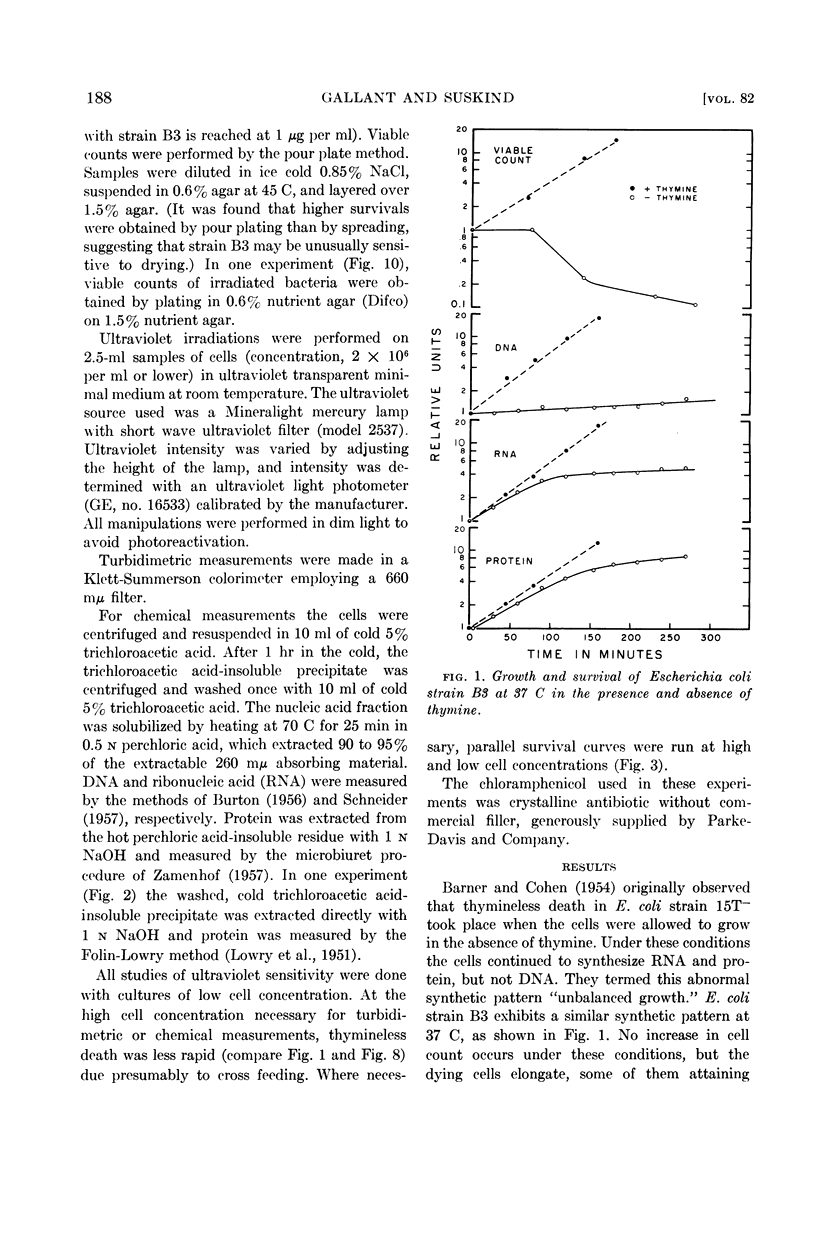
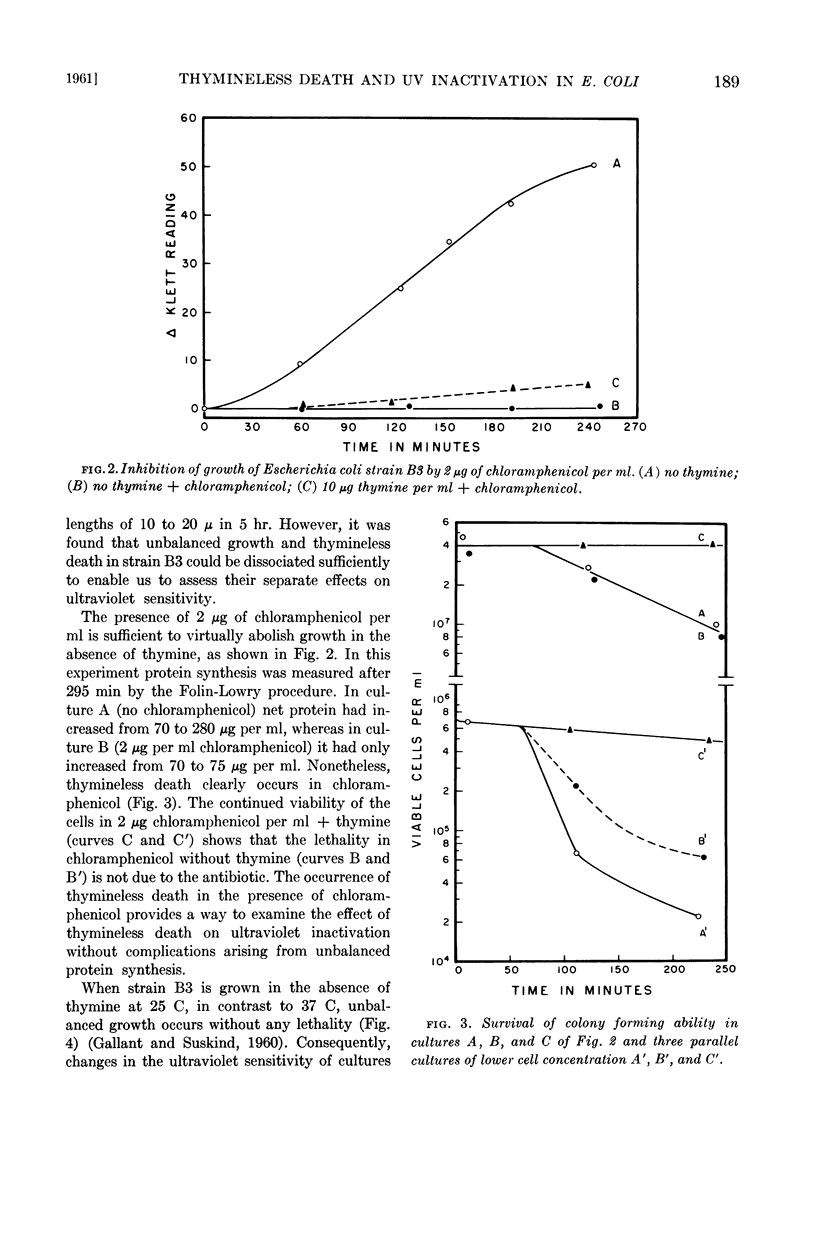
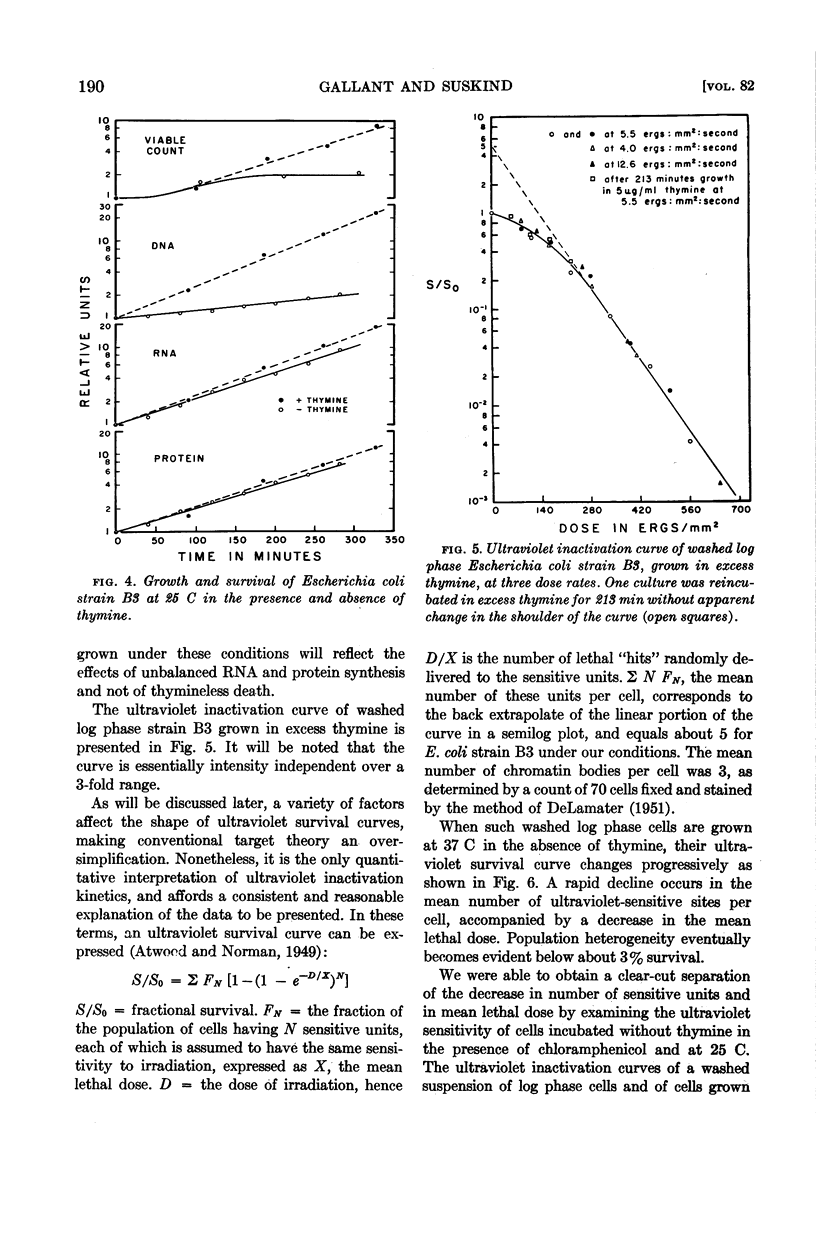
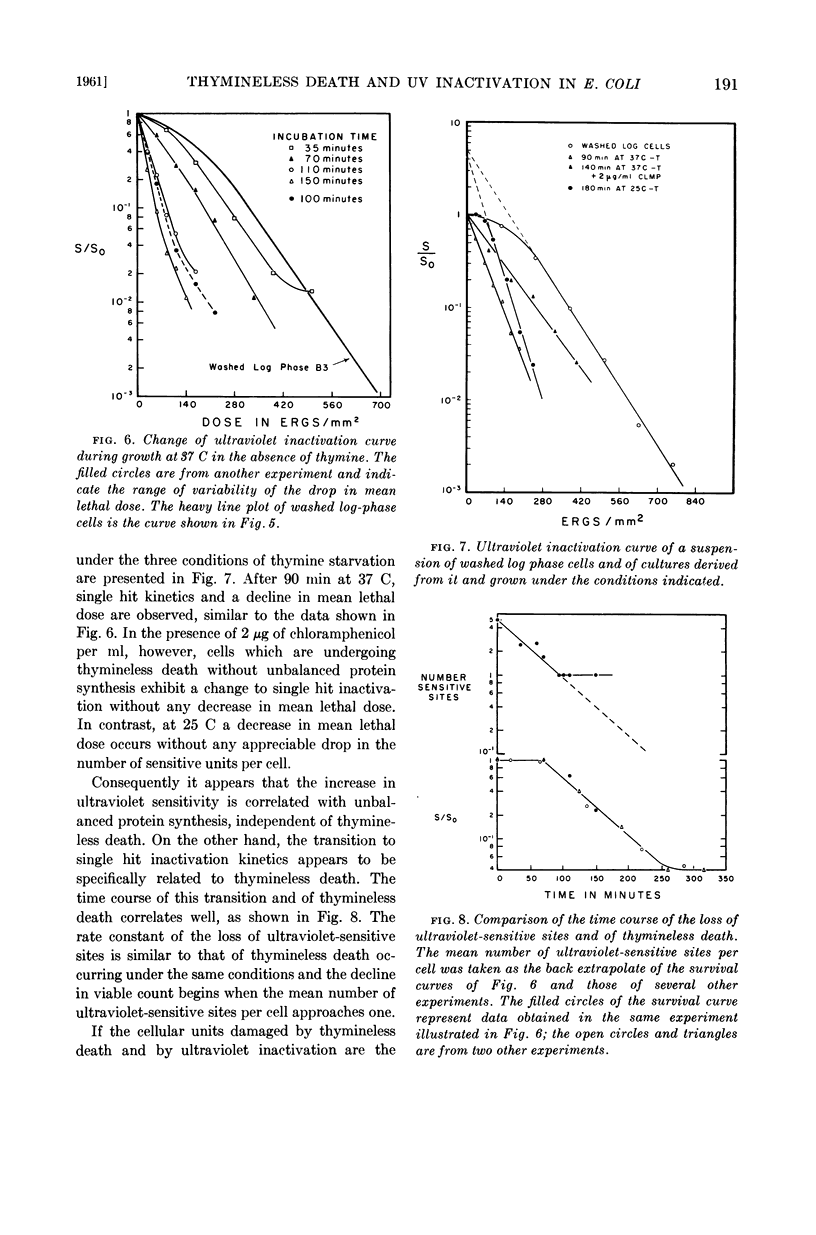
Abstract
Gallant, J. (Johns Hopkins University, Baltimore, Md.), and S. R. Suskind. Relationship between thymineless death and ultraviolet inactivation in Escherichia coli. J. Bacteriol. 82:187–194. 1961.—At 37 C, cultures of Escherichia coli strain B3 growing in the absence of thymine undergo unbalanced growth and thymineless death. Thymineless death also occurs at 37 C in 2 μg of chloramphenicol per ml despite virtually complete inhibition of cell growth and net protein synthesis. Thymineless cultures growing at 25 C exhibit unbalanced synthesis of ribonucleic acid and of protein without lethality. Examination of the kinetics of ultraviolet inactivation of cells grown under these three conditions indicates that the mean lethal dose of ultraviolet irradiation decreases with unbalanced protein synthesis whether or not thymineless death occurs, whereas the latter is specifically correlated with a transition from multihit to single hit inactivation kinetics.
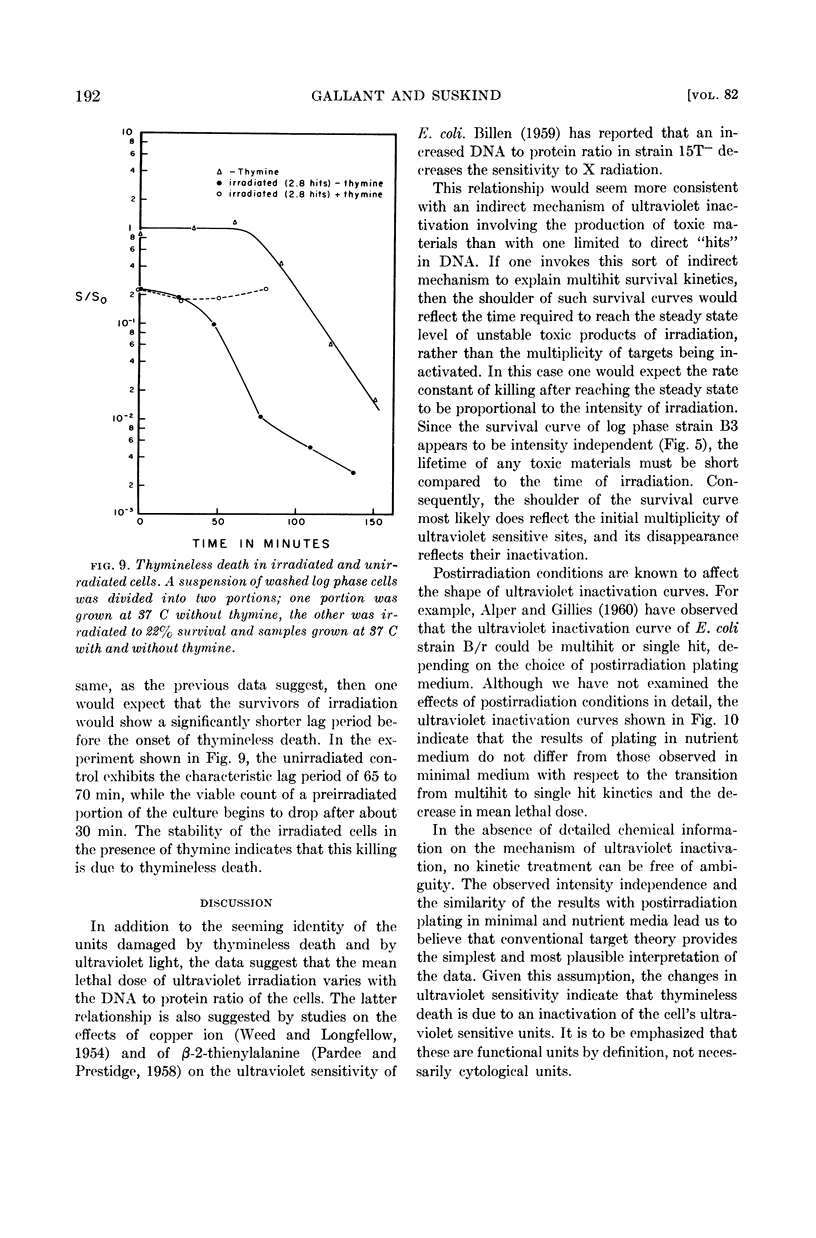
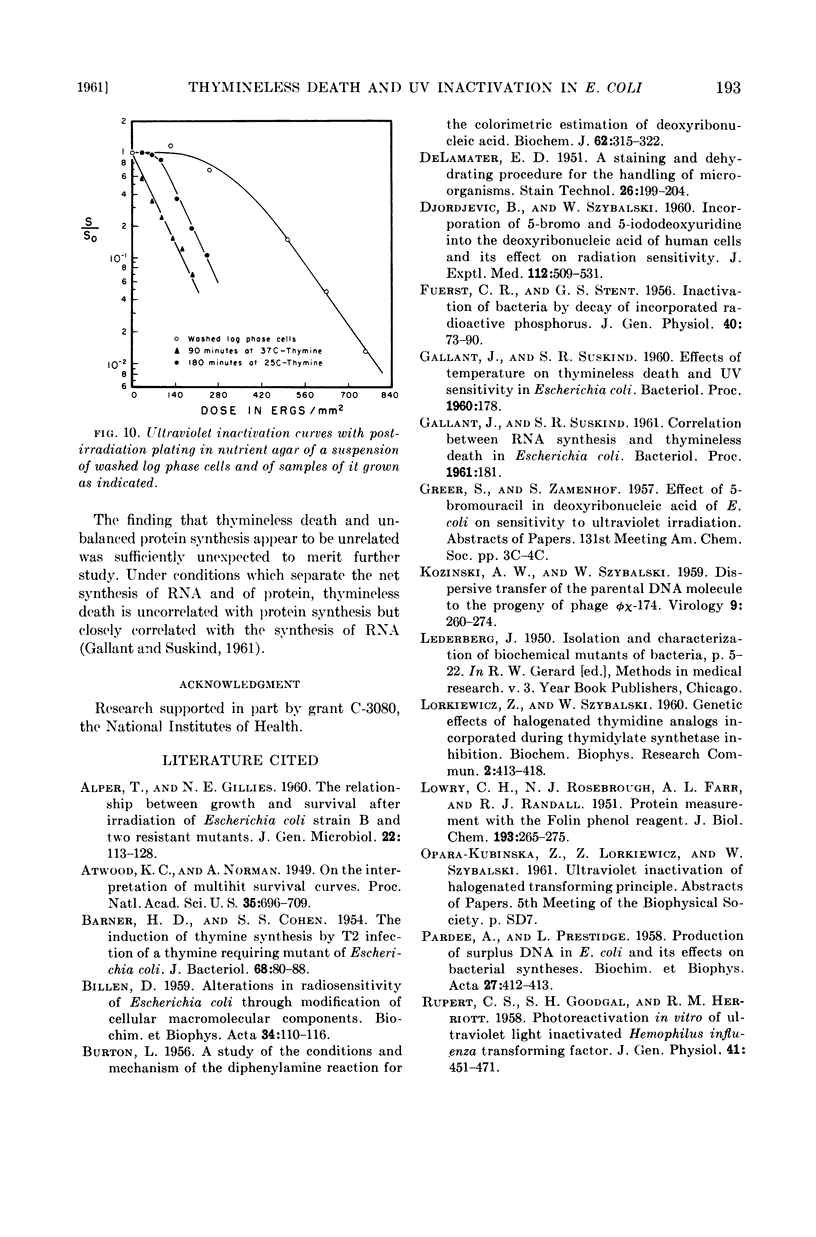
The time course of this transition correlates well with that of thymineless death, and preirradiated cells show a shorter lag period before the onset of thymineless death. These results, therefore, suggest that thymineless death is due to an inactivation of those cellular units which are sensitive to ultraviolet irradiation, presumably nuclei or some nuclear function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPER T., GILLIES N. E. The relationship between growth and survival after irradiation of Escherichia coli strain B and two resistant mutants. J Gen Microbiol. 1960 Feb;22:113–128. doi: 10.1099/00221287-22-1-113. [DOI] [PubMed] [Google Scholar]
- Atwood K. C., Norman A. On the Interpretation of Multi-Hit Survival Curves. Proc Natl Acad Sci U S A. 1949 Dec;35(12):696–709. doi: 10.1073/pnas.35.12.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNER H. D., COHEN S. S. The induction of thymine synthesis by T2 infection of a thymine requiring mutant of Escherichia coli. J Bacteriol. 1954 Jul;68(1):80–88. doi: 10.1128/jb.68.1.80-88.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILLEN D. Alterations in the radiosensitivity of Escherichia coli through modification of cellular macromolecular components. Biochim Biophys Acta. 1959 Jul;34:110–116. doi: 10.1016/0006-3002(59)90238-0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DJORDJEVIC B., SZYBALSKI W. Genetics of human cell lines. III. Incorporation of 5-bromo- and 5-iododeoxyuridine into the deoxyribonucleic acid of human cells and its effect on radiation sensitivity. J Exp Med. 1960 Sep 1;112:509–531. doi: 10.1084/jem.112.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLAMATER E. D. A staining and dehydrating procedure for the handling of microorganisms. Stain Technol. 1951 Jul;26(3):199–204. doi: 10.3109/10520295109113208. [DOI] [PubMed] [Google Scholar]
- FUERST C. R., STENT G. S. Inactivation of bacteria by decay of incorporated radioactive phosphorus. J Gen Physiol. 1956 Sep 20;40(1):73–90. doi: 10.1085/jgp.40.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZINSKI A. W., SZYBALSKI W. Dispersive transfer of the parental DNA molecule to the progeny of phage phiX-174. Virology. 1959 Oct;9:260–274. doi: 10.1016/0042-6822(59)90119-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Production of surplus DNA in E. coli, and its effects on bacterial syntheses. Biochim Biophys Acta. 1958 Feb;27(2):412–413. doi: 10.1016/0006-3002(58)90352-4. [DOI] [PubMed] [Google Scholar]
- RUPERT C. S., GOODGAL S. H., HERRIOTT R. M. Photoreactivation in vitro of ultraviolet-inactivated Hemophilus influenzae transforming factor. J Gen Physiol. 1958 Jan 20;41(3):451–471. doi: 10.1085/jgp.41.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R., BOYCE R. The ultraviolet light inactivation of phi-X174 bacteriophage at different wave lengths and pH's. Biophys J. 1960 Sep;1:29–41. doi: 10.1016/s0006-3495(60)86873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEED L. L., LONGFELLOW D. Morphological and biochemical changes induced by copper in a population of Escherichia coli. J Bacteriol. 1954 Jan;67(1):27–33. doi: 10.1128/jb.67.1.27-33.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]



