA, centrifuged at 200×g for 5 min, mixed with the nucleofection solution, and nucleofected with program I-13. Immediately, 0.5 ml warm, complete DMEM medium was added, and cells were transferred to a 100 mm plate containing 10 ml pre-warmed, complete DMEM medium. Plates were incubated overnight at 37°C in a 5% CO2 atmosphere. The next day cells were arrested at prometaphase by incubation in 100 ng/ml nocodazole for 12 hrs, collected by mechanical shaking, and released in fresh, complete DMEM medium for 1.5 hrs.
Immunofluorescence
Cells (5×104) were seeded onto glass coverslips, and after 48 hrs growth incubation, were arrested in 200 mM thymidine for 14 hrs. Cells were then released from the cell cycle block for 8 hrs, arrested a second time with 200 mM thymidine for an additional 14 hrs, released for 8, 9, or 10 hrs, and prepared for immunofluorescent microscopy. Coverslips were washed with PBS, fixed in 4% paraformaldehyde for 20 min at room temperature, washed again with PBS, and incubated with 0.2% Triton X-100 in PBS for 5 min at room temperature. Monolayers were then blocked with 20% heat-inactivated goat serum in PBS for 1 hr, incubated with primary antibodies (1:50) for 1 hr at room temperature, washed with PBS, incubated with secondary antibodies (1:1000) for 30 min, and washed again. DAPI was added to the cells for 3 min at room temperature just prior to mounting. Coverslips were mounted onto microscope slides with VectaShield (Vector Laboratories, Inc. Burlingame, CA), sealed with Cytoseal-60 (Richard-Allan Scientific, Kalamazoo, MI), and examined by a LEICA fluorescent microscope (LEITZ DMRBE; Leica Microsystems; Bannockburn, IL).
Western Blotting
Cells grown to 80–90% confluency in 6-well dishes were washed with ice cold PBS and lysed in 50 µl ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM PMSF, and 1x protease inhibitor cocktail (Calbiochem, San Diego, CA). Protein concentration of cleared lysates was measured by the BCA assay (Pierce,), according to the manufacturer’s protocol. 30 µg lysate protein was fractionated by 6% SDS-PAGE and transferred to PVDF membrane. Membranes were blocked in 5% non-fat milk in TBS-T (25 mM Tris-HCl, pH 8.0, 125 mm NaCl, and 0.1% Tween 20) for 1 hr at room temperature, incubated in primary antibodies (p190; 1:1000, Flag; 1:10,000, ECT2; 1:10,000, and β-actin; 1:500,000) overnight at 4°C, washed in TBS-T, and incubated in secondary antibody (1:5000) for 1 hr at room temperature. Binding of secondary antibody was visualized by treating the membrane in ECL reagent (Pierce) for 5 min and exposing to X-Ray film.
Co-Immunoprecipitation
Thymidine-nocodazole blocks were performed to arrest cells in prometaphase. Briefly, transfected cells (1×106) were seeded in 100 mm dishes, and at 60–70% confluency were incubated with 200 mM thymidine (Sigma) for 14 hrs, removed from thymidine for 6 hrs, and treated with 100 ng/ml nocodazole (Sigma) for 15 additional hrs. Mitotic cells were collected by mechanical shake-off (designated as M) and released from the block for two hrs to allow cells to progress through mitosis.
Cells were then washed with ice-cold PBS and lysed with cold RIPA buffer. The lysate was clarified by centrifugation at 15,000 × g for 10 min at 4°C, followed by incubation of 1 mg supernatant with 30 µl of a 50% slurry of protein A or G agarose beads (UBI) for 30 min at 4°C and centrifugation at 10,000 × g for 10 min in the cold. Clarified lysate was transferred to a new 1.5 ml centrifuge tube and incubated with 5 µg primary antibody overnight at 4°C. Samples were precipitated with 50 µl of 50% slurry of protein A or G agarose beads for 1 hr at 4°C, washed with ice-cold PBS, and stored in 40 µl 2x Laemmlli sample buffer at 20°C until analyzed by SDS-PAGE and Western immunoblotting.
Rho-pull down assay
The Rho pull-down assay was performed as previously described (Fiordalisi et al., 2006). Briefly, RBD-GST fusion proteins were generated from E. coli and coupled to glutathione-agarose beads (Sigma). HeLa cells were washed once with ice-cold PBS and lysed in Rho lysis buffer (50 mM, Tris-HCl, pH 7.4, 500 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 10% glycerol) supplemented with 1x protease inhibitor cocktail (Roche, Indianapolis, IN). Five hundred µg protein lysate was incubated with 30 µg GST-RBD-conjugated agarose beads for 30 min at 4°C for complex formation between RBD and RhoGTP. Beads were then washed with ice-cold wash buffer (25 mM Tris, pH7.5, 40 mM NaCl, and 30 mM MgCl2), suspended in 40 µl 2x Laemmli buffer, and boiled. Proteins were separated by 12% SDS-PAGE, transferred to Immobilon-P membranes (Millipore, Bedford, MA), and immunoblotted for Rho, as described above. The blot was quantitated with the alphaEC Innoteck program (San Leandro, CA).
Cell Fractionation
Hela cells (1.6×106 cells) were plated in 100 mm plates and after reaching 80% confluency were incubated in buffer A (10 mM Tris-HCl pH 7.5, 1.5 mM MgCl2, 10 mM NaCl, 1 mM EDTA, 1x protease inhibitor cocktail and 200 mM Na vanadate) for 10 min and dounce homogenized 100 times on ice. Fractions were separated by centrifugation at 1000 × g for 10 min at 4°C and individually lysed with RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM PMSF), and 1x protease inhibitor cocktail (Calbiochem, San Diego, CA). ECT2 or p190 was immunoprecipitated from 500 µg lysate protein from each fraction or from 1 mg protein from a combination of the two fractions. Precipitates were then immunoblotted for both proteins.
Supplementary Material
Acknowledgments
We thank J. Fiordalisi and A. Cox of the University of North Carolina at Chapel Hill for teaching us the Rho pull-down assay and J. Dasilva for critical reading of the manuscript. We thank T. Miki for providing the pCEV3F-ECT2 plasmid. We are also grateful to the S.J. Parsons’ and C. Silva’s laboratories for constructive discussions. This work was supported by grant CA39438 (SJP) from the National Cancer Institute.
Reference List
- 1.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bement WM, Miller AL, von DG. Rho GTPase activity zones and transient contractile arrays. Bioessays. 2006;28:983–993. doi: 10.1002/bies.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J, Bronson RT, Settleman J. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891–4903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- 4.Brouns MR, Matheson SF, Settleman J. p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nat. Cell Biol. 2001;3:361–367. doi: 10.1038/35070042. [DOI] [PubMed] [Google Scholar]
- 5.Chalamalasetty RB, Hummer S, Nigg EA, Sillje HH. Influence of human Ect2 depletion and overexpression on cleavage furrow formation and abscission. J. Cell Sci. 2006;119:3008–3019. doi: 10.1242/jcs.03032. [DOI] [PubMed] [Google Scholar]
- 6.Chang JH, Gill S, Settleman J, Parsons SJ. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J. Cell Biol. 1995;130:355–368. doi: 10.1083/jcb.130.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem. J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr. Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- 9.Eda M, Yonemura S, Kato T, Watanabe N, Ishizaki T, Madaule P, Narumiya S. Rho-dependent transfer of Citron-kinase to the cleavage furrow of dividing cells. J. Cell Sci. 2001;114:3273–3284. doi: 10.1242/jcs.114.18.3273. [DOI] [PubMed] [Google Scholar]
- 10.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu. Rev. Biochem. 2006;75:543–566. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 11.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 12.Fincham VJ, Chudleigh A, Frame MC. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J. Cell Sci. 1999;112(Pt 6):947–956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- 13.Fiordalisi JJ, Keller PJ, Cox AD. PRL tyrosine phosphatases regulate rho family GTPases to promote invasion and motility. Cancer Res. 2006;66:3153–3161. doi: 10.1158/0008-5472.CAN-05-3116. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Mata R, Burridge K. Catching a GEF by its tail. Trends Cell Biol. 2007;17:36–43. doi: 10.1016/j.tcb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Glotzer M. Cleavage furrow positioning. J. Cell Biol. 2004;164:347–351. doi: 10.1083/jcb.200310112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 17.Haskell MD, Nickles AL, Agati JM, Su L, Dukes BD, Parsons SJ. Phosphorylation of p190 on Tyr1105 by c-Src is necessary but not sufficient for EGF-induced actin disassembly in C3H10T1/2 fibroblasts. J. Cell Sci. 2001;114:1699–1708. doi: 10.1242/jcs.114.9.1699. [DOI] [PubMed] [Google Scholar]
- 18.Jantsch-Plunger V, Gonczy P, Romano A, Schnabel H, Hamill D, Schnabel R, Hyman AA, Glotzer M. CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J. Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee JS, Miki T. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol. Biol. Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JE, Billadeau DD, Chen J. The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J. Biol. Chem. 2005;280:5733–5739. doi: 10.1074/jbc.M409298200. [DOI] [PubMed] [Google Scholar]
- 21.Kimura K, Fukata Y, Matsuoka Y, Bennett V, Matsuura Y, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase. J. Biol. Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]
- 22.Kimura K, Tsuji T, Takada Y, Miki T, Narumiya S. Accumulation of GTP-bound RhoA during cytokinesis and a critical role of ECT2 in this accumulation. J. Biol. Chem. 2000;275:17233–17236. doi: 10.1074/jbc.C000212200. [DOI] [PubMed] [Google Scholar]
- 23.Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J. Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzi MV, Castagnino P, Chen Q, Hori Y, Miki T. Distinct expression patterns and transforming properties of multiple isoforms of Ost, an exchange factor for RhoA and Cdc42. Oncogene. 1999;18:4742–4755. doi: 10.1038/sj.onc.1202851. [DOI] [PubMed] [Google Scholar]
- 25.Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organisation of the contractile ring in dividing sand dollar eggs. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- 26.Madaule P, Furuyashiki T, Eda M, Bito H, Ishizaki T, Narumiya S. Citron, a Rho target that affects contractility during cytokinesis. Microsc. Res. Tech. 2000;49:123–126. doi: 10.1002/(SICI)1097-0029(20000415)49:2<123::AID-JEMT3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Maddox AS, Burridge K. RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J. Cell Biol. 2003;160:255–265. doi: 10.1083/jcb.200207130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakahara H, Mueller SC, Nomizu M, Yamada Y, Yeh Y, Chen WT. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J. Biol. Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- 29.Narumiya S, Yasuda S. Rho GTPases in animal cell mitosis. Curr. Opin. Cell Biol. 2006;18:199–205. doi: 10.1016/j.ceb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.O'Connell CB, Wheatley SP, Ahmed S, Wang YL. The small GTP-binding protein rho regulates cortical activities in cultured cells during division. J. Cell Biol. 1999;144:305–313. doi: 10.1083/jcb.144.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridley AJ, Self AJ, Kasmi F, Paterson HF, Hall A, Marshall CJ, Ellis C. rho family GTPase activating proteins p190, bcr and rhoGAP show distinct specificities in vitro and in vivo. EMBO J. 1993;12:5151–5160. doi: 10.1002/j.1460-2075.1993.tb06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Settleman J, Albright CF, Foster LC, Weinberg RA. Association between GTPase activators for Rho and Ras families. Nature. 1992;359:153–154. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- 33.Solski PA, Wilder RS, Rossman KL, Sondek J, Cox AD, Campbell SL, Der CJ. Requirement for C-terminal sequences in regulation of Ect2 guanine nucleotide exchange specificity and transformation. J. Biol. Chem. 2004;279:25226–25233. doi: 10.1074/jbc.M313792200. [DOI] [PubMed] [Google Scholar]
- 34.Su L, Agati JM, Parsons SJ. p190RhoGAP is cell cycle regulated and affects cytokinesis. J. Cell Biol. 2003;163:571–582. doi: 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suetsugu S, Miki H, Takenawa T. Distinct roles of profilin in cell morphological changes: microspikes, membrane ruffles, stress fibers, and cytokinesis. FEBS Lett. 1999;457:470–474. doi: 10.1016/s0014-5793(99)01086-8. [DOI] [PubMed] [Google Scholar]
- 36.Tatsis N, Lannigan DA, Macara IG. The function of the p190 Rho GTPase-activating protein is controlled by its N-terminal GTP binding domain. J. Biol. Chem. 1998;273:34631–34638. doi: 10.1074/jbc.273.51.34631. [DOI] [PubMed] [Google Scholar]
- 37.Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J. Cell Biol. 1999;147:921–928. doi: 10.1083/jcb.147.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol. Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 39.Tikoo A, Czekay S, Viars C, White S, Heath JK, Arden K, Maruta H. p190-A, a human tumor suppressor gene, maps to the chromosomal region 19q13.3 that is reportedly deleted in some gliomas. Gene. 2000;257:23–31. doi: 10.1016/s0378-1119(00)00387-5. [DOI] [PubMed] [Google Scholar]
- 40.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol. Cell. 2000;5:13–25. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 41.Wang DZ, Nur-E-Kamal MS, Tikoo A, Montague W, Maruta H. The GTPase and Rho GAP domains of p190, a tumor suppressor protein that binds the M(r) 120,000 Ras GAP, independently function as anti-Ras tumor suppressors. Cancer Res. 1997;57:2478–2484. [PubMed] [Google Scholar]
- 42.Yoshizaki H, Ohba Y, Parrini MC, Dulyaninova NG, Bresnick AR, Mochizuki N, Matsuda M. Cell type-specific regulation of RhoA activity during cytokinesis. J. Biol. Chem. 2004;279:44756–44762. doi: 10.1074/jbc.M402292200. [DOI] [PubMed] [Google Scholar]
- 43.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zrihan-Licht S, Fu Y, Settleman J, Schinkmann K, Shaw L, Keydar I, Avraham S, Avraham H. RAFTK/Pyk2 tyrosine kinase mediates the association of p190 RhoGAP with RasGAP and is involved in breast cancer cell invasion. Oncogene. 2000;19:1318–1328. doi: 10.1038/sj.onc.1203422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.