Abstract
Bacteria of the genus Yersinia cause disease in humans ranging from enteritis and lymphadenitis to plague. These organisms have the ability to bind to host cells and, in a complex interaction, are able to deliver a specific subset of proteins into the host cell. These proteins alter host structures and functions, and in the case of macrophages, prevent phagocytosis of the organism. In Yersinia enterocolitica, one of these proteins, Yop51, is known to be a protein tyrosine phosphatase. By using a directed approach, we have raised murine CTL that recognize a peptide epitope from Yop51 in the context of the MHC class I molecule H-2 Db. This epitope, Yop51 249–257, seems to be presented both by cells that produce Yop51 endogenously and by epithelial cells infected with virulent Y. enterocolitica. In vivo, Y. enterocolitica is believed to remain mostly in an extracellular niche. Therefore, detection of this organism by cytotoxic T cells and, possibly, resistance to disease may depend on recognition of epitopes from a subset of virulence determinants delivered into the cytoplasm of host cells by surface-bound organisms.
Bacterial pathogens have evolved the ability to survive and multiply within a mammalian host. One challenge to these pathogens is to avoid or subvert the immune response directed against them, and bacteria that infect the deeper tissues of the host must cope with the natural and specific immune responses directed against them. A subset of these organisms has evolved the means to enter and survive within mammalian cells. Infected cells may be professional phagocytic cells or nonprofessional cells such as epithelial cells. Pathogens that enter host cells, and that can survive within them, have entered into an environment protected from elements of the natural and humoral immune systems. One of the challenges of such an intracellular lifestyle is sequestration of foreign Ag within the host cell. Failure to sequester Ag leads to specific T cell responses directed against the infected host cell. In the case of pathogens that can enter nonprofessional phagocytes, the ability to remain within a vacuole may provide such sequestration. These cells, which normally express only MHC class I, present epitopes primarily derived from cytoplasmic proteins (1).
Ags brought into phagocytes are processed within the phagocytic vacuole, where they are cleaved into peptides and presented by MHC class II molecules (2). T cells that recognize these Ags are usually of the CD4+ helper class. This type of T cell response is thought to play a role in the clearance of pathogens such as Salmonella, which, for the most part, remain within phagocytic vacuoles (3). In contrast, Ag that is introduced directly into the cytoplasm is processed by the cell and presented by MHC class I (1). The T cells which recognize such endogenous Ags are primarily CD8+ CTL. Organisms such as Listeria, which have the ability to break out of the phagosome, are known to introduce Ags into the cytoplasm where they can be processed and presented by MHC class I. One such Ag, the listeriolysin O (LLO)3 protein from Listeria monocytogenes, has been shown to be presented by MHC class I in a murine infection model (4, 5), and CTL that recognize LLO can transfer immunity against the organism to naive mice (6).
The genus Yersinia encompasses three species that cause human disease. Yersinia enterocolitica and Yersinia pseudotuberculosis are fecal-oral pathogens causing enteritis and mesenteric lymphadenitis. Yersinia pestis is the causative agent of plague. The Yersiniae have a large number of well-characterized virulence determinants (7). Many of these virulence determinants are contained on a 70-kb plasmid shared by all three species. A subset of the plasmid-encoded genes are known as Yops and are coordinately regulated in response to environmental cues (8, 9). The Yops are up-regulated at 37°C and low Ca2+. In the case of Y. enterocolitica or Y. pseudotuberculosis, the organism is able to navigate from the luminal surface of the intestinal tract into the deeper tissues and the bloodstream (10). Although these organisms have the ability to bind to and enter into mammalian cells in culture, it is unclear to what extent entry into cells occurs in vivo (11–14). Intimate binding of bacteria to host cell integrins requires the activity of at least one of several bacterial proteins (9, 15, 16), and during this interaction, the Yersinia are thought to inhibit phagocytosis (16). This inhibition of phagocytosis is mediated through the activity of at least two Yersinia proteins (17–20), including Yop51 (YopH in Y. pseudotuberculosis), which is known to be a protein tyrosine phosphatase (21). It seems unlikely that Yop51 plays a regulatory role in the bacterium as there is no known target of this protein in Yersinia. The antiphagocytic activity of Yop51 is dependent on its phosphatase activity (16), and abolishment of its catalytic activity also causes a marked decrease in virulence (22). Yop51 does not seem to act on cellular targets when purified and applied exogenously to eucaryotic cells (9); it seems that Yop51 must be produced by bacteria bound to the cell surface and that it is subsequently translocated into the cytoplasm of the host cell (9, 23). In addition to specifically inhibiting phagocytosis, Yop51 may play a more general role in subverting proper signal transduction within infected cells (9, 21, 22, 24).
Previous studies have demonstrated the importance of T cells in the immune response to Yersinia; however, the Ags recognized by these T cells have not been identified (25, 26). Because the Yop51 phosphatase is thought to be introduced into the cytoplasm of host cells, we sought to raise murine cytotoxic T cells that recognize a Yop51 epitope. In addition, we sought to investigate whether such T cells recognize an epitope from Yop51 presented by MHC class I molecules when Yop51 is produced endogenously and when it is introduced into the cell by infecting Yersinia.
Here, we present evidence suggesting a novel mechanism by which CTL can recognize bacterial pathogens. We show that a natural epitope derived from a Yersinia protein is presented by MHC class I despite the fact that these organisms are found mostly extracellularly and, when seen in an intracellular compartment, remain entirely within the vacuole. Such presentation suggests that Yersinia bound to host cells have the ability to translocate into the cytoplasm of those cells proteins that contribute to their virulence, and in the process subject the infected cell to recognition by CTL.
Materials and Methods
Mice
Female C57BL/6 mice (H-2b) were obtained from The Jackson Laboratory (Bar Harbor, ME) and used at 6 to 12 wk of age.
Tissue culture
The medium used for all tissue culture was RP-10, consisting of RPMI 1640 supplemented with l-glutamine, 50 µM 2-ME, antibiotics (except when noted), and 10% FCS. Transfected cell lines were maintained in RP-10 containing G418 at a concentration of 400 µg/ml. All cultured cells were maintained at 37°C in 7% CO2. The cell lines used in this study are as follows: EL-4 (H-2b) is derived from a C57BL/6 thymoma; P815 (H-2d) is from a DBA/2 mastocytoma. Clones of P815 transfected with either the gene for Kb (27) or the gene for Db (S. Vukmanović, unpublished observations) were also used. A murine thymic epithelial cell line, 1308.1 (H-2b) (28), or L-929 cells transfected with the gene for Db, L-Db (N. Hosken, unpublished observations), were used for in vitro infections.
Bacterial strains and media
All bacterial strains were grown on solid LB-agar medium. Liquid cultures were grown in LB-broth (29). Bacterial strains containing recombinant plasmids were supplemented with ampicillin at a final concentration of 100 µg/ml. Yersinia enterocolitica 8081 (30), used in the infection of tissue culture cells, was grown overnight at 30°C, diluted in LB-broth, and grown to midlogarithmic phase at 37°C. Escherichia coli strains used in the T7 overexpression system are described elsewhere (31–33).
Immunization with Yop51 protein-coated spleen cells
Purified rYop51 C403S protein was generously provided by Dr. Jack Dixon (University of Michigan, Ann Arbor, MI). Spleen cells from B6 mice were harvested, washed, and treated to lyse the RBCs selectively. The cells were then irradiated (2000 rad) and incubated for 10 min at 37°C in HBSS containing Yop51 C403S at a concentration of 2 mg/ml. After the incubation, 3 × 107 cells were injected into the lateral tail vein of C57BL/6 mice (34).
Stimulation and maintenance of Yop51-specific CTL
CTL populations were stimulated and maintained as previously described (35). Briefly, spleen cells from immunized mice were harvested 13 days after inoculation. The splenocytes were washed in RP-10, and cultures containing 3.5 × 107 splenocytes and 3 × 106 irradiated (2 × 104 rad) APCs were established in 10 ml of RP-10 in upright 25-cm2 flasks. The cells used for stimulation in the cultures were EL-4 tumor cells that had undergone cytoplasmic loading of Yop51 C403S as described below. Primary cytotoxic effector populations were harvested after 7 days, and 5 × 106 recovered cells were restimulated in a culture containing 3.5 × 107 irradiated (2000 rad) syngeneic spleen cells and 3 × 106 irradiated (2 × 104 rad) Yop51 C403S-loaded EL-4 cells in 10 ml RP-10. Subsequent weekly stimulations were conducted with 2.5 × 106 responder cells, 3.5 × 107 irradiated (2000 rad) syngeneic spleen cells, and 2.5 × 106 irradiated (2 × 104 rads) Yop51 C403S-loaded EL-4 cells in a medium containing RP-10 supplemented with 5% supernatant from Con A-stimulated rat spleen cells and 50 mM α-methyl-mannoside.
Cytoplasmic loading of Yop51 C403S
Exogenous protein was introduced into the cytoplasm of tumor cells essentially as described (35). In short, tumor cells were pelleted and resuspended in 50 µl of PBS containing 1 mg/ml rYop51 C403S. The cells and protein were mixed with an equal volume of a hypertonic medium containing RPMI 1640, 20 mM HEPES, 1 M sucrose, and 20% w/v PEG 1000, and incubated at 37°C for 10 min. The suspension was then diluted with 10 ml of isotonic RPMI 1640 containing 10 mM HEPES, and incubation was continued for an additional 3 min. The cells were then pelleted, resuspended in RP-10, and allowed to rest for 1 h at 37°C in 7% CO2 before use.
CTL assays
The activity of CTL was determined by using a chromium release assay. Target cells were resuspended in 100 µl to which 100 µCi sodium 51chromate was added. The cells were incubated at 37°C for 1 h, washed three times with RP-10, and diluted for use at 104 cells/well. Serial dilutions of CTL were added to assay wells such that the final assay volume was 200 µl in RP-10. In cases in which peptide preparations were added to test for targeting, the peptide was incubated with the target cells in the assay wells for 30 min before addition of the effector cells. Spontaneous release was determined in wells with target cells but without CTL. Maximum release was determined by adding detergent to wells containing target cells but no CTL. After 4-h incubation at 37°C, 100 µl of supernatant was evaluated on a Beckman 5500B gamma counter. Percentage of specific lysis was determined as follows:
Spontaneous release in the absence of CTL was less than 10% of maximal release by detergent in all experiments.
CTL proliferation assays
The ability of CTL to proliferate in response to Y. enterocolitica-infected cells was determined. L-Db cells grown in antibiotic-free RP-10 were infected with Y. enterocolitica at a multiplicity of infection of 100:1. The monolayers had been seeded 24 h before infection in 96-well tissue culture plates at 105 cells/well. The bacteria were centrifuged onto the monolayers at 1000 × g for 5 min and incubated at 37°C for 75 min. The medium was then removed and replaced with medium containing RP-10 (with antibiotics) supplemented with 5% supernatant from Con A-stimulated rat spleen cells and 50 mM α-methyl-mannoside. The assay plates were irradiated (2 × 104 rad) and 7 × 105 irradiated (2000 rad) syngeneic spleen cells and 3 × 104 CTL were added to each well. The assay plates were returned to the tissue culture incubator for 48 h. [3H]thymidine was then added to a final concentration of 2.5 µCi/ml. The assay plates were incubated for an additional 12 h, after which incorporation of [3H]thymidine into CTL was measured. All assays were conducted in triplicate.
Expression of Yop51 in E. coli
Plasmids containing the yop51 wild-type (36) and yop51 C403S genes under the control of the T7 bacteriophage promoter were provided by Dr. Jack Dixon. These plasmids were introduced into the E. coli strain K38 (31), previously transformed with pGPl-2 containing the T7 RNA polymerase under the control of the λp1 promoter that is repressed by a temperature-sensitive repressor (cI857) (32). Overexpression of the recombinant yop51 genes in these E. coli strains using this T7 polymerase/promoter expression system (32, 33) was accomplished by growing the doubly transformed bacteria at 30°C to an OD of 1.5. The temperature was then shifted to 42°C for 25 min. The cells were then grown an additional 2 h at 37°C in the presence of 100 µg/ml rifampicin. The bacterial cells were then harvested and used to generate targeting peptide as described below.
Generation of targeting peptide from recombinant protein
E. coli strains overexpressing the yop51 genes were processed to generate targeting peptide. Bacteria were induced for overexpression as described above. Samples containing 1 ml of the bacteria were pelleted, resuspended in 100 µl of 10 mg/ml Iysozyme in TE (10 mM Tris-Cl, pH 8.0, 0.5 mM EDTA), and allowed to incubate at room temperature for 30 min. The samples were then subjected to a single freeze-thaw cycle, boiled for 1 min, treated with 0.1 vol of 10 N NaOH, and boiled for an additional 15, 30, 45, 75, and 90 s. Samples were then neutralized with 1 N HC1 and placed on ice. Six microliters of each sample was used for each 104 target cell sample.
Determination of the targeting peptide epitope
Unidirectional deletion of the yop51 wild-type and yop51 C403S T7 plasmids was conducted on plasmid DNA cut with EcoRI and PstI, using Exonuclease III and S1 nuclease as described (29). The set of plasmids containing progressively shorter yop51 genes were religated and introduced into E. coli K38 (pGPl-2).
Yop51 249–257 was synthesized using an Applied Biosystems (Foster City, CA) 432A peptide synthesizer. Peptide concentration was determined using a Micro BCA reagent kit (Pierce, Rockford, IL).
Transfection and expression of yop51 in EL-4
A 1.6-kb DNA fragment containing the yop51 C403S gene was amplified from the T7 expression plasmids using PCR (37, 38). The upstream primer used in the PCR reaction (5′-GAA GGG TCG ACC GCC ATG AAC TTA TCA TTA AGC-3′) contains additional nonhomologous nucleotides at the 5′ end which introduce (5′ to 3′) a SalI recognition sequence and a consensus sequence for eucaryotic translation initiation (39) immediately upstream of the initiation codon. The second primer (5′-ATC GAT GAT AAG CTT GG-3′) was homologous to the sequence downstream of the termination codon and, in addition, amplified a segment of the vector polylinker containing a BamHI recognition sequence. The amplified product was cleaved with SalI and BamHI and ligated into the SalI and BamHI sites of the eucaryotic expression vector pHβAPr-1-neo (40). The resulting plasmid was linearized with PvuI, and 10 µg of DNA was electroporated (1500 mF, 50 µs) into EL-4 (41). Transfectants were selected using 400 µg/ml G418, and cloned by limiting dilution.
Immunoprecipitation and elution of peptides from Db molecules
Immunoprecipitation of Db molecules from the EL-4 (yop51 C403S) transfectant was conducted using the anti-Db Ab 28.14.8 and protein A-sepharose as previously described (27). Peptides were eluted from the immunoprecipitated Db molecules using 0.1% trifluoroacetic acid (TFA) and purified through a Centricon-10 microconcentrator (Amicon, Beverly, MA).
Elution of peptides from infected cells
Monolayers of the thymic epithelial cell line 1308.1 (H-2b), grown in antibiotic-free RP-10, were infected with Y. enterocolitica at a multiplicity of infection of 50:1. The monolayers had been seeded 24 h before infection on 100-mm tissue culture dishes at 107 cells/dish. The bacteria were centrifuged onto the monolayers at 1000 × g for 2 min, and incubated at 37°C for 3 h. The monolayers were then washed twice with antibiotic-free RP-10 and overlayed with RP-10 containing only gentamicin at a concentration of 100 µg/ml, killing all extracellular bacteria (42). The cells were then returned to 37°C for 3 h to allow for processing and presentation of Ag. The cells were removed from the tissue culture dishes with PBS, washed twice in PBS, and processed for elution of peptides (43). The cells were resuspended in 0.1% TFA, sonicated, and centrifuged for 30 min at 35,000 rpm in a Beckman SW-41 swinging bucket rotor. The supernatant was collected, passed through a Centriprep-10 microconcentrator (Amicon, Beverly, MA), and lyophilized. The pellet was resuspended in 0.1% TFA and fractionated by HPLC.
HPLC fractionation of peptides
Synthetic YopSl 249–257 peptides from Db molecules on the EL-4 (yop51 C403S) transfectant, and peptides from Y. enterocolitica infected 1308.1 cells, were each fractionated by reverse-phase (RP)-HPLC using a Delta-Pak C18 column (Waters, Milford, MA) on Waters HPLC instruments. Eluants used in the gradient were 0.1% TFA in H2O and 0.1% TFA in acetonitrile. The gradient conditions are as described elsewhere (5). Fractions were collected, lyophilized, resuspended in RPMI 1640, diluted in RP-10, and used in targeting assays.
Results
Derivation of Yop51-specific CTL
A previous report from this laboratory demonstrated that class I-restricted processing and presentation of exogenous Ag can occur in vivo after immunization with cells coated with protein (34). Splenocytes from C57BL/6 mice (H-2b) were incubated with purified rYop51 C403S protein, washed, and injected i.v. into syngeneic animals. The rYop51 protein used in this study has two single amino acid changes. The first, C235R, allows for a 10-fold increase in production of the recombinant protein in E. coli (36). The second, C403S, eliminates the phosphatase activity. This catalytic mutant protein was used to eliminate any possible toxic effects.
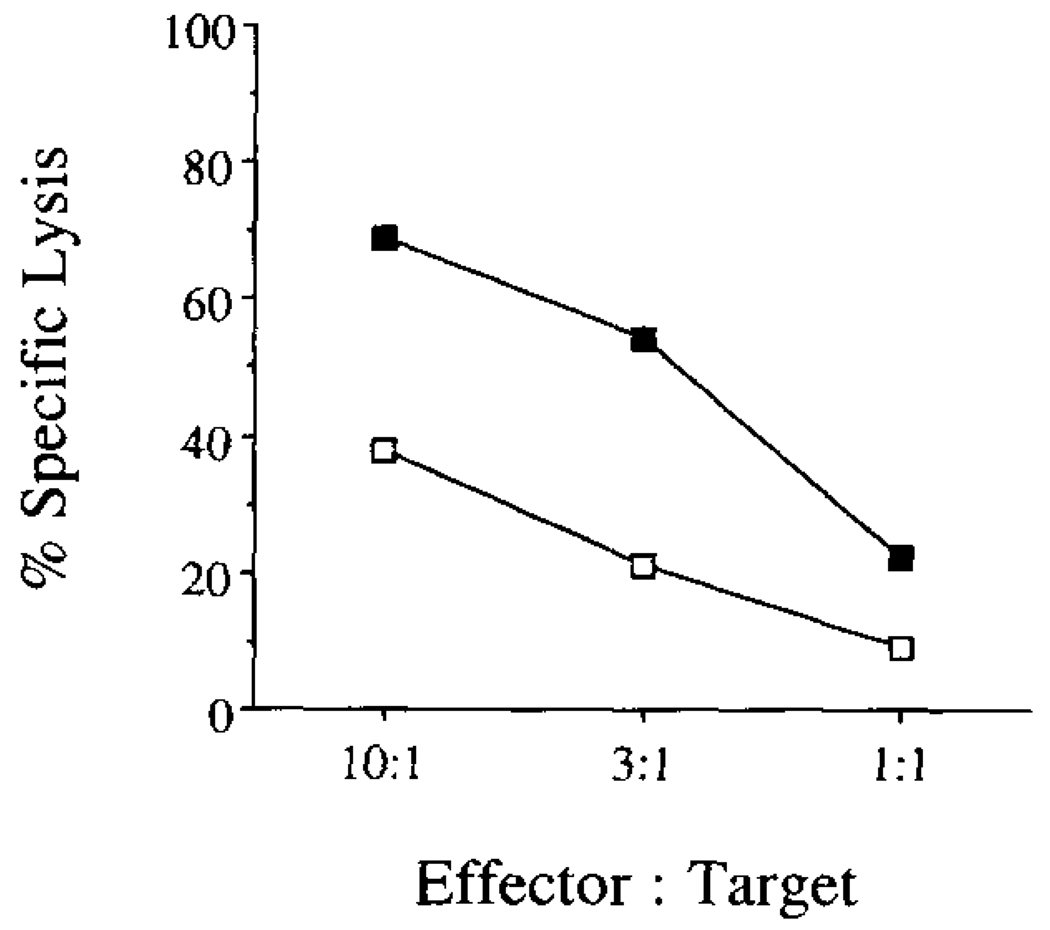
At day 13, the immunized mice were killed and spleen cells were stimulated on a syngeneic tumor cell line EL-4 that had undergone osmotic loading of Yop51 C403S protein into the cell cytoplasm. After 5 days of in vitro stimulation, the responding cells were tested for their ability to lyse the tumor cell line EL-4 which had undergone osmotic loading of Yop51 C403S protein into the cell cytoplasm. Specific lysis of the protein-loaded APCs was measured in a 4-h chromium release assay. As can be seen in Figure 1, there is specific lysis of APCs that have undergone loading with Yop51, indicating the presence of CTL specific for Yop51. The CTL line generated in this manner was numbered Yop51 line 6.0.
FIGURE 1.
Lysis of protein-loaded target cells by Yop51-specific CTLs in a 51Cr release assay. Spleen cells from immunized mice were used after 5-day stimulation in vitro as effector cells on either EL-4 cells cytoplasmically loaded with Yop51 C403S (—■—) or untreated (—□—).
Yop51-specific CTL proliferate in response to Yersinia-infected cells
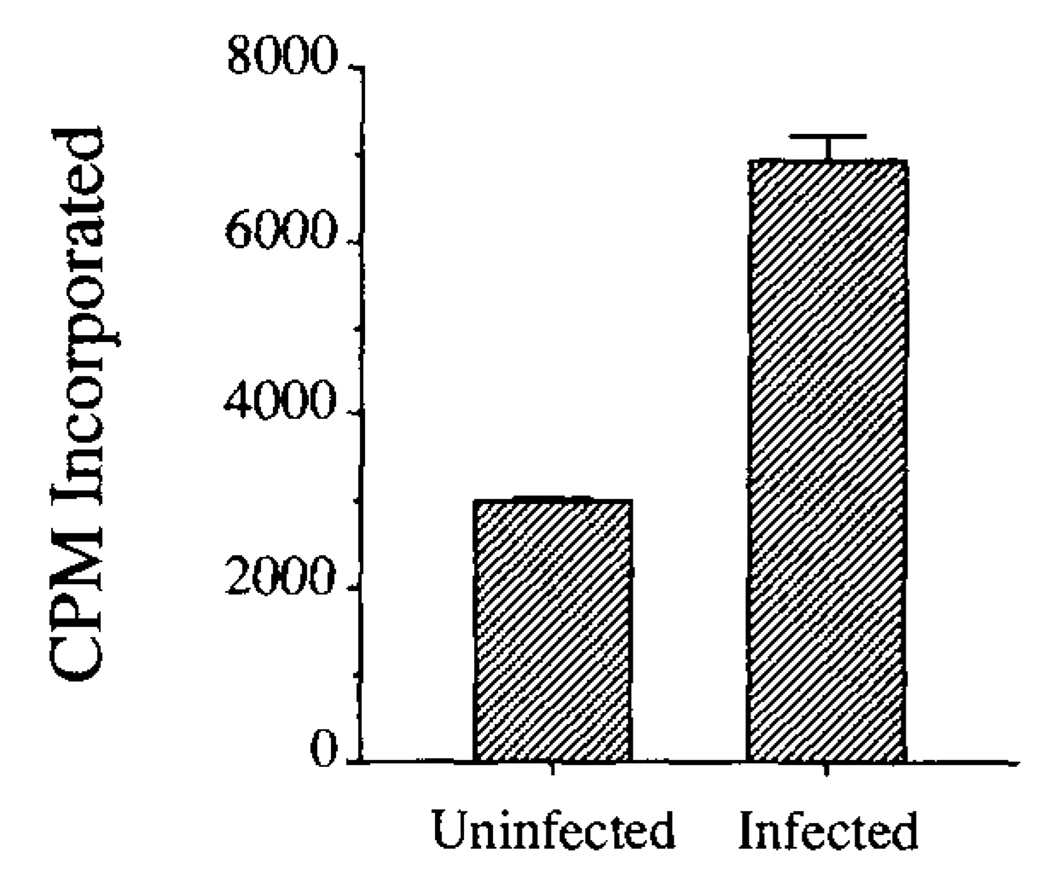
Cytotoxic T lymphocytes proliferate in response to cells presenting cognate Ag. One way to test whether the Yop51 epitope recognized by the CTL was presented on infected cells was to determine whether cultured cells infected with Y. enterocolitica could stimulate the proliferation of Yop51 CTL line 6.0. The ability of infected and uninfected cells to stimulate proliferation was compared. As shown in Figure 2, the Yersinia-infected cells were better able to stimulate proliferation of the Yop51-specific CTL. These data suggested that an epitope from Yop51 was presented on cells during Yersinia infection.
FIGURE 2.
Proliferation of Yop51-specific CTL in response to Yersinia-infected cells. The ability of infected and uninfected L-Db cells to stimulate proliferation of the Yop51-specific CTL was measured by incorporation of [3H]thymidine in a proliferation assay. Error bars delineate SEM.
Yop51-specific CTL recognize Ag in the context of H-2 Db
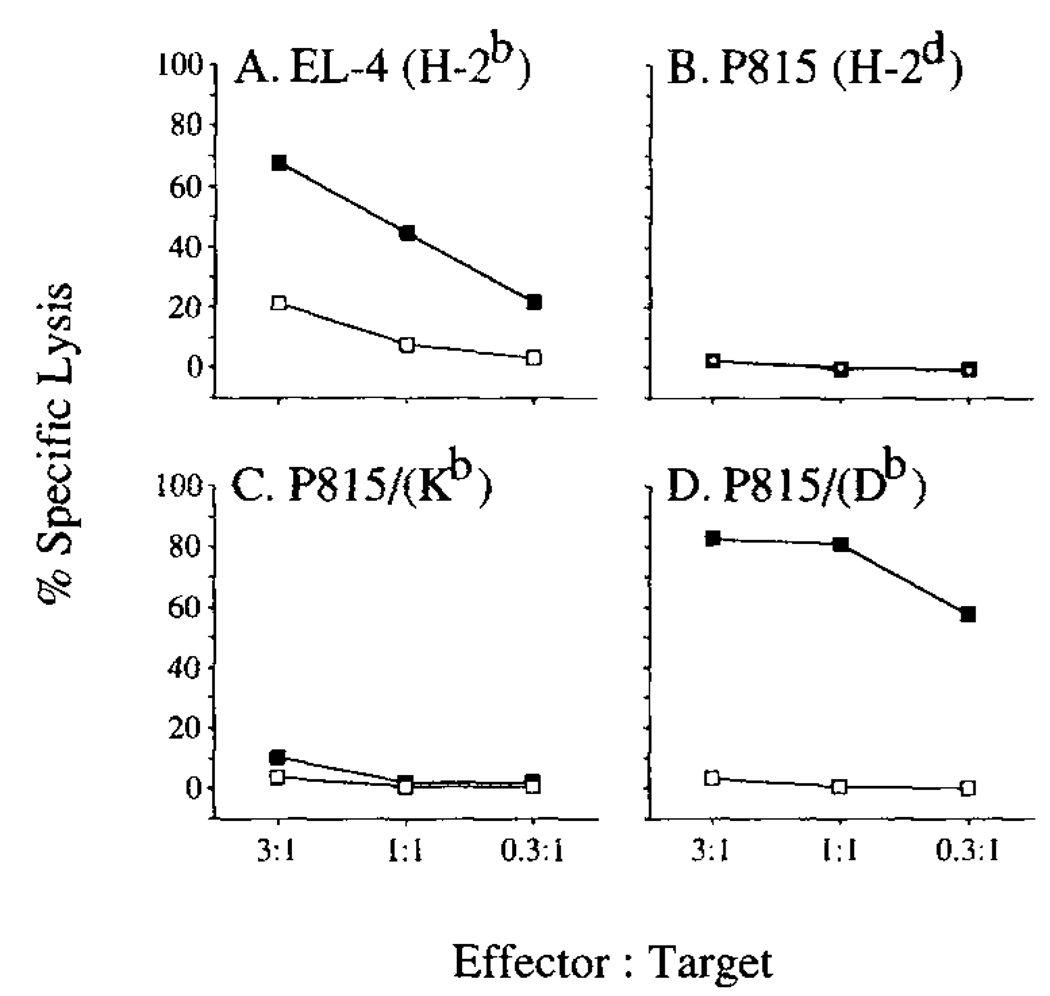
To define the MHC restriction of the epitope recognized by Yop51-specific CTL, we tested the ability of Yop51 line 6.0 to recognize an allogeneic tumor cell line P815 (H-2d) which had been transfected with either the gene for H-2 Kb or H-2 Db, and which had been cytoplasmically loaded with Yop51 C403S. The results of this chromium release assay are shown in Figure 3. The data in Figure 3A show specific lysis of EL-4 cells loaded with Yop51 C403S. In Figure 3B, the data show no lysis of the protein loaded allogeneic tumor cells P815. When P815 cells transfected with the gene for Kb were loaded with purified Yop51 C403S, they were not significantly lysed by Yop51 line 6.0 (Fig. 3C). However, when P815 cells transfected with the gene for Db were loaded with purified Yop51 C403S, there was extensive lysis of the presenting cells by Yop51 line 6.0 (Fig. 3D). This suggested that the predominant activity of Yop51 line 6.0 was restricted to H-2 Db.
FIGURE 3.
Determination of the MHC class I molecule presenting Ag to the Yop51-specific CTL. Target cells are shown at the top of each panel. The ability of the Yop51-specific CTL line to recognize Ag presented by these APCs was tested in a 51Cr release assay. Cells were cytoplasmically loaded with Yop51 C403S (—■—) or untreated (—□—).
Expression of Yop51 in E. coli can be used to generate targeting peptide
Unlike protein Ag, which must be introduced into the cytoplasm of the APC and processed for presentation by MHC class I, peptide can be exogenously added to surface MHC molecules (44). Recent work in our laboratory has shown that overexpression of various genes in an E. coli expression system can be used to generate peptides that will allow targeting of specific CTL (45). Here, the yop51 wild-type and yop51 C403S genes were each introduced into an E. coli overexpression system driven by the bacteriophage T7 promoter and T7 RNA polymerase. E. coli induced to overexpress the genes were lysed and treated by boiling in NaOH for various periods of time. This treatment cleaves some of the protein of interest into peptides of a size able to bind to MHC molecules on the surface of APCs.
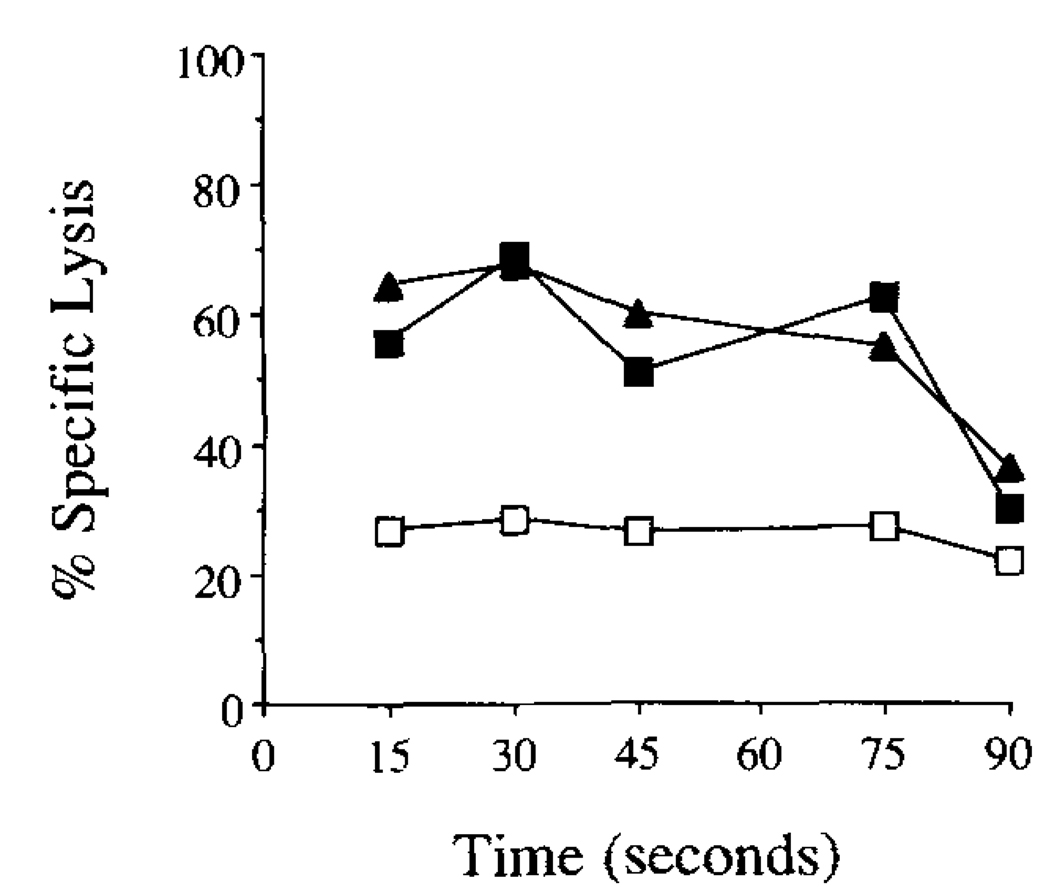
As can be seen in Figure 4, boiling the E. coli strain containing either yop51 gene in 1 N NaOH is sufficient to release peptide able to target EL-4 APCs for lysis by Yop51 line 6.0 CTL. This also suggested that the epitope presented to these CTL was shared by the wild-type and the catalytic mutant enzyme.
FIGURE 4.
Targeting of APCs for lysis using peptide generated from recombinant E. coli overexpressing yop51. Samples obtained by treatment of recombinant E. coli overexpressing either wild-type Yop51 (—■—), Yop51 C403S (—▲—), or without the plasmid containing a recombinant protein gene (—□—). Samples containing the recombinant E. coli were treated and subjected to boiling in 1 N NaOH for various periods of time. The samples were then incubated with EL-4 and tested as targets for the Yop51-specific CTL line in a 51Cr release assay. The E:T ratio used in the assay was 10:1.
Yop51 line 6.0 recognizes a specific nonamer peptide, Yop51 249–257
To determine the specific peptide that is presented to the CTL on the H-2 Db molecule, we examined the published amino acid sequence of Yop51 (46). Falk et al. (47) and others have shown that the peptides known to bind Db are typically nine amino acids long and contain an asparagine at position 5. The 21 peptides from the sequence of Yop51 that meet these criteria are shown in Table I.
Table 1.
List of peptides from Yop51 that fit the Db peptide binding motifa
| Position |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 26 | K | L | R | G | N | V | A | A | N |
| 30 | N | V | A | A | N | K | E | T | T |
| 61 | S | H | V | A | N | I | V | L | T |
| 80 | T | V | K | H | N | L | N | N | Y |
| 82 | K | H | N | L | N | N | Y | E | L |
| 83 | H | N | L | N | N | Y | E | L | R |
| 91 | R | S | V | G | N | G | N | S | V |
| 93 | V | G | N | G | N | S | V | L | V |
| 202 | T | T | L | R | N | T | L | A | P |
| 209 | A | P | A | T | N | D | P | R | Y |
| 223 | G | E | K | L | N | R | F | R | D |
| 241 | R | A | D | L | N | A | N | Y | I |
| 243 | D | L | N | A | N | Y | I | Q | V |
| 249 | I | Q | V | G | N | T | R | T | I |
| 273 | M | L | A | E | N | R | T | P | V |
| 289 | S | E | I | A | N | Q | R | F | G |
| 349 | V | H | V | G | N | W | P | D | Q |
| 377 | E | T | K | R | N | M | Y | E | S |
| 416 | A | M | C | M | N | D | S | R | N |
| 420 | N | D | S | R | N | S | Q | L | S |
| 437 | R | V | Q | R | N | G | I | M | V |
Shown are the 21 peptides that fit the motif reported for Db binding peptides. The number to the left of each sequence is the amino acid number of the N-terminal residue. The numbers at the top are the position of each amino acid. Requirements described for binding of peptides to Db are that the peptide be 9-amino acids in length and have an asparagine at position 5.
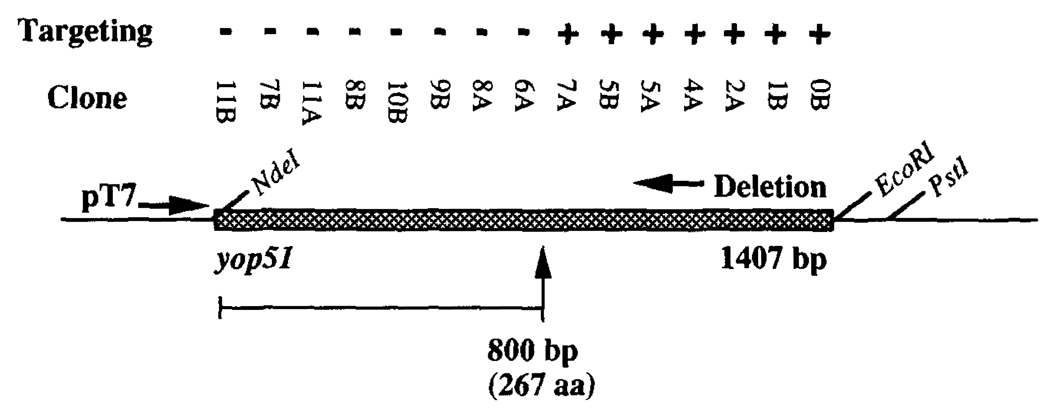
We then generated a series of nested unidirectional deletions in the plasmid used to express yop51 in the T7 expression system (Fig. 5). The deletions removed increasing lengths of the gene from the 3’ end. The plasmids of this deletion series were then reintroduced into the E. coli T7 expression system, grown, induced, and boiled for 30 s in NaOH as described above to release targeting peptide. The smallest plasmid that yielded targeting activity contained only the first 800 bp of the yop51 gene. The targeting activity was lost in all smaller plasmids. Among the peptides listed in Table I, the peptide coded for immediately upstream of the point where targeting activity was last observed is peptide 249–257.
FIGURE 5.
Deletions in the yop51 gene and targeting using peptide from recombinant E. coli clones. Shown is the strategy used to make nested deletions in the plasmid used to overexpress wild-type yop51 in E. coli. These plasmids were returned to the T7 expression system, treated as described, and assayed for the ability to target EL-4 for lysis in a 51Cr release assay. The ability of each clone to generate targeting peptide is indicated with a (+) or (−). Designation with a (+) indicates at least 10% specific lysis. The E:T ratio used in the assay was 10:1.
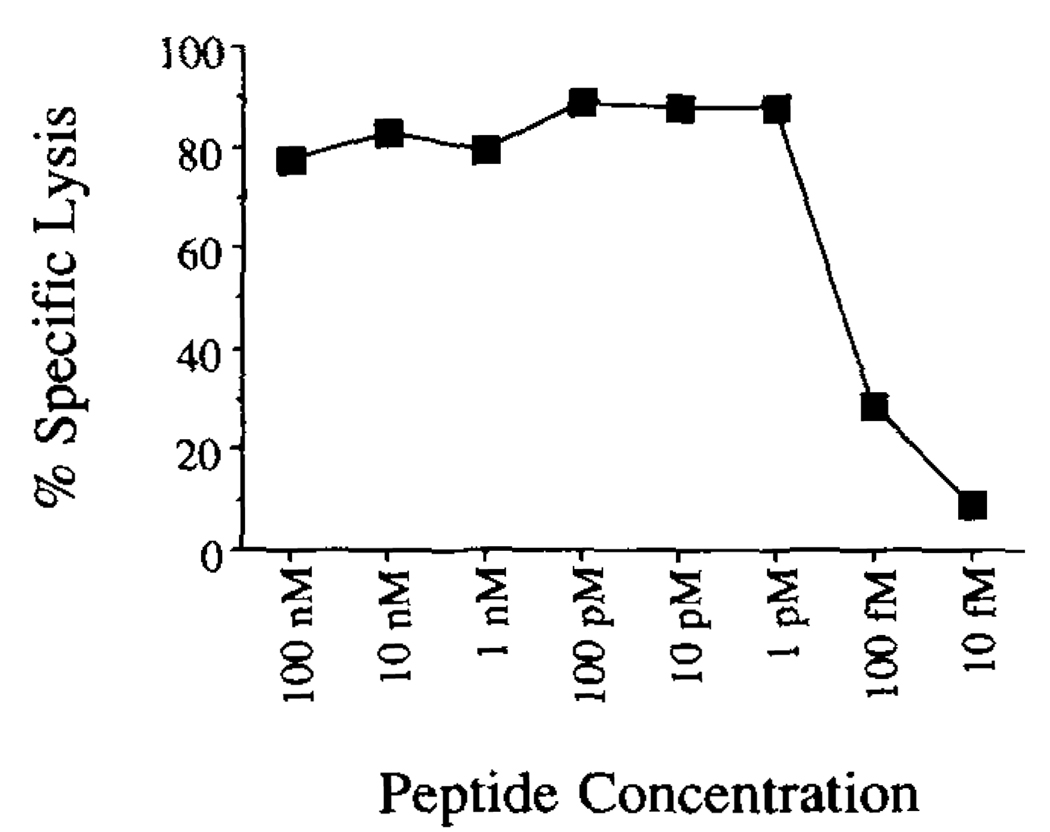
Yop51 249–257 was chemically synthesized and tested for the ability to target EL-4 for lysis by Yop51 line 6.0 CTL. As shown in Figure 6, the synthetic peptide is able to target EL-4 for lysis at concentrations as low as 1 pmol. Targeting activity diminished to background levels when the peptide was diluted to 10 femtomolar. The activity of this peptide is consistent with other natural peptides which have been characterized (43).
FIGURE 6.
Ability of synthetic peptide Yop51 249–257 to target EL-4 for lysis by the Yop51-specific CTL. The product of the chemical synthesis of Yop51 249–257 was fractionated by RP-HPLC and the fraction containing the most material (fraction 27) was lyophilized, resuspended in PBS, subjected to peptide quantitation, and diluted into RP-10 to test for targeting. Various dilutions of the peptide were tested for the ability to target EL-4 for lysis in a 51Cr release assay. The E:T ratio used in the assay was 8:1.
Construction of an APC line expressing Yop51
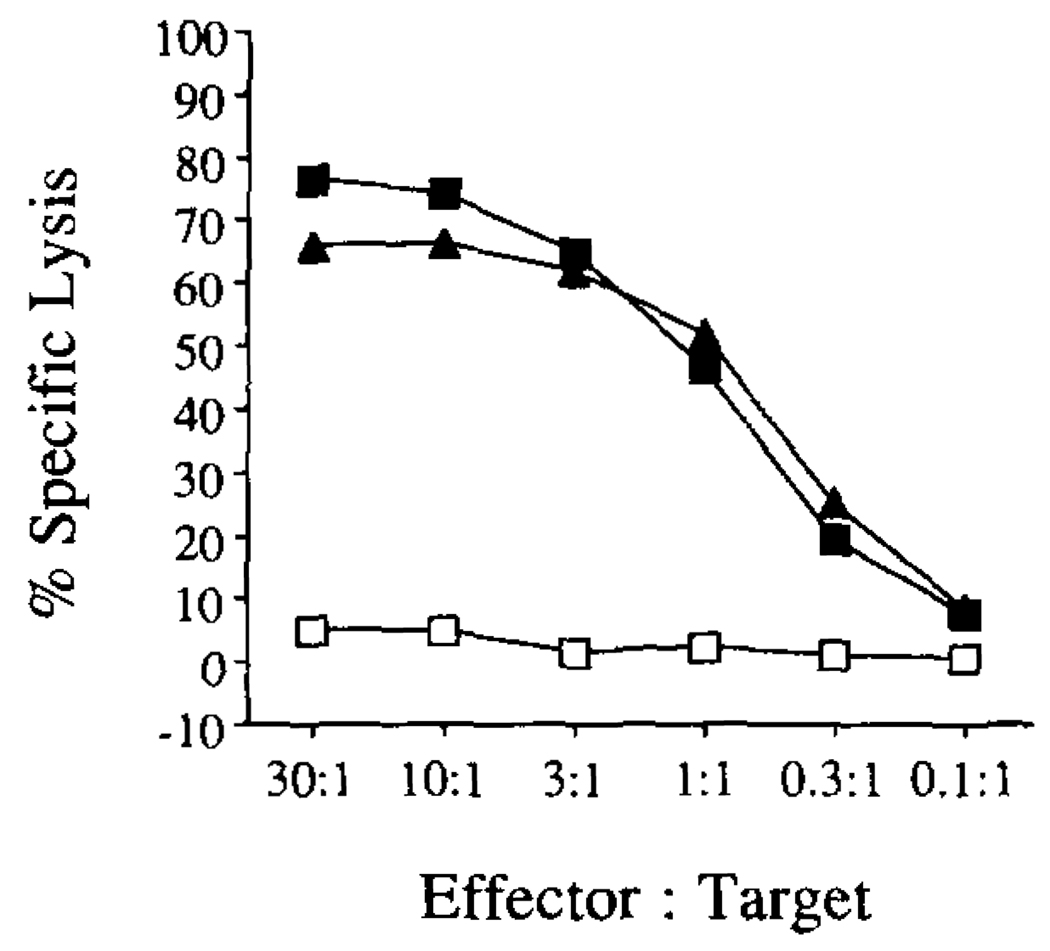
To study the processing and presentation of Yop51 within a eucaryotic cell, we introduced the yop51 C403S gene into EL-4. The gene was introduced into a eucaryotic expression vector, the vector was linearized, and then electroporated into EL-4. Stable transfectants were selected using G418, and clones were tested for their ability to present Ag to the Yop51-specific CTL line. One clone was chosen for additional study. The ability of the Yop51 line 6.0 CTL to recognize this transfectant is shown in Figure 7.
FIGURE 7.
Ability of Yop51-specific CTL to recognize EL-4 transfected with the yop51 C403S gene. EL-4 transfected with the yop51 C403S gene (—▲—) was tested for its ability to present Yop51 Ag to the Yop51-specific CTL line in a 51Cr release assay. Also tested was EL-4 transfected with the eucaryotic expression vector alone (—□—), and the vector control cytoplasmically loaded with Yop51 C403S protein (—■—).
In addition to the studies described below, this transfectant has been the APC used in the continued propagation of the Yop51-specific CTL line.
Natural targeting peptide from the yop51 C403S-transfected APC coelutes with synthetic Yop51 249–257 upon HPLC
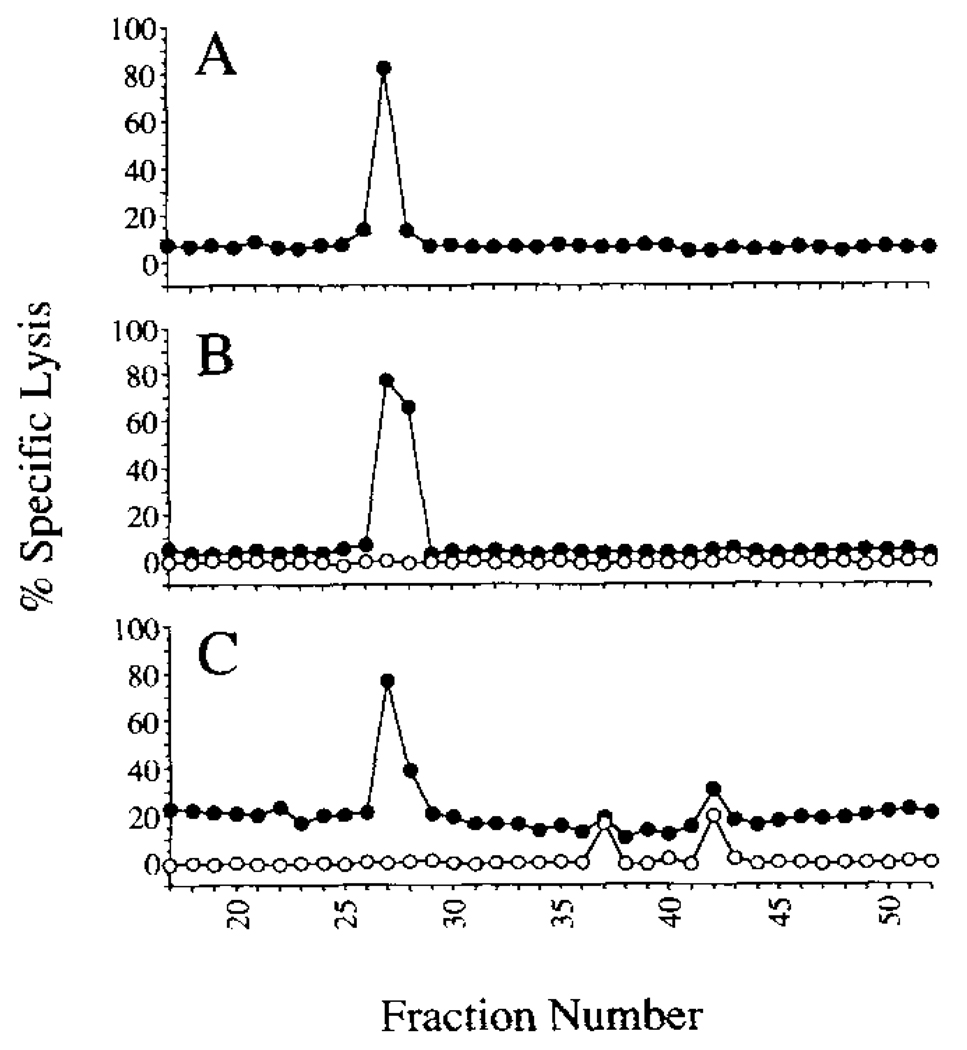
To determine what natural peptide is being presented by the yop51 C403S-transfectant to the Yop51-specific CTL line 6.0, we immunoprecipitated lysates of the transfectant with Ab specific for the Db molecule. The immunoprecipitated material was then treated with 0.1% TFA to elute peptide from the Db molecules and was separated by RP-HPLC. Fractions were collected and tested for the ability to target EL-4 for lysis by the Yop51-specific CTL. Fractions were similarly collected and tested after HPLC separation of the synthetic peptide Yop51 249–257. As shown in Figure 8, A and B, the targeting activity from both the synthetic peptide and the natural peptides from the transfectant was found to be contained within fraction 27. This result suggests that Yop51 249–257 is the dominant epitope on Db recognized by the Yop51-specific CTL line 6.0.
FIGURE 8.
Targeting activity of HPLC-fractionated peptides for Yop51-specific CTL Samples containing targeting peptides were fractionated by RP-HPLC. Each fraction was lyophilized, resuspended in RP-10, and tested in a 51Cr release assay for the ability to target EL-4 for recognition by the Yop51-specific CTL line. In panel A, the product of the chemical synthesis of Yop51 249–257 was fractionated and tested (—●—). In panel B, the sample consisted of peptides eluted from Db molecules immunoprecipitated from EL-4 transfected with yop51 C403S (—●—). As a control for toxicity of cellular components that might co-elute with peptides, each fraction was also tested for the ability to cause chromium release in the absence of CTL (—○—). In panel C, the sample consisted of total peptide eluted from the surface of 1308.1 epithelial cells infected with Y. enterocolitica (—●—). Chromium release in the absence of CTLs was also assessed for each of these fractions (—○—). In all of the preceding CTL assays the E:T ratio used was 10:1.
The targeting activity of peptides eluted from Yersinia-infected cells coelutes with Yop51 249–257
To determine whether tissue culture cells infected with Y. enterocolitica can present a Yop51 epitope to the Yop51-specific CTL, we infected the H-2b epithelial cell line 1308.1 with Y. enterocolitica and eluted MHC-bound peptides from infected cells. The eluted peptides were then fractionated by HPLC, and each of the fractions was tested for targeting activity using the Yop51-specific CTL. As seen in Figure 8C, the targeting activity resided in a single major peak centered on fraction 27. Fractions causing lysis of target cells in later fractions are not CTL-dependent and likely result from toxic material which eluted in these fractions. As described above, the Yop51 249–257 peptide also elutes in fraction 27. This suggests that Yop51 is introduced into the class I processing pathway and presented on the surface of infected cells within Db peptide binding grooves. Because MHC class I typically draws from proteins found in the cytosol, this supports the hypothesis that Yop51 is introduced into the cytoplasm of these cells during infection.
Discussion
The Yersinia Yop proteins are unique not only in the mechanism used in their secretion (48, 49), but also in the presumption that some Yops are introduced into the host cell cytoplasm following intimate binding of bacteria to the host cell surface (9, 23, 50). This led us to consider whether these translocated Yops are processed in the cytoplasm of host cells and presented by MHC class I molecules. Very few studies have been able to identify specific bacterial proteins which serve as targets for T cell recognition. Here, we show that one of the Yop proteins, Yop51, made by members of the pathogenic Yersiniae is processed by host cells and presented by MHC class I molecules. Yop51, which is known to be a tyrosine phosphatase (21), and is presumed to act primarily on host proteins (22, 24), contains a CTL epitope presented in the context of H-2 Db. We used a combination of approaches to determine the amino acid sequence of this epitope and to provide evidence that this epitope, Yop51 249–257, is presented on eucaryotic cells expressing this protein endogenously and in cells infected with Y. enterocolitica.
It is clear from recent studies that T cells play an important role in host resistance to Yersinia. Athymic nude C57BL/6 mice are more susceptible to Y. enterocolitica infection than their euthymic littermates, suggesting an important role for T lymphocytes in resistance to this pathogen (25). Both CD4+ and CD8+ T lymphocytes are found in liver lesions induced by Y. enterocolitica infection; however, there is no evidence that these T cells are Yersinia-specific (51). Others have shown that both helper and cytotoxic T cells are generated in C56BL/6 mice in response to Y. enterocolitica infection, that these cells recognize APC fed heat-killed Y. enterocolitica, and that transfer of both helper and cytotoxic clones could mediate partial immunity to Y. enterocolitica (26).
Much of the focus of other reports of T cell immunity to the Yersiniae has been on the autoimmune sequelae of Yersinia infections. A subset of patients who recover from these infections later develop a reactive arthritis (52–54), in a process which has been linked with the MHC haplotype of the host. Interestingly, these spondyloarthropathies occur most frequently in patients expressing HLA-B27, a class I MHC molecule (55); however, this association may result from the effects of other closely linked genes. There have been several reports that have characterized T cells from the synovial fluid of human patients with reactive arthritis. One study examined the activation of T helper type 1-like population in response to Y. enterocolitica sonicates (56); however, it is possible that the expansion of some T cell populations in the presence of whole Yersinia protein may result from the action of a recently described Yersinia superantigen (57). Others have also described T helper clones derived from patient synovial fluid that respond to Y. enterocolitica, one of which was stimulated in response to heat-stressed APC, as well as the mycobacterial and human 65-kDa heat shock protein (58). There is also evidence that synovial fluid from patients with reactive arthritis contains T cells that can recognize Y. enterocolitica-infected target cells in a MHC unrestricted manner (59). The T cells responsible for this recognition were CD8+ CTL. None of these previous studies defined a specific Yersinia protein or peptide epitope recognized by responding CTL.
The role of Yop-specific CTL in the course of Yersinia infection has not been previously explored. One may speculate that cells infected with Yersinia and presenting peptide Ags from proteins such as Yop51 may elicit a CTL response. At least two possible roles can be envisioned for Yop51-responsive CTL in Yersinia infection. First, IFN-γ is produced by Ag-stimulated CTL. IFN-γ is known to activate macrophages to phagocytose and to kill extracellular microorganisms by inducing enzymes that stimulate the respiratory burst (60, 61). Cells in intimate contact with extracellular Yersinia may present the Yop51 peptide Ag to CTL stimulating IFN-γ production. This may be a critical response in the recognition and clearance of a pathogen able to inhibit phagocytosis by macrophages. A recent report has shown that when Abs against IFN-γ are introduced into infected mice, the animals are more susceptible to Y. enterocolitica, suggesting an important role for this lymphokine in host resistance (62). An alternative mechanism for Yop51 249–257 presentation by MHC class I which would also result in IFN-γ production by responding CTL is suggested by a recent report in which Gram-negative bacteria expressing foreign proteins were incubated with macrophages, resulting in the targeting of peptides from the foreign protein onto the surface of neighboring cells (63). There is no evidence, however, that such a result could be demonstrated using nonprofessional phagocytes as described here. Second, it is possible that the mechanisms used by Yersinia to enter into nonprofessional cells in vitro occurs to some extent in vivo. Bacteria that are able to enter into such cells are generally protected from the immune system unless proteins from the bacteria are introduced into the class I MHC processing pathway which samples proteins found in the cytoplasm. The translocation of Yop51 into the cytoplasm of these cells may mark the host cell for lysis by CTL and expose the intracellular bacteria to elements of natural and humoral immunity. In addition, the response of CTL to these infected cells, and the resulting production of IFN-γ, may induce production of class II MHC molecules on nonprofessional cells. Such up-regulation of MHC class II in response to IFN-γ has been described for a variety of tissues and cells (64), and may allow for a response by T helper populations.
We are presently investigating the role of Yop51 presentation in the pathogenesis of Yersinia to determine if Yop51 249–257 is presented during murine infection, whether Yop51-specific CTL are generated in vivo during infection, and what effect CTL specific for Yop51 may have on the course of infection. In addition, at least two other Yersinia proteins are thought to be translocated into the cytoplasm of infected cells, YopE and YpkA (9, 50). YopE is a cytotoxin, which directs the destruction of host cell microfilaments, causing rounding of cultured cells and detachment of the cell monolayer from the extracellular matrix (18, 50). YpkA has recently been described as a Ser/Thr protein kinase (65). Presentation of epitopes from these additional Yersinia proteins may also occur during infection, increasing the number of candidate proteins available for presentation by multiple MHC alleles. In addition, the role of IFN-γ in resistance to Yersinia can now be explored further using mice, recently described, in which the gene for IFN-γ has been disrupted (66).
Acknowledgments
We are grateful to Drs. J. Dixon and J. Clemens for generously providing the yop51 expression vectors and purified recombinant protein. We thank Drs. J. Harty, S. Jameson, and K. Hogquist for critical review of the manuscript.
Footnotes
This work was supported by Public Health Service Grant A119335 and the Howard Hughes Medical Institute.
Abbreviations used in this paper: LLO, listeriolysin O (protein); TFA, trifluoroacetic acid; RP-HPLC, reverse-phase HPLC; RP-10, medium consisting of RPMI 1640 supplemented with l-glutamine, 50 µM 2-ME, antibiotics (except when noted), and 10% FCS; LB, Luria-Bertani.
References
- 1.Monaco JJ. A molecular model of MHC class-I-restricted antigen processing. Immunol. Today. 1992;13:173. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- 2.Braciale TJ, Braciale VL. Antigen presentation: structural themes and functional variations. Immunol. Today. 1991;12:124. doi: 10.1016/0167-5699(91)90096-C. [DOI] [PubMed] [Google Scholar]
- 3.Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J. Immunol. 1990;145:1265. [PubMed] [Google Scholar]
- 4.Bouwer HG, Nelson CS, Gibbins BL, Portnoy DA, Hinrichs DJ. Listeriolysin O is a target of the immune response to Listeria monocytogenes. J. Exp. Med. 1992;175:1467. doi: 10.1084/jem.175.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pamer EG, Harty JT, Bevan MJ. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353:852. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harty JT, Bevan MJ. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J. Exp. Med. 1992;175:1531. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brubaker RR. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol. Rev. 1991;4:309. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis GR, Biot T, Lambert de Rouvroit C, Michiels T, Mulder B, Sluiters C, Sory M-P, Van Bouchaute M, Vanooteghem J-C. The Yersinia yop regulon. Mol. Microbiol. 1989;3:1455. doi: 10.1111/j.1365-2958.1989.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 9.Straley SC, Skrzypek E, Piano GV, Bliska JB. Yops of Yersinia spp. pathogenic for humans. Infect. Immun. 1993;61:3105. doi: 10.1128/iai.61.8.3105-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonet M, Falkow S. Invasin expression in Yersinia pseudotuberculosis. Infect. Immun. 1992;60:4414. doi: 10.1128/iai.60.10.4414-4417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian C-J, Hwang WS, Kelly JK, Pai CH. Invasiveness of Yersinia enterocolitica lacking the virulence plasmid: an in-vivo study. J. Med. Microbiol. 1987;24:219. doi: 10.1099/00222615-24-3-219. [DOI] [PubMed] [Google Scholar]
- 12.Hanski C, Kutschka U, Schmoranzer HP, Naumann M, Stallmach A, Hahn H, Menge H, Riecken EO. Immunohisto-chemical and electron microscopic study of interaction of Yersinia enterocolitica serotype 08 with intestinal mucosa during experimental enteritis. Infect. Immun. 1989;57:673. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimura Y, Kihara T, Mine H. Membranous cells as a portal of Yersinia pseudotuberculosis entry into rabbit ileum. J. Clin. Electron. Microsc. 1992;25:35. [Google Scholar]
- 14.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 1990;58:841. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isberg RR. Mammalian cell adhesion functions and cellular penetration of enteropathogenic Yersinia species. Mol. Microbiol. 1989;3:1449. doi: 10.1111/j.1365-2958.1989.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 16.Bliska JB, Copass MC, Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect. Immun. 1993;61:3914. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect. Immun. 1988;56:2139. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosqvist R, Forsberg Å, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 1990;4:657. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 19.Lian CJ, Hwang WS, Pai CH. Anti-phagocytic role of plasmid-associated outer membrane proteins of Yersinia enterocolitica. Contrib. Microbiol. Immunol. 1987;9:186. [PubMed] [Google Scholar]
- 20.Lian C-J, Hwang WS, Pai CH. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect. Immun. 1987;55:1176. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan K, Dixon JE. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 22.Bliska JB, Guan K, Dixon JE, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. USA. 1991;88:1187. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bliska JB, Galán JE, Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993;73:903. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- 24.Bliska JB, Clemens JC, Dixon JE, Falkow S. The Yersinia tyrosine phosphatase: specificity of a bacterial virulence determinant for phosphoproteins in the J774A.1 macrophage. J. Exp. Med. 1992;176:1625. doi: 10.1084/jem.176.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Autenrieth IB, Vogel U, Preger S, Heymer B, Heesemann J. Experimental Yersinia enterocolitica infection in euthymic and T-cell deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect. Immun. 1993;61:2585. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autenrieth IB, Tingle A, Reske KA, Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect. Immun. 1992;60:1140. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandea AG, III, Bevan MJ. Single-residue changes in class I major histocompatibility complex molecules stimulate responses to self peptides. Proc. Natl. Acad. Sci. USA. 1992;89:2794. doi: 10.1073/pnas.89.7.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faas SJ, Rothstein JL, Kreider BL, Rovera G, Knowles BB. Phenotypically diverse mouse thymic stromal cell lines which induce proliferation and differentiation of hematopoietic cells. Eur. J. Immunol. 1993;23:1201. doi: 10.1002/eji.1830230602. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 30.Portnoy DA, Moseley SL, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect. Immun. 1981;31:775. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russel M, Model P. Replacement of the fip gene of Escherichia coli by an inactive gene cloned on a plasmid. J. Bacteriol. 1984;159:1034. doi: 10.1128/jb.159.3.1034-1039.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabor S, Richardson CC. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA. 1985;82:1074. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabor S. Expression using the T7 RNA polymerase/promoter system. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Current Protocols; 1990. Unit 16.2. [Google Scholar]
- 34.Carbone FR, Bevan MJ. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J. Exp. Med. 1990;171:377. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z-Y, Clemens JC, Schubert HL, Stuckey JA, Fischer MWF, Hume DM, Saper MA, Dixon JE. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J. Biol. Chem. 1992;267:23759. [PubMed] [Google Scholar]
- 37.Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 38.Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 39.Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984;12:857. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunning P, Leavitt J, Muscat G, Ng S-Y, Kedes L. A human β-actin expression vector system directs high-level accumulation of antisense transcripts. Proc. Natl. Acad. Sci. USA. 1987;84:4831. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potter H, Weir L, Leder P. Enhancer-dependent expression of human κ immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc. Natl. Acad. Sci. USA. 1984;81:7161. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isberg RR, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 43.Rötzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee H-G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990;348:252. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- 44.Townsend ARM, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 45.Gavin MA, Gilbert MJ, Riddell SR, Greenberg PD, Bevan MJ. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J. Immunol. 1993;151:3971. [PubMed] [Google Scholar]
- 46.Michiels T, Cornelis G. Nucleotide sequence and transcription analysis of yop51 from Yersinia enterocolitica W22703. Microb. Pathog. 1988;5:449. doi: 10.1016/0882-4010(88)90006-x. [DOI] [PubMed] [Google Scholar]
- 47.Falk K, Rötzschke O, Stevanović S, Jung G, Rammensee H-G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 48.Michiels T, Wattiau P, Brasseur R, Ruysschaert J-M, Cornelis G. Secretion of Yop proteins by Yersiniae. Infect. Immun. 1990;58:2840. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michiels T, Cornelis GR. Secretion of hybrid proteins by the Yersinia Yop export system. J. Bacteriol. 1991;173:1677. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosqvist R, Forsberg Å, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 1991;59:4562. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Autenrieth IB, Hantschmann P, Heymer B, Heesemann J. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiol. 1993;187:1. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- 52.Winblad S. Arthritis associated with Yersinia enterocolitica infections. Scand. J. Infect. Dis. 1975;7:191. doi: 10.3109/inf.1975.7.issue-3.07. [DOI] [PubMed] [Google Scholar]
- 53.Solem JH, Lassen J. Reiter’s disease following Yersinia enterocolitica infection. Scand. J. Infect. Dis. 1971;3:83. doi: 10.3109/inf.1971.3.issue-1.16. [DOI] [PubMed] [Google Scholar]
- 54.Leirisalo-Repo M. Yersinia arthritis. Acute clinical picture and long-term prognosis. Contrib. Microbiol. Immunol. 1987;9:145. [PubMed] [Google Scholar]
- 55.Aho K, Ahvonen P, Lassus A, Sievers K, Tilikainen A. HL-A antigen 27 and reactive arthritis. Lancet. 1973;2:157. doi: 10.1016/s0140-6736(73)93109-7. [DOI] [PubMed] [Google Scholar]
- 56.Lahesmaa R, Yssel H, Batsford S, Luukkainen R, Möttönen T, Steinman L, Peltz G. Yersinia enterocolitica activates a T helper type 1-like T cell subset in reactive arthritis. J. Immunol. 1992;148:3079. [PubMed] [Google Scholar]
- 57.Stuart PM, Woodward JG. Yersinia enterocolitica produces superantigenic activity. J. Immunol. 1992;148:225. [PubMed] [Google Scholar]
- 58.Hermann E, Lohse AW, Van der Zee R, Van Eden W, Mayet WJ, Probst P, Poralla T, Meyer zum Büschenfelde K-H, Fleischer B. Synovial fluid-derived Yersinia-reactive T cells responding to human 65-kDa heat-shock protein and heat-stressed antigen-presenting cells. Eur. J. Immunol. 1991;21:2139. doi: 10.1002/eji.1830210923. [DOI] [PubMed] [Google Scholar]
- 59.Hermann E, Mayet W-J, Meyer zum Büschenfelde KH, Fleischer B. MHC-unrestricted recognition of bacteria-infected target cells by human CD8+ cytotoxic T lymphocytes. Cell. Immunol. 1992;143:253. doi: 10.1016/0008-8749(92)90023-i. [DOI] [PubMed] [Google Scholar]
- 60.Murray HW. The interferons, macrophage activation, and host defense against nonviral pathogens. J. Interferon Res. 1992;12:319. doi: 10.1089/jir.1992.12.319. [DOI] [PubMed] [Google Scholar]
- 61.Murray HW. Interferon-γ, the activated macrophage, and host defense against microbial challenge. Ann. Intern. Med. 1988;108:595. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- 62.Autenrieth IB, Heesemann J. In vivo neutralization of tumor necrosis factor-α and interferon-γ abrogates resistance to Yersinia enterocolitica infection in mice. Med. Microbiol. Immunol. Berl. 1992;181:333. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- 63.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 64.Klein J. Natural history of the major histocompatibility complex. New York: Wiley; 1986. [Google Scholar]
- 65.Galyov EE, Håkansson S, Forsberg Å, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993;361:730. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- 66.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]