Abstract
In recent preclinical studies, the role of nitric oxide (NO) in nicotine dependence has become increasingly evident. Inhibition of NO synthesis blocks acquisition of conditioned place preference, and attenuates the nicotine abstinence syndrome in rodents. These findings have not been followed up in human studies. In order to obtain preliminary data on NO inhibition in human smokers, we conducted a randomized, double-blind, crossover study (N = 12) of minocycline, a tetracycline derivative antibiotic, that inhibits the neuronal nitric oxide (NO) synthase enzyme with resultant inhibition of NO production. Medication effects were assessed through a smoking choice procedure as well as subjective and physiological responses to nicotine administered via the intravenous route (IV). Minocycline treatment did not affect smoking self-administration in our choice procedure and did not affect most of the subjective responses to IV nicotine or sample smoking. Following IV nicotine administration, there was a greater reduction in craving for cigarettes under minocycline, compared to placebo. Similarly, smokers had greater reduction in their craving for cigarettes following sample smoking under minocycline treatment. These findings provide limited support for the potential use of minocycline as a treatment of nicotine dependence.
Keywords: nitric oxide, nitric oxide synthase, minocycline, nicotine dependence, intravenous nicotine
Despite advances in pharmacotherapy for nicotine dependence, including nicotine replacement therapy (NRT), sustained release (SR) bupropion, and varenicline (Cahill et al. 2007; Fiore 2008; Stead et al. 2008), generally 70-90% resume smoking within a year of treatment. Accordingly, the mission to identify novel effective nicotine dependence pharmacotherapies continues, with a broad range of antidepressant, antianxiety, and other agents already evaluated or undergoing assessment. Given the grave consequences of the tobacco epidemic, innovative approaches to medications development are warranted.
Recently, the role of nitric oxide (NO) in nicotine dependence has become increasingly evident. NO serves as a second messenger for the glutamate and dopamine receptors and facilitates nicotine's effects in the reward circuit (Schilstrom et al. 2004; Vleeming et al. 2002). Moreover, inhibition of NO synthesis by L-Nitro-amino-methyl-ester (L-NAME) or 7-nitroindazole (7-NI) (Martin and Itzhak 2000; Sahraei et al. 2004), blocked the acquisition of nicotine-induced place preference in mice (Martin and Itzhak 2000; Sahraei et al. 2004), a model of the rewarding effects of nicotine. Of clinical interest, blockade of NO synthesis by nitro-L-arginine (L-NNA) also attenuated nicotine abstinence symptoms precipitated by the nicotinic antagonist mecamylamine (Malin et al. 1998). A number of clinically approved medications affect NO synthesis and other NO synthesis inhibitors are in development (Erdal et al. 2005). One widely used medication of the former class, minocycline, is a tetracycline derivative antibiotic that crosses the blood brain barrier (Allen 1976; Macdonald et al. 1973) and has diverse CNS effects including anti-inflammatory and neuroprotective actions in doses used clinically (Jonas and Cunha 1982; Stirling et al. 2005). Minocycline also inhibits the neuronal nitric oxide (NO) synthase enzyme, with resultant inhibition of NO production (Du et al. 2001). Based on this background, it is of interest to evaluate minocycline's effects on nicotine dependence.
In order to obtain preliminary data on minocycline in nicotine dependent humans, we conducted a human laboratory study with a randomized, double-blind, crossover design. Medication effects were assessed through a smoking choice procedure as well as subjective and physiological responses to nicotine administered via intravenous route. We hypothesized that minocycline would result in reduced preference for cigarette puffs, nicotine withdrawal and craving, and physiological and subjective response to IV nicotine.
Method
Participants
Seven female and 5 male non-treatment seeking smokers were recruited from the New Haven, Connecticut area. Four additional participants were enrolled but dropped out of the study and were not included in the analyses. Eight participants were African-American, 3 were Caucasian, and 1 was Hispanic. The average age (SD) of the participants was 35.3 (7.4). On average, participants smoked 17.9 (4.5) cigarettes/day, and had a Fagerstrom Test for Nicotine Dependence (Heatherton et al. 1991) score of 6.6 (1.4). Participants had normal physical, laboratory and psychiatric examinations and were not dependent on drugs or alcohol other than nicotine. Participants provided written, signed consent before participating in the study. This study was approved by the VA Connecticut Healthcare System Human Subjects Subcommittee. Experimental sessions were conducted in the Biostudies Unit located at the VA Connecticut Healthcare System and participants were paid for participation.
Design and Procedures
This was an outpatient randomized, double-blind, crossover study. The design is presented in Table 1. Following an adaptation session, participants had two 4-day treatment periods, in which they were assigned to a random sequence of minocycline (200 mg/day) or placebo treatment. These treatment periods were separated by a washout period lasting a minimum of 4 days. In the adaptation session, participants first received an IV saline injection followed by 2 escalating doses of IV nicotine (0.5 and 1.0 mg/70 kg), given 30 minutes apart. This procedure ensured that participants tolerated the IV saline and nicotine doses that were used in the experimental sessions. During the first 2-days of each treatment period, participants had daily clinic visits to receive the study medications and to complete outcome measures. Starting at midnight of Day 1, participants were asked to stop smoking until the morning of Day 3. To assure compliance with non-smoking instructions, participants were paid extra for not smoking. Abstinence from smoking was verified with expired carbon monoxide (CO; < 10 parts-per-million).
Table 1.
Experimental Design
| Treatment Period 1 | Treatment Period 2 | ||||||
|---|---|---|---|---|---|---|---|
| Adaptation Session | Day 1-2 Outpatient Visits | Day 3 (ES1) Smoking behavior | Day 4 ES 2 IV nicotine responses | W | Day 1-2 Outpatient Visits | Day 3 (ES1) Smoking behavior | Day 4 ES 2 IV nicotine responses |
Note: ES: Experimental Session; W: Washout.
On Day 3 (Choice Session), two hours following study medication (minocycline or placebo) administration, smoking behavior was assessed using a choice procedure, as described below. Participants returned to the clinic on Day 4 (IV Nicotine Session), after overnight abstinence from smoking, where responses to IV nicotine were obtained. The two experimental sessions and the outpatient visits were scheduled at 8 AM.
Smoking Choice Procedure (Table 2 a)
Table 2.
| Table 2a Schedule of Events: Experimental Session 1 | |
|---|---|
| Time | Measures and Events |
| Baseline | CO, HR/BP, POMS, NWSC, PANAS |
| 8:00 AM | Medication treatment (Minocycline or Placebo) |
| 8: 30 AM | Snack |
| 10:00 AM | Cognitive testing, HR/BP, DEQ, NWSC, PANAS Smoking Period Starts |
| 12:30 | Smoking Period ends HR/BP, POMS, NWSC, PANAS |
| Table 2b Schedule Of Events: Experimental Session 2 | |
| Time | Measures and Events |
| Baseline | CO, Nicotine levels, HR/BP, POMS, NWSC, PANAS |
| 8:00 AM | Medication treatment (Minocycline or Placebo) |
| 8: 15 AM | Snack |
| 10:00 AM | HR/BP, DEQ, NWSC Nicotine 0 mg |
| 10:01 | HR/BP, DEQ |
| 10:02 | HR/BP |
| 10:03 | HR/BP, DEQ |
| 10:05 | HR/BP, DEQ |
| 10:08 | HR/BP, DEQ |
| 10:10 am | HR/BP, DEQ |
| 10:15 min | HR/BP, DEQ Nicotine 0.5 mg /70 kg |
| 10:45 | Nicotine 1 mg/70 kg |
| 12:15 | HR/BP, POMS, NWSC, PANAS |
Note: CO: Alveolar carbon monoxide; HR/BP: Heart rate/Blood pressure; POMS: Profiles of Mood States; NWSC: Nicotine Withdrawal Symptom Checklist; PANAS: Positive and Negative Affect Schedule; DEQ: Drug Effects Questionnaire.
Note: The same measures were obtained following saline and each nicotine administration. For brevity, only the measures after saline are shown. For abbreviations see Table 2a
Participants received 10 tokens at the beginning of the Smoking Choice Session, which could later be exchanged for money ($0.75/token) or 2 cigarette puffs. The choice procedure has been shown to be sensitive in demonstrating medication effects on smoking behavior (Tidey et al. 2000). We chose $0.75 as the token value, since this token value is expected to be sensitive to both increases and decreases in smoking behavior (Bisaga et al. 2007). This session started with sample smoking consisting of 2, 3 sec cigarette puffs separated by a 20 sec interval. The sample smoking allowed measurement of subjective responses to smoking following abstinence. Starting 15 minutes after the sample smoking episode and every 15 minutes thereafter until 2.5 hours had elapsed (i.e., 15 min × 10 tokens), participants had the opportunity to exchange tokens for cigarette puffs or money.
Intravenous Nicotine Administration (Table 2 b)
For this procedure, participants had an indwelling catheter placed in an antecubital vein. After baseline measures were obtained, participants received an oral dose of either minocycline or placebo. Two hours after the medication administration, participants first received saline followed by two ascending doses of IV nicotine, 0.5 and 1.0 mg/70 kg, 30 min apart. Both 0.5 and 1.0 mg/70 kg dose of nicotine have been well tolerated and produced robust physiological and subjective responses in male and female smokers in our previous studies (Sofuoglu et al. 2003; Sofuoglu et al. 2005; Sofuoglu et al. 2006). These doses are within the range of nicotine delivered by smoking one cigarette, which is approximately 0.5 to 2 mg.
Drugs
Nicotine and Minocycline Administration
Nicotine bitartrate was obtained from Interchem Corporation (Manchester, Connecticut). Nicotine samples were prepared by a research pharmacist at the VA CT Healthcare System. A saline injection was followed by two nicotine injections (0.5 and 1.0 mg/70 kg). Nicotine was administered in 5 ml volume over 60 seconds intravenously via a catheter located in a forearm vein. The injections were given 30 minutes apart.
Minocycline (Dynacin®) was administered at 200 mg/day, as a single dose, for 4 days. This dose is within the range of usual daily dose of minocycline used for the treatment of infections (Jonas and Cunha 1982). Further, two recent trials have utilized a 200 mg/day dose of minocycline in studies of Parkinson's disease and acute stroke (Lampl et al. 2007; The NINDS NET-PD Investigators 2006). Following oral administration, peak plasma levels of minocycline are reached within 1-4 hours. The elimination half-life of minocycline ranges from 11 to 24 hours. Minocycline was administered in the clinic daily by the study nurse.
Measures
The outcome measures tapped behavioral, biochemical, physiological, subjective, and cognitive domains. Smoking behavior was assessed through a Choice Procedure, i.e., number of cigarette puff selections. Biochemical measures were expired CO and plasma cotinine concentrations to verify abstinence from smoking and level of smoking, respectively. Blood samples were obtained at baseline and before the IV Nicotine Sessions. The physiological measures were systolic and diastolic blood pressure and heart rate, which were taken before medication treatment and every 20 min for 2 hours afterwards. Additional physiological measures were taken at -5 and 1, 2, 3, 5, 8, 10, and 15 min in relation to saline or nicotine deliveries.
The subjective measures included the Drug Effects Questionnaire (DEQ), Cigarette Evaluation Scale (CES), Nicotine Withdrawal Symptom Checklist (NWSC), Positive and Negative Affect Schedule (PANAS) and Profile of Mood States (POMS). The DEQ was used to measure acute effects from intravenous nicotine and consisted of 5 items: drug strength, good effects, bad effects, head rush and like the drug. Participants rated these effects on a 100 mm scale, from “not at all” to “extremely.” The DEQ was given just before and 1, 3, 5, 8, 10, and 15, minutes after saline or nicotine administration. The Cigarette Evaluation Scale (CES) was used to measure the subjective effects of smoking under each of the treatment conditions (Cappelleri et al. 2007; Westman et al. 1992). This scale assesses satisfaction, taste, and smoking-induced changes in dizziness, calm, concentration, wakefulness, hunger, nausea, irritability, throat and chest sensations, and craving on a seven-point ratings (from 1 = “not at all” to 7 = “extremely”).
The POMS bipolar, a 72–item rating scale (McNair et al. 1988), was used to measure the effects of medication treatments on the mood. The POMS has 6 subscales: (1) composed-anxious; (2) agreeable-hostile; (3) elated-depressed; (4) confident-unsure; (5) energetic-tired; (6) clear headed-confused. The PANAS is a 20-item scale that assesses both negative and positive affective states (Watson et al. 1988). Participants rate adjectives describing affective states on a scale of 1 to 5 using a specified time period (i.e., now, today, past week, etc.). This scale is sensitive to the affective symptoms of tobacco withdrawal (Kenford et al. 2002). The NWSC measures withdrawal symptoms from tobacco and includes items of cigarette craving, irritability/anger, anxiety, difficulty concentrating, restlessness, increased appetite, depressed or sad mood, and insomnia (Hughes and Hatsukami 1986; Hughes and Hatsukami 1997). We used a modified version of the NWSC in which participants were asked to rate these symptoms on a 100 mm scale, from “not at all” to “extremely” (e.g., Buchhalter et al. 2005). The “cigarette craving” item from the NWSC was also given during the session on Day 4 to measures changes in urges to smoke in response to intravenous nicotine. The NWSC, POMS, and PANAS were given daily during the study, On Day 4, the cigarette craving item from the NWSC was given at baseline, before saline and nicotine administrations and at the end of the session.
Cognitive Performance was measured with the Sustained Attention to Response Test (SART) (Robertson et al. 1997; Sofuoglu et al. 2008a). The SART assesses the ability to withhold responses to an infrequently occurring target. The SART was administered on Days 1, 2 and 3 of each treatment phase. On Day 3, the SART was administered 2 hours after medication treatment. Due to experimenter error, one participant did not complete the SART. Data from 2 sessions was missing for an additional two participants.
Statistical Analysis
Study outcomes were analyzed using a mixed-effect repeated-measures crossover models using the Statistical Analysis System, Version 9.1.3. (SAS Institute Inc. 2007). Each model included fixed main effect terms for treatment (placebo or minocycline), and time of measurement (day in the study or time since medication administration), as well as the interaction of these two effects. We also included a random effect for participant and a blocking factor for treatment sequence. Values of p< .05 were considered statistically significant, based on two-tailed tests, unless otherwise specified. Significant treatment or treatment by time interactions (p<0.05) were followed by post hoc comparisons, with Tukey adjustments to prevent the Type I error rate. For blood pressure, heart rate and DEQ measurements during IV nicotine administration, where multiple measurements were collected before and after each dose, a change score (maximum post dose score minus predose baseline) was used in the analysis.
Results
Smoking behavior
There was no treatment effect on smoking self-administration [F(1,11) = 0.7; p>0.05]. Participants exchanged an average (SD) of 7.3 (0.9) tokens for cigarette puffs under placebo treatment, and 7.4 (1.0), under minocycline treatment.
Plasma cotinine measurements and breath CO levels
Baseline cotinine (SEM) levels were 236 ng/mL (32). Cotinine levels (SEM) on the last day of each treatment (IV Nicotine Session) were 207 ng/mL (32) for the placebo, and 184 ng/mL (29) ng/mL for the minocycline treatment [F(1, 11) =1.5; p>0.2]. Smoking abstinence before the sessions were verified by CO levels <10 ppm.
Physiological response to IV nicotine
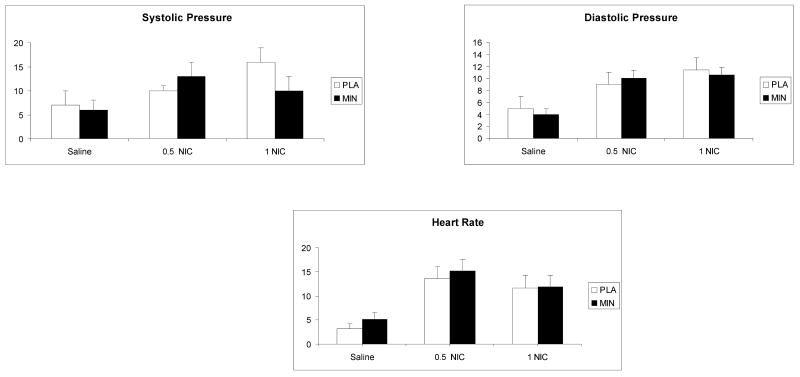
There was no treatment effect on IV nicotine responses for heart rate [F(1, 55) =1.5; p>0.05], systolic [F(1,55) =1.2; p>0.05] or diastolic [F(1,55) =0.2; p>0.05] blood pressure. As shown in Figure 1, there was a significant nicotine dose effect for the heart rate, systolic and diastolic blood pressure (p<0.001). Pairwise comparisons indicated that responses for all 3 outcomes were higher under the 0.5 mg or 1.0 mg nicotine than under the placebo condition (p<0.05).
Fig. 1.
The average (with standard error of the mean - SEM) systolic blood pressure, diastolic blood pressure and heart rate responses to saline, 0.5 and 1.0 mg/ 70 kg intravenous nicotine under placebo and minocycline conditions. Bars represent the change (maximum post dose-baseline). Measurements were taken 5 minutes before and 1, 2, 3, 5, 8 and 10 minutes after each injection.
Tobacco withdrawal severity and mood measures
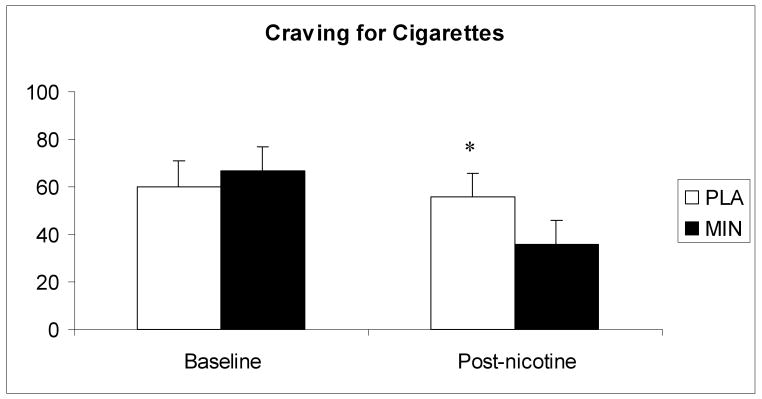
There was no treatment effect on the total NWSC scores during the first 3 days of each treatment period [F(1, 55) = 0.2; p>0.05]. There was a significant main effect for time [F(3, 77) = 5.1; p<0.01], with increased withdrawal severity over time. For the individual items, a significant treatment-by-time interaction was observed for “depressed mood,” with lower rating under minocycline treatment [F(3, 77) = 2.9; p<0.05]. In response to IV nicotine administration, cigarette craving showed a significant treatment-by- time interaction, (Figure 2) with greater decreases under the minocycline condition [F(4, 99) = 2.9; p<0.05].
Fig 2.
The average (with SEM) ratings of cigarette craving in response to saline and nicotine administration. The measurements shown were taken at baseline and 30 minutes after 1.0 mg nicotine administration. * indicate a significant treatment-by-time interaction.
No treatment effects were observed for any of the PANAS and POMS subscales
Subjective responses to cigarette smoking and IV nicotine
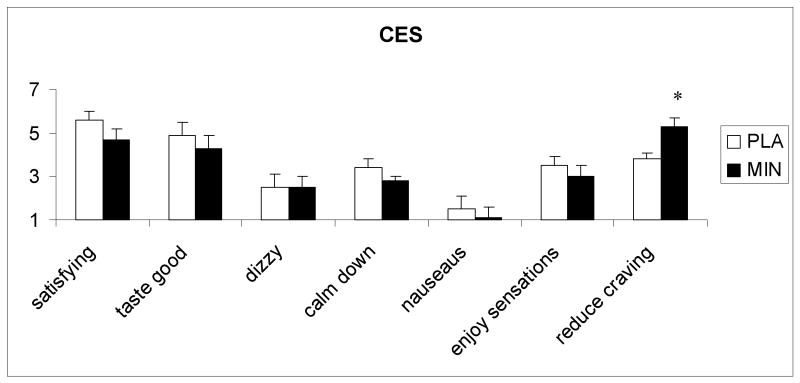
The subjective responses to sample smoking, measured with CES, a significant treatment effect was observed for the rating of “reduced craving for cigarettes” [F (1, 11) = 8.5; p<0.05], 5.4 (1.3) and 3.7 (2.0) under minocycline and placebo conditions, respectively. Other CES items did not show treatment effects (Fig 3.)
Fig. 3.
The average (with SEM) subjective responses to saline, 0.5 and 1.0 mg/ 70 kg intravenous nicotine under placebo and minocycline conditions. Bars represent the change (maximum post dose-baseline). Measurements were taken just before and 1, 3, 5, 8 and 10 minutes after each injection.
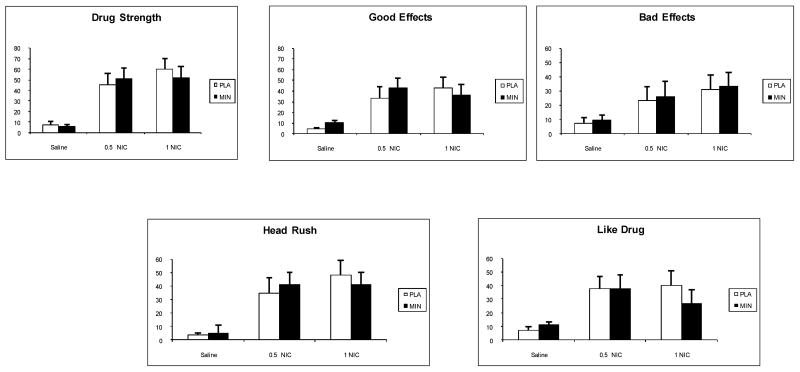
The treatment effects on the subjective response to IV nicotine measured with DEQ are shown in Figure 4 There was no treatment effect for the rating of drug strength [F(1,54) =0.07; p>0.05], good effects [F(1,54) =0.02; p>0.05], bad effects [F(1,54) = 0.02; p>0.05], head rush [F(1,54) =0.04; p>0.05] or drug liking [F(1,54) =0.3; p>0.05]. As expected, there was a significant nicotine dose effect for all 5 DEQ items (p<0.001). Pairwise comparisons indicated that the responses under the placebo were less than the 0.5 or 1.0 mg nicotine conditions (p<0.05).
Fig. 4.
The average (with SEM) ratings of subjective responses to sample smoking measured with the Cigarette Evaluation Scale (CES). The measurements were obtained after 2 puffs of a cigarette.* indicate a significant treatment effect.
Performance Measures
Table 3 summarizes the results of the SART. No effects involving treatment were observed on the SART (p>0.05).
Table 3.
Summary statistics on the SART (n = 11) (Note * due to missing data, n=10).
| Placebo | Minocycline | |||||
|---|---|---|---|---|---|---|
| Day 1* | Day 2 | Day 3 | Day 1* | Day 2 | Day 3 | |
| Errors on 3s (/25) | 11.8 (6.09) | 12.5 (6.8) | 11.0 (7.0) | 9.9 (6.8) | 10.5 (6.7) | 10.5 (5.5) |
| Errors on non-3s (/200) | 8.40 (15.1) | 11.7 (19.9) | 5.54 (6.3) | 6.7 (7.9) | 11.4 (15.2) | 7.73 (10.7) |
| Mean RT for correct presses (ms) | 355.1 (81.4) | 377.6 (92.4) | 380.4 (77.8) | 383.1 (120.0) | 412.0 (95.4) | 400.9 (73.4) |
Discussion
Minocycline treatment did not affect smoking self-administration in our choice procedure. Smokers chose cigarette puffs in approximately 70 percent of opportunities under placebo or minocycline treatment, with a token value of $0.75. These findings are consistent with a recent study by Bisaga and coworkers (2007) which demonstrated an increase in token exchange rate for cigarette puffs in a range of token values from $0.5 to $2. In that study, token exchange rate was 100 and 80 percent, for token values of $0.50 and $1 respectively, demonstrating the sensitivity of the choice-procedure to changes in alternative reinforcer values (i.e., money).
Our model examined responses to IV nicotine, in addition to cigarette smoking. The advantage of IV nicotine is fast and accurate dose delivery and the ability to produce consistently both subjective and physiological effects. As demonstrated in previous studies, IV nicotine is reinforcing and readily self-administered by smokers (Harvey et al. 2004; Sofuoglu et al. 2008b). Examining nicotine as well as cigarette smoking responses helped to assess medication effects on both nicotinic and non-nicotinic components of tobacco addiction, since cigarette smoke contains many other compounds other than nicotine (Hoffmann and Wynder 1986). Minocycline did not affect most of the subjective responses to IV nicotine or sample smoking. One exception was the rating of cigarette craving. Following IV nicotine administration, there was a greater reduction in craving for cigarettes under minocycline, compared to placebo. Similarly, smokers had greater reduction in their craving for cigarettes following sample smoking under minocycline treatment, compared to placebo. As demonstrated in a recent field study, smoking reliably reduces craving (Carter et al. 2008); a medication that facilitates this craving reduction may attenuate ab libitum smoking. Future research can also determine whether minocycline reduces cue-induced craving.
Consistent with many previous studies, tobacco withdrawal severity increased over 3 days of cigarette abstinence. Minocycline treatment did not affect the severity of tobacco withdrawal symptoms. One exception was feeling depressed or sad mood, which was attenuated under the minocycline treatment condition. However, minocycline did not affect the rating of elated-depressed subscale of the POMS. Interestingly, preclinical studies and anecdotal evidence from humans support antidepressant effects of minocycline (Pae et al. 2008). Whether minocycline will have efficacy for depression or depressed mood associated with tobacco withdrawal remains to be examined in future studies.
What are the implications of our findings? As mentioned before, NO inhibitors have shown promising results for nicotine addiction in preclinical studies. Unfortunately, the NO inhibitors used in these preclinical studies are not approved for human use. Minocycline, in clinically used doses, has been shown to inhibit NO synthesis (Kim et al. 2004; Sadowski and Steinmeyer 2001); however, it has not been examined in preclinical models for nicotine addiction. To fully evaluate the potential use of NO inhibitors for nicotine addiction, further studies using other NO inhibitors, especially those selective for neuronal type, as they become available, is warranted.
This study also had several limitations. First, dose-dependent effects of minocycline were not examined. We selected a clinically used dose of minocycline, which has also been used in many clinical trials for neuropsychiatric conditions including Huntington's disease, Alzheimer's disease, stroke and schizophrenia (Thomas et al. 2004). Second, the study had a 4-day treatment duration. It is possible that longer treatment duration might be associated with different treatment effects. Third, participants were non-treatment seeking smokers who were not motivated to quit. The generalizibility of our findings to treatment-seeking smokers may be limited.
To summarize, we detected no differences between minocycline and placebo on most of the measures of behavioral, biochemical, physiological, subjective, and cognitive domains. Minocycline treatment was associated with modest improvement in depressed mood and greater reduction in craving for cigarettes following smoking in abstinent smokers.
Acknowledgments
This research was supported by the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) and NIH grants P50AA015632, K02-DA021304 (MS) and K01-DA-019446 (MM), and K05 AA014715 (SSO). We would like to thank Ellen Mitchell, R.N., Lance Barnes, and Stacy Minnix for technical assistance.
Contributor Information
Mehmet Sofuoglu, Yale University, School of Medicine, Department of Psychiatry and VA Connecticut Healthcare System, West Haven, CT.
Andrew J. Waters, Uniformed Services University of the Health Sciences, Bethesda, Maryland
Marc Mooney, University of Minnesota, Minneapolis, Minnesota.
Stephanie S. O'Malley, Yale University, School of Medicine, Department of Psychiatry and VA Connecticut Healthcare System, West Haven, CT
References
- Allen JC. Minocycline. Ann Intern Med. 1976;85:482–7. doi: 10.7326/0003-4819-85-4-482. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Padilla M, Garawi F, Sullivan MA, Haney M. Effects of alternative reinforcer and craving on the choice to smoke cigarettes in the laboratory. Hum Psychopharmacol. 2007;22:41–7. doi: 10.1002/hup.816. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–9. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2007:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32:912–23. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, W DW, Cinciripini PM. Real-time craving and mood assessments before and after smoking. Nicotine and Tobacco Research. 2008 doi: 10.1080/14622200802163084. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:14669–74. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdal EP, Litzinger EA, Seo J, Zhu Y, Ji H, Silverman RB. Selective neuronal nitric oxide synthase inhibitors. Curr Top Med Chem. 2005;5:603–24. doi: 10.2174/1568026054679317. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. USDoHaHSPH; Rockville, MD: 2008. In: Service. ed. [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175:134–42. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Wynder EL. Chemical constituents and bioactivity of tobacco smoke. IARC Sci Publ. 1986:145–65. [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Effects of three doses of transdermal nicotine on post-cessation eating, hunger and weight. J Subst Abuse. 1997;9:151–9. doi: 10.1016/s0899-3289(97)90013-4. [DOI] [PubMed] [Google Scholar]
- Jonas M, Cunha BA. Minocycline. Ther Drug Monit. 1982;4:137–45. [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70:216–27. [PubMed] [Google Scholar]
- Kim SS, Kong PJ, Kim BS, Sheen DH, Nam SY, Chun W. Inhibitory action of minocycline on lipopolysaccharide-induced release of nitric oxide and prostaglandin E2 in BV2 microglial cells. Arch Pharm Res. 2004;27:314–8. doi: 10.1007/BF02980066. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–10. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Macdonald H, Kelly RG, Allen ES, Noble JF, Kanegis LA. Pharmacokinetic studies on minocycline in man. Clin Pharmacol Ther. 1973;14:852–61. doi: 10.1002/cpt1973145852. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Shenoi M, Upchurch TP, Johnson SC, Schweinle WE, Cadle CD. The nitric oxide synthesis inhibitor nitro-L-arginine (L-NNA) attenuates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 1998;140:371–7. doi: 10.1007/s002130050778. [DOI] [PubMed] [Google Scholar]
- Martin JL, Itzhak Y. 7-Nitroindazole blocks nicotine-induced conditioned place preference but not LiCl-induced conditioned place aversion. Neuroreport. 2000;11:947–9. doi: 10.1097/00001756-200004070-00010. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Dropperman L. Profile of Mood States: Bipolar Form. Educational and Industrial Testing Service. Educational and Industrial Testing Service; 1988. [Google Scholar]
- Pae CU, Marks DM, Han C, Patkar AA. Does minocycline have antidepressant effect? Biomed Pharmacother. 2008 doi: 10.1016/j.biopha.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–58. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Sadowski T, Steinmeyer J. Minocycline inhibits the production of inducible nitric oxide synthase in articular chondrocytes. J Rheumatol. 2001;28:336–40. [PubMed] [Google Scholar]
- Sahraei H, Falahi M, Zarrindast MR, Sabetkasaei M, Ghoshooni H, Khalili M. The effects of nitric oxide on the acquisition and expression of nicotine-induced conditioned place preference in mice. Eur J Pharmacol. 2004;503:81–7. doi: 10.1016/j.ejphar.2004.08.054. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows. SAS Institute Inc.; Cary, NC: 2007. [Google Scholar]
- Schilstrom B, Mameli-Engvall M, Rawal N, Grillner P, Jardemark K, Svensson TH. Nitric oxide is involved in nicotine-induced burst firing of rat ventral tegmental area dopamine neurons. Neuroscience. 2004;125:957–64. doi: 10.1016/j.neuroscience.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb D, Hatsukami DK. Labetalol treatment enhances the attenuation of tobacco withdrawal symptoms by nicotine in abstinent smokers. Nicotine Tob Res. 2003;5:947–53. doi: 10.1080/14622200310001615312. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Yoo S, Culligan K, Kosten T. Effects of tiagabine in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl) 2005;181:504–10. doi: 10.1007/s00213-005-0010-y. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Mouratidis M, Kosten T. Effects of topiramate in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl) 2006;184:645–51. doi: 10.1007/s00213-005-0296-9. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, Kosten T. Riluzole and d-amphetamine interactions in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2008a;32:16–22. doi: 10.1016/j.pnpbp.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Yoo S, Hill KP, Mooney M. Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology. 2008b;33:715–20. doi: 10.1038/sj.npp.1301460. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–22. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- The NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–71. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- Thomas M, Ashizawa T, Jankovic J. Minocycline in Huntington's disease: a pilot study. Mov Disord. 2004;19:692–5. doi: 10.1002/mds.20018. [DOI] [PubMed] [Google Scholar]
- Tidey JW, O'Neill SC, Higgins ST. d-amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology (Berl) 2000;153:85–92. doi: 10.1007/s002130000600. [DOI] [PubMed] [Google Scholar]
- Vleeming W, Rambali B, Opperhuizen A. The role of nitric oxide in cigarette smoking and nicotine addiction. Nicotine Tob Res. 2002;4:341–8. doi: 10.1080/14622200210142724. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Westman EC, Levin ED, Rose JE. Smoking while wearing the nicotine patch:is smoking satisfying or harmful? Clin Res. 1992;40:871A. [Google Scholar]