Abstract
The affinities of Ca2+-saturated and Ca2+-free calmodulin for a fluorescent reporter construct containing the PEP19 IQ domain differ by a factor of ~100, with Kd values of 11.0±1.2 and 1128.4±176.5 µM, while the affinities of a reporter containing the neuromodulin IQ domain are essentially identical, with respective Kd values of 2.9±0.3 and 2.4±0.3 µM. When Ca2+ is bound only to the C-ter pair of Ca2+-binding sites in calmodulin the Kd value for the PEP19 reporter complex is decreased ~5-fold, while the value for the the neuromodulin reporter complex is increased by the same factor. When Ca2+ is bound only to the N-ter pair of Ca2+-binding sites the Kd value for the PEP19 reporter complex is unaffected, but the value for the complex with the neuromodulin reporter is increased ~12-fold. These functional differences are largely accounted for by three differences in the CaM-binding sequences of the two reporters. Replacement of a central Gly in the neuromodulin IQ domain with the Lys at this position in the PEP19 sequence almost entirely accounts for the distinctive patterns of Ca2+-dependent stability changes exhibited by the two complexes. Replacement of a Lys immediately before the “IQ” amino acid pair in the neuromodulin sequence with an Ala accounts for the remaining Ca2+-dependent differences. Replacement of an Ala in the N-ter half of the IQ domain with a Gln accounts for about half the Ca2+-independent difference in the stabilities of the two reporter complexes, with the Ca2+-independent effect of the Lys replacement accounting for most of the remainder. Since the central Gly in the neuromodulin IQ domain is conserved in half of all known IQ domain sequences, these results suggest that the presence or absence of this residue defines two major functional classes of IQ domains.
The Ca2+-binding protein calmodulin (CaM1) participates at multiple levels in essentially all cellular processes. It interacts with an estimated 100 intracellular proteins, whose activities it modulates to varying degrees with varying dependencies on the intracellular free Ca2+ concentration. CaM contains two pairs of EF hand Ca2+-binding domains, each comprising a globular structure which we term a “lobe”. The two lobes are joined by a solvent-exposed flexible helix (1). Due to cooperativity within each pair of Ca2+-binding sites (Ca2+)2CaM, with either the N-ter or C-ter EF hand pair occupied, and (Ca2+)4CaM are the major Ca2+ liganded species produced (2). A number of proteins, including neuromodulin, neurogranin and PEP19, the unconventional myosins, ion channels (3–5), and modulators of small G-proteins, bind CaM through so called “IQ domains”. Depending upon their amino acid sequences, these domains can bind Ca2+-free and Ca2+-bound forms of CaM with quite different degrees of preference, allowing for a variety of Ca2+-dependent switching behaviors (4).
IQ domains were first identified as light chain subunit binding sites in conventional myosins, and the consensus sequence was defined as IQxxxRGxxxR (4, 6). As additional homologous domains have been discovered in other proteins a less restricted consensus sequence has emerged: [I,L,V]QxxxR[G,x]xxx[R,K] (4, 6). Structural studies demonstrate that a narrow hydrophobic cleft in the Ca2+-free C-ter CaM lobe and in the homologous regions in the myosin light chain subunits is involved in recognition of the “IQ” amino acid pair in the IQ domain (7–9). The Ca2+-free N-ter lobes in CaM and the light chains contain no such cleft, and their interactions with the IQ domain appear to be variable (7–9).
Structural and biophysical studies indicate that in the absence of Ca2+ there are two types of light chain or CaM complexes with IQ domains: a compact structure in which the N-ter lobe interacts closely with the IQ domain, and an extended form in which the N-ter lobe is only weakly associated (7, 8). The compact structure is formed if the semi-conserved central Gly residue in the IQ consensus sequence is present, and the extended structure is formed if there is a bulky amino acid at this position (7, 8). Extensive hydrophobic surfaces are exposed on both CaM lobes when they are replete with Ca2+, and these appear to enfold IQ domains to form structures similar to those observed with other types of Ca2+-saturated CaM complexes (10, 11). Structural and biophysical studies further suggest that IQ domains adopt an α-helical conformations when they are bound to Ca2+-free or Ca2+-saturated CaM (7–11).
The semi-conserved Gly in the IQ domain consensus sequence is present in neuromodulin, but has been replaced by a Lys in PEP19. The complexes between CaM and the IQ domains in these proteins are therefore expected to belong to different structural classes. In order to investigate the possible functional correlates of these classes, we compared the affinities of the Ca2+-free, Ca2+-saturated and intermediate Ca2+-bound CaM complexes with the neuromodulin and PEP19 IQ domains. Our results suggest that the IQ domains in neuromodulin and PEP19 represent two major functional classes whose complexes with CaM each exhibit a distinctive pattern of Ca2+-dependent stability changes.
Materials and Methods
The cDNA encoding BIQ, a fluorescent protein reporter that binds CaM via an IQ domain insert sequence derived from neuromodulin, has been described in detail elsewhere (12–14). The CaM-binding sequences in these reporter constructs are inserted between cyan and yellow variants of green fluorescence protein. When CaM is bound fluorescence resonance transfer from the cyan donor to the yellow acceptor is decreased (12–14). The BIQ reporter construct, which contains the neuromodulin IQ domain, and intact neuromodulin have previously been shown to bind CaM with similar affinities (12). The insert sequences in the reporter constructs we have used in these studies are listed in Fig. 1. Fluorescent protein reporter constructs were expressed in E. coli BL21(DE3) and purified as described previously (15). Native and mutant vertebrate CaMs were expressed in E. coli and purified as described in detail elsewhere(16, 17). Two mutant CaMs were employed to determine the effects of Ca2+-binding solely to the N-ter and C-ter EF hand pair: NxCCaM (NxC), in which Ca2+ ligands at positions 31 and 67 in the N-ter EF hand pair have been replaced with alanines, and NCxCaM (NCx), in which the homologous ligands at positions 104 and 140 in the C-ter EF hand have been replaced (12). We have previously determined that the mutant EF hand pairs in these proteins do not bind Ca2+ under the experimental conditions used for these investigations, and that the mutant CaM lobes mimic the properties of Ca2+-free native lobes (12).
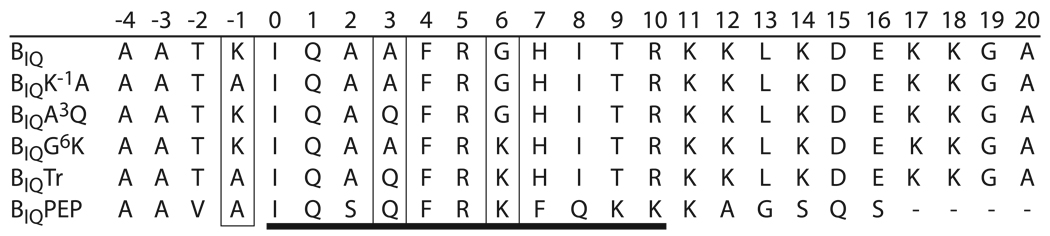
FIG 1. Native and mutant IQ domain sequences that were investigated.
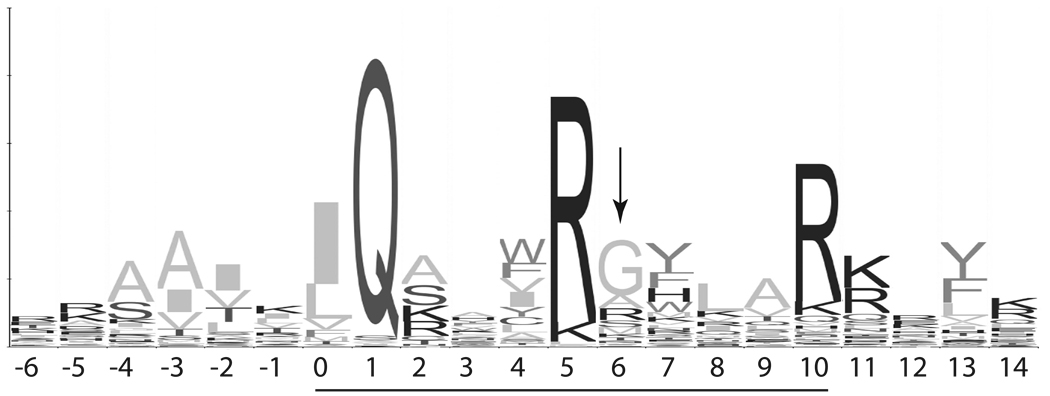
The sequences listed are inserts in a previously described reporter construct that responds to CaM binding with decreases in fluorescence resonance energy transfer between two fluorescent protein variants (12–14). These decreases were monitored based on fluorescence emission at 525 nm (430 nm excitation). The aligned insert sequences are numbered in relation to the first residue (0) in the 11 residue consensus IQ motif sequence: [I,L,V]QxxxR[G,x]xxx[R,K], which is underscored by a heavy line. Positions -1, 3 and 6 (boxed) were the focus of these investigations. The insert sequences in BIQ and BIQPEP are from neuromodulin and PEP19. The others were derived by replacing amino acids in the BIQ sequence with those in the BIQPEP sequence at one or all three (BIQTr) of the indicated positions.
Fluorescence Measurements
A Photon Technologies International (Monmouth Junction, NJ) QM-1 fluorometer operated in photon-counting mode was used for all equilibrium fluorescence measurements. Monochromator excitation and emission slit widths were set to produce bandwidths of ~2.5 nm. All experiments were performed at 23 °C. The standard experimental buffer contained 25 mM Tris (pH 7.5), 100 mM KCl, 100 µg/mL BSA, 25 µM TPEN, and other components as specified in the text or captions. Nominally Ca2+-free conditions were produced by including 3 mM BAPTA. Saturation of functional Ca2+ binding sites in native or mutant CaMs was achieved by adding CaCl2 to the standard buffer and CaM stock solutions to produce a final free Ca2+ concentration of ~250 µM.
Analysis of Fluorescence Data
Decreases in yellow acceptor fluorescence protein emission at 525 nm (cyan donor excited at 430 nm) due to CaM binding were used to determine fractional binding to reporter IQ domains as described in detail elsewhere (12). Apparent Kd values were derived from these measurements using a standard hyperbolic binding equation: FR = [CaM]/([CaM] + Kd). Fractional response (FR) is defined as (Fmax - F)/(Fmax - Fmin), where F corresponds to the 525 nm fluorescence emission measured after each addition of CaM, and Fmax and Fmin correspond to the fluorescence of the CaM-free and CaM-saturated reporter. In cases of very low affinity interactions where Fmin could not be approached, its value was allowed to float during fitting calculations. The Fmin values thus obtained were not found to differ significantly from the experimental values determined for higher affinity interactions. A hyperbolic binding equation is applicable to these data because the 15–100 nM reporter concentrations used are at least 5-fold below the lowest CaM concentrations used. Thus, the free and total CaM concentrations can be considered to be the same. This equation describes a single-site dependence of the fractional response on [CaM], so the excellent fits obtained support a 1:1 binding stoichiometry. We have previously confirmed this by performing stoichiometric titrations of the reference BIQ reporter (data not shown). Reported Kd values are the means of 3–5 independent determinations. Errors are expressed as the standard error of the mean (SEM). The difference between two values is considered statistically significantly if the p value derived from an unpaired t-test is less than 0.05.
Results
CaM binding to BIQ and BIQPEP
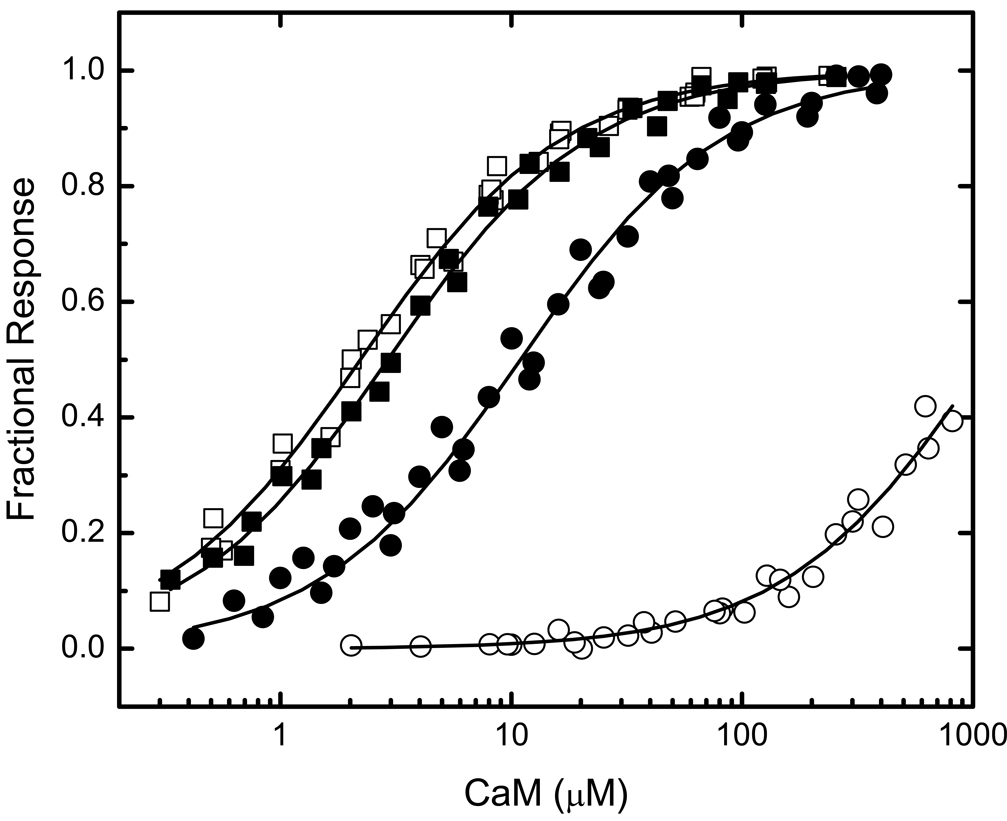
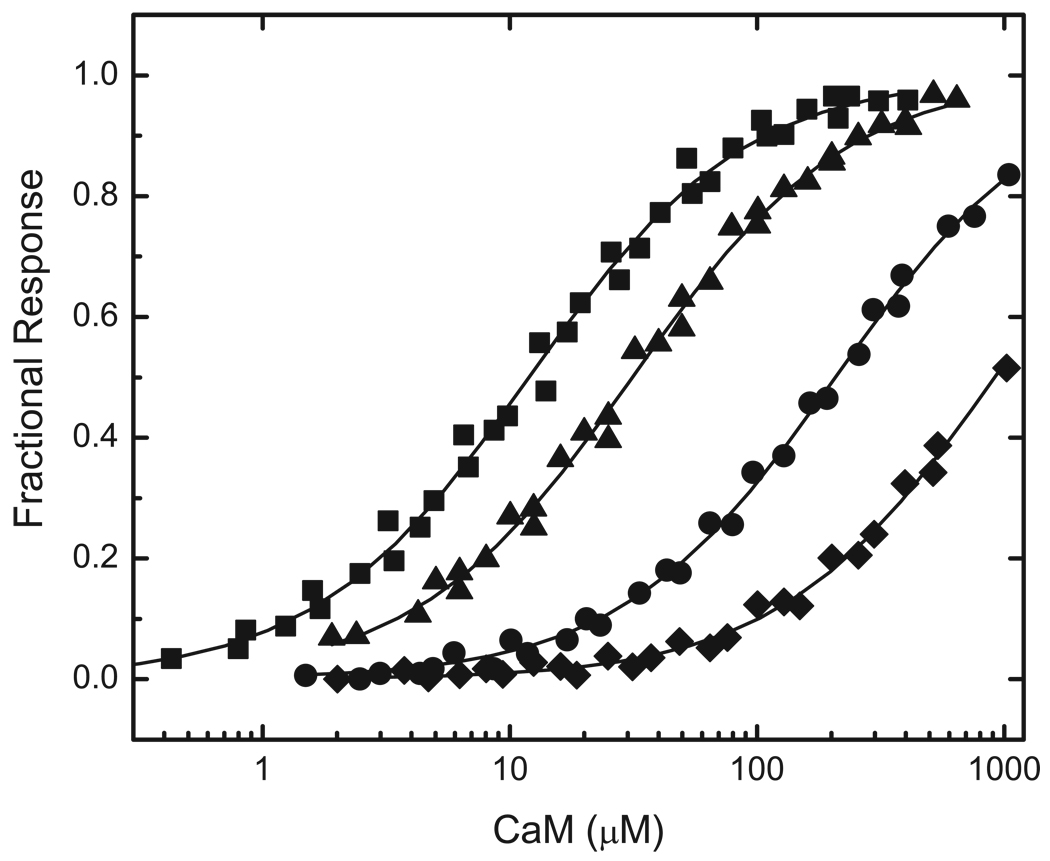
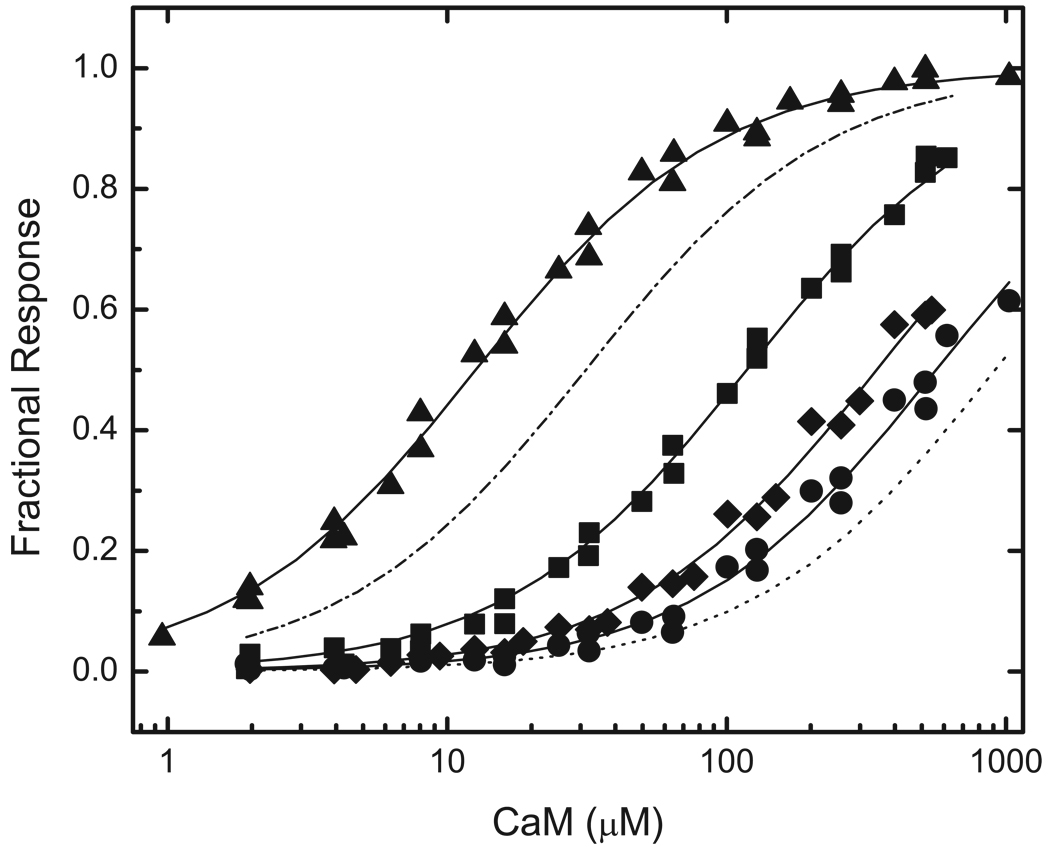
The first step in these investigations was to evaluate binding by BIQ and BIQPEP of CaM in its four principal Ca2+-liganded states, namely Ca2+-free, fully Ca2+-saturated, and partially saturated; with Ca2+ bound only to the N-ter (N2CCaM) or C-ter EF (NC2CaM) hand pair. Representative data are presented in Fig 2 and Fig 3, and the Kd values derived from these and similar data are listed in Table 1. Determinations performed with BIQ confirm results obtained previously with this construct (12). Thus, Ca2+-free and Ca2+-saturated CaM bind this reporter with similar Kd values of 2.4±0.3 and 2.9±0.3 µM. NC2CaM and NC2CaM are both bound more weakly, with respective Kd values of 12.7±1.1 and 31.3±2.7 µM. In contrast, BIQPEP binds Ca2+-free and Ca2+-saturated CaM with significantly different Kd values of 1128.4±176.5 and 11.0±1.2 µM, and it binds NC2CaM and N2CCaM with respective Kd values of 207.6±16.5 and 988.4±146.8 µM. Thus, Ca2+ binding to only one EF hand pair either increases the affinity of the BIQPEP complex or has little effect, dependent upon which pair of sites is occupied.
FIG 2. Comparison of the CaM-binding properties of reporters containing the neuromodulin and PEP19 IQ domains.
Binding was evaluated based on the fractional changes in the fluorescence emission of the BIQ (■, □) or BIQPEP (●,○) reporter constructs. Binding experiments were performed in the presence of ~250 µM free Ca2+ (filled symbols) or 3 mM BAPTA (empty symbols). Apparent Kd values derived from fits of these (solid lines) and similar data to a hyperbolic binding equation are listed in Table 1. Fluorescent reporter concentrations were 25–100 nM. The standard experimental buffer contained 25 mM Tris (pH 7.5), 100 mM KCl, 100 µg/mL BSA, 25 µM TPEN, and other components as specified. The fitted Fmin value for binding of Ca2+-free CaM to BIQPEP is 0.55±0.07. This is not different from the value of 0.56±0.07 derived for the Ca2+-free CaM complex with BIQ.
FIG 3. The effects of Ca2+ binding to the N-ter or C-ter EF hand pair in CaM on its affinities for reporters containing the neuromodulin and PEP19 IQ domains.
Data are presented for binding of BIQ to NxC (■) or NCx (▲) CaM and for binding of BIQPEP to NxC (●) or NCx (◆) CaM. At the ~250 µM free Ca2+ concentration used for these experiments the native EF hand pairs in NxCCaM and NCxCaM are Ca2+ saturated. Apparent Kd values derived from fits of a hyperbolic binding equation to these (solid lines) and similar data are listed in Table 1. Additional experimental details are given in the caption to Fig. 2. The fitted Fmin values for binding of NC2CaM and N2CCaM CaM to BIQPEP are 0.72±0.03 and 0.70±0.04. These are not different from the respective values of 0.74±0.03 and 0.71±0.01 derived for the complexes with BIQ.
Table 1. Kd and ΔΔGB values for binding of Ca2+-free and Ca2+-bound CaM.
N and C refer to the N-terminal and C-terminal EF hand pairs in CaM, respectively, with subscripts indicating their Ca2+-liganded states (2 or no Ca2+ bound). Kd values are given as means ± standard error of the mean calculated from 3 – 5 individual determinations. The row labeled “SUM” contains the arithmetic sums of the ΔΔGB values for the BIQ variants containing single amino acid replacements. The nomenclature for IQ domain reporter constructs is defined in Fig. 1. Differences in binding energy (ΔΔGB) were calculated according to the relation: ΔΔGB = RTln(KdBIQN/KdBIQ), where KdBIQ is the Kd value for a Ca2+-free or Ca2+-bound CaM-BIQ complex and KdBIQN is the Kd value for the corresponding complex with BIQPEP or a substituted BIQ variant, as specified in the table.
| NC | NC2* | N2C* | N2C2 | |||||
|---|---|---|---|---|---|---|---|---|
| Construct | Kd (µM) | ΔΔGB (kJ/mol) |
Kd (µM) | ΔΔGB (kJ/mol) |
Kd (µM) | ΔΔGB (kJ/mol) |
Kd (µM) | ΔΔGB (kJ/mol) |
| BIQ | 2.4±0.3 | - | 12.7±1.1 | - | 31.3±2.7 | - | 2.9±0.3 | - |
| BIQK−1A | 21.1±2.8 | 5.4±0.9 | 46.6±3.9 | 3.2±0.4 | 118.1±8.4 | 3.3±0.4 | 5.0±0.4 | 1.3±0.2 |
| BIQA3Q | 18.6±1.8 | 5.0±0.7 | 83.6±14.0 | 4.6±0.9 | 562.7±60.0 | 7.1±0.9 | 12.3±1.1 | 3.6±0.5 |
| BIQG6K | 5.8±0.8 | 2.2±0.4 | 1.4±0.2 | −5.4±0.8 | 12.7±1.3 | −2.2±0.3 | 0.3±0.03 | −5.6±0.8 |
| SUM | - | 12.6±1.8 | - | 2.4±1.3 | - | 8.2±1.0 | - | −0.7±1.0 |
| BIQTr | 413.5±23.4 | 12.7±1.7 | 47.2±4.2 | 3.2±0.4 | 344.2±46.1 | 5.9±0.9 | 7.9±0.7 | 2.5±0.4 |
| BIQPEP | 1128.4±176.5 | 15.1±2.3 | 207.6±16.5 | 6.9±0.7 | 988.4±146.8 | 8.5±1.5 | 11.0±1.2 | 3.3±0.5 |
The affinities of complexes with these Ca2+-liganded states of CaM were determined in the presence of 250 µM free Ca2+ using NxCCaM or NCxCaM, which contain defective N-ter and C-ter EF hand pairs, as indicated by the subscript “x”.
For comparative purposes it is preferable to express differences in the affinities of reporter complexes in terms of their stabilities instead of their Kd values, which do not scale linearly with stability. We have calculated the stability differences (ΔΔGB) between the CaM complexes with BIQPEP or BIQ variants and the corresponding BIQ complexes (Table 1). A negative ΔΔGB value means that BIQPEP or BIQ variant complex is more stable than the BIQ complex, while a positive value means it is less stable.
The ΔΔGB values for the BIQPEP complexes with Ca2+-free and Ca2+-saturated CaM are 15.1±2.3 and 3.3±0.5 kJ/mol, and the values for the complexes with NC2CaM and N2CCaM are 6.9±0.7 and 8.5±1.5 kJ/mol. Although the BIQPEP complex is less stable overall than the BIQ complex, this difference is significantly reduced when Ca2+ is bound to either EF hand pair in CaM, and is reduced still further when Ca2+ is bound to both EF hand pairs. The two complexes therefore differ both in terms of their overall stabilities and the changes in stability produced when Ca2+ is bound to calmodulin.
CaM binding to BIQ variants
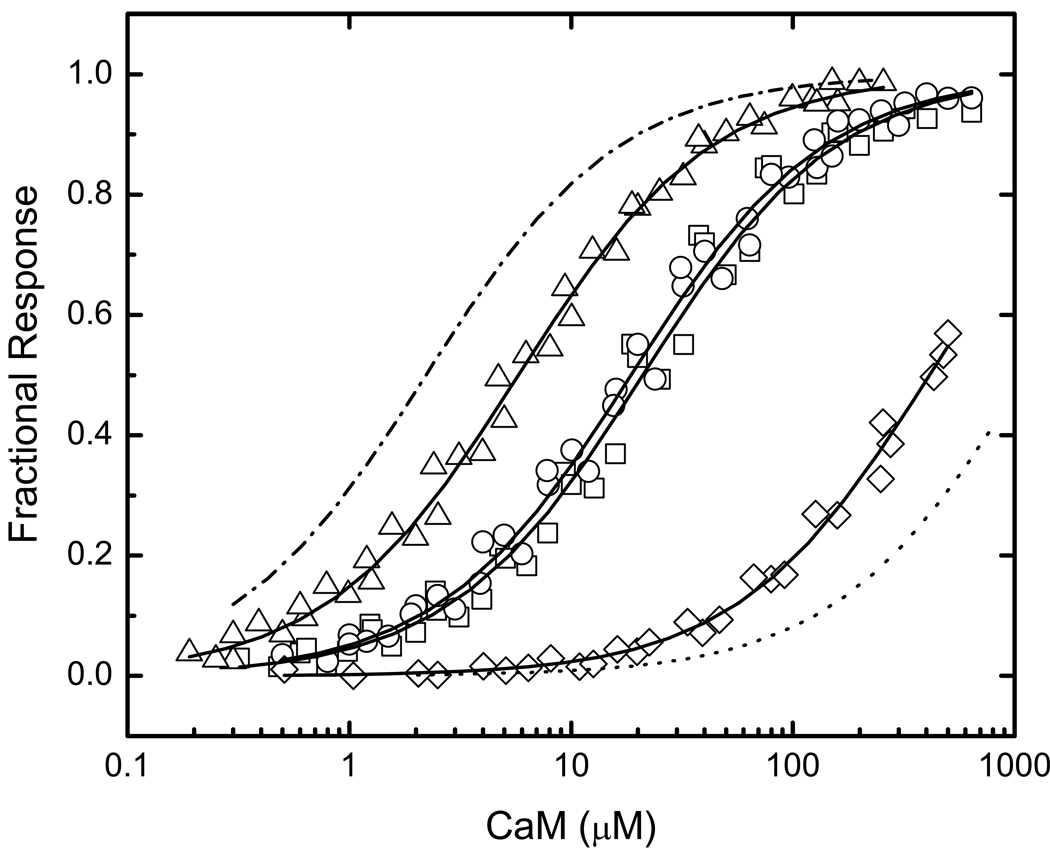
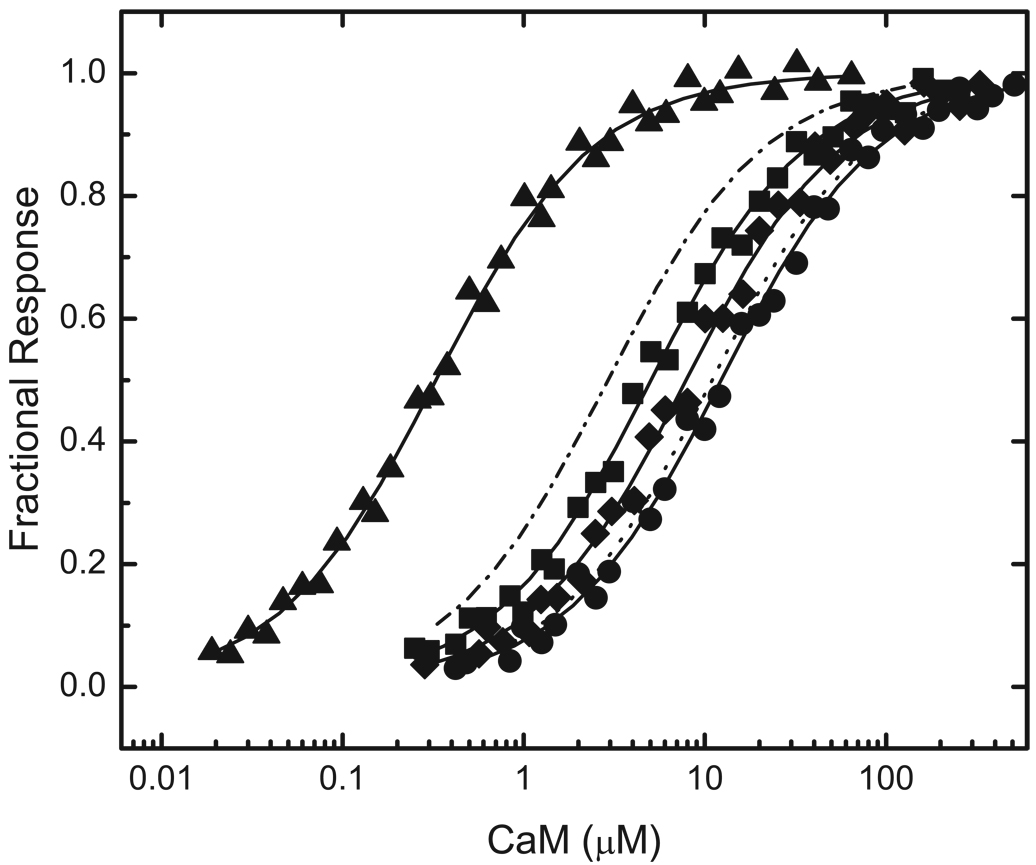
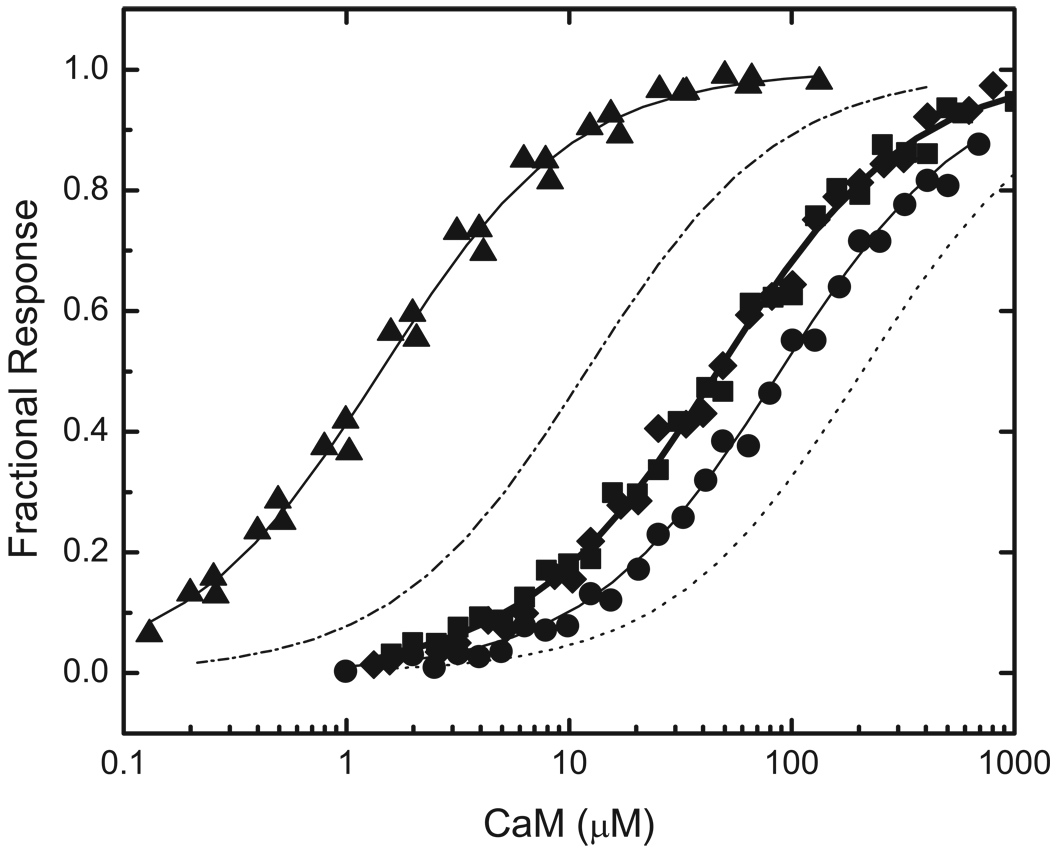
As seen in Fig 1, the inserts in BIQ and BIQPEP differ in length and in amino sequence at several positions. For these initial investigations the functional significance of amino acid sequence differences at positions -1, 3 and 6 was examined. The Gly at position 6 in the neuromodulin IQ domain has been replaced by a Lys in the PEP19 sequence. Since a Gly is present at this position in about half of all IQ domains, we were particularly interested in the functional consequences of this replacement. The other two replacements are of interest because structural data suggest that they are likely to interact with the C-ter CaM lobe, which plays a key role in Ca2+-free CaM-IQ domain complexes (7–9). Three variants of BIQ with single amino acid replacements were generated: BIQK−1A, BIQA3Q and BIQG6K, together with one containing all three replacements: BIQTr. The complete insert sequences for these reporter constructs are listed in Fig 1. Representative data for binding of Ca2+-free and Ca2+-saturated CaM, NC2CaM, and N2CCaM by all of these reporters are presented in Fig 4 through Fig 7. The Kd and ΔΔGB values derived from these and similar data are presented in Table 1.
FIG 4. Binding of Ca2+-free CaM to reporters containing mutant neuromodulin IQ domains altered to match the PEP19 sequence at positions -1, 3 and 6.
Data for the following reporter constructs are presented: BIQK−1A (□), BIQA3Q (○), BIQG6K (△), and BIQTr (◇), which contains all three amino acid changes The complete amino acid sequences of the IQ domains in these reporters are listed in Fig. 1. Apparent Kd values derived from fits of a hyperbolic binding equation to these (solid lines) and similar data are listed in Table 1. Nominally Ca2+-free conditions were produced by addition of 3 mM BAPTA. Additional experimental details are given in “Experimental Procedures” or in the caption to Fig. 2. Fitted curves for binding of Ca2+-free CaM to BIQ (-.-) and BIQPEP (…) are presented for comparative purposes (see Fig. 2.). The fitted Fmin value for BIQTr is 0.60±0.01. This is not different from the values derived for the other reporter constructs, which range from 0.57±0.01 to 0.59±0.01.
FIG 7. The effects of Ca2+ binding to the N-terminal EF hand pair in CaM on its affinities for reporters containing mutant neuromodulin IQ domains altered to match the PEP19 sequence at positions -1, 3 and 6.
Data for binding of NCxCaM to the following reporter constructs are presented: BIQK−1A (■), BIQA3Q (●), BIQG6K (▲), and BIQTr (◆), which contains all three amino acid changes. The complete amino acid sequences of the IQ domains in these reporters are listed in Fig. 1. At the ~250 µM free Ca2+ concentration used for these experiments the native EF hand pair in NCxCaM is Ca2+ saturated. Apparent Kd values derived from fits of a hyperbolic binding equation to these (solid lines) and similar data are listed in Table 1. Additional experimental details are given in “Experimental Procedures” or in the caption to Fig. 2. Fitted curves for binding of Ca2+-saturated NCxCaM to BIQ (-.-) and BIQPEP (…) are presented for comparative purposes (see Fig. 2). The fitted Fmin values for BIQA3Q and BIQTr are 0.70±0.03 and 0.70±0.04. These are not different from the values derived for BIQK−1A and BIQG6K, which are 0.69±0.01 and 0.71±0.01.
The respective ΔΔGB values for the Ca2+-free CaM complexes with BIQK−1A, BIQA3Q and BIQG6K are 5.4±0.9, 5.0±0.7 kJ/mol and 2.2±0.4 kJ/mol. Thus, the K−1A and A3Q replacements significantly decrease the stability of the complex, while the G6K replacement has a relatively small effect, corresponding with only a ~2-fold increase in the Kd value (Table 1). The ΔΔGB values for the various Ca2+-bound forms of the BIQA3Q complex are similar to the value for the Ca2+-free form of this complex (Table 1). The effects of the A3Q replacement on stability thus appear to be Ca2+-independent. The ΔΔGB values for the intermediate Ca2+-bound forms of the BIQK−1A complex both appear to be less than the 5.4±0.9 kJ/mol value for the Ca2+-free complex, but the differences are not quite statistically significant. The 1.3±0.2 kJ/mol ΔΔGB value for the Ca2+-saturated form of this complex is significantly lower (Table 1). The K−1A replacement therefore appears to have significant Ca2+-dependent and independent effects on stability. The ΔΔGB values for the complexes between BIQG6K and the Ca2+-bound forms of CaM all are negative, with values ranging from −5.6±0.8 to −2.2±0.3 kJ/mol (Table 1), and the effects of the G6K replacement on stability are primarily Ca2+-dependent.
The ΔΔGB values for the CaM complex with BIQTr, which contains all three amino replacements, are similar to the corresponding values for the BIQPEP complex (Table 1). The only statistically significant difference is between the values for the two NC2CaM complexes. Thus, the combination of Ca2+-independent and Ca2+-dependent changes in stability produced by the three amino acid replacements appear to largely explain the functional differences between the BIQ and BIQPEP complexes. The arithmetic sums of the ΔΔGB values for the CaM complexes with BIQ variants containing single replacements are similar to the ΔΔGB values for the BIQTr reporter complex (Table 1). The only statistically significant difference is between the two values for the Ca2+-saturated complex. This additivity suggests that there is little interaction among positions -1, 3 and 6.
Energy coupling in the CaM complexes with BIQ and BIQPEP
It is evident that Ca2+-binding has quite distinct effects on the stabilities of the BIQ and BIQPEP complexes. To examine these differences we have derived energy coupling (ΔΔGC) values for the various reporter complexes from ratios of the Kd values for the different Ca2+-free and Ca2+ liganded forms of CaM, as indicated in Table 2. These values define the changes in stability produced when Ca2+ is initially bound to the C-ter or N-ter EF hand pair in CaM (NC2/NC or N2C/NC ratio), and the changes produced when the remaining EF hand pair is occupied (N2C2/NC2 or N2C2/N2C ratio). The overall effect of Ca2+ binding on stability is defined by the ΔΔGC value derived from the N2C2/N2C Kd ratio (Table 2). This value is, by definition, independent of whether the C-ter (C-ter→N-ter binding order) or N-ter (N-ter→C-ter binding order) EF hand pair is occupied first. Although ΔΔGC values could in principle be calculated from ΔΔGB values, calculating them from the Kd values for a single reporter complex is preferable because it reduces the number of experimentally determined values in the calculations.
Table 2. ΔΔGC values for CaM-IQ domain complexes.
The indicated Kd ratios were calculated for each reporter construct from the Kd values in Table 1 for binding of the different Ca2+− liganded forms of CaM (NC, NC2, N2C or N2C2). Energy coupling (ΔΔGC) values were calculated from these Kd ratios according to the relation: ΔΔGC = RTln(Kd ratio). A positive ΔΔGC value means that Ca2+ binding to the indicated EF hand pair(s) increases the Kd for the complex; a negative value means that Ca2+ binding decreases the Kd for the complex.
| NC2/NC | N2C2/NC2 | N2C/NC | N2C2/N2C | N2C2/NC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Construct | Kd Ratio | ΔΔGC (kJ/mol) |
Kd Ratio | ΔΔGC (kJ/mol) |
Kd Ratio | ΔΔGC (kJ/mol) |
Kd Ratio | ΔΔGC (kJ/mol) |
Kd Ratio | ΔΔGC (kJ/mol) |
| BIQ | 5.4±0.8 | 4.1±0.6 | 0.2±0.03 | −3.5±0.5 | 14.1±1.9 | 6.5±0.9 | 0.09±0.012 | −5.8±0.8 | 1.3±0.2 | 0.7±0.1 |
| BIQK−1A | 2.2±0.4 | 1.9±0.3 | 0.1±0.01 | −5.5±0.6 | 5.6±0.9 | 4.2±0.7 | 0.04±0.005 | −7.8±0.8 | 0.2±0.09 | −3.5±0.6 |
| BIQA3Q | 4.8±1.0 | 3.8±0.8 | 0.1±0.03 | −4.8±1.1 | 33.3±4.9 | 8.6±1.4 | 0.02±0.003 | −9.2±1.7 | 0.7±0.08 | −0.7±0.2 |
| BIQG6K | 0.2±0.04 | −3.4±0.6 | 0.2±0.04 | −3.6±0.6 | 1.8±0.3 | 1.5±0.3 | 0.03±0.004 | −8.5±1.2 | 0.06±0.008 | −7.0±1.1 |
| BIQTr | 0.1±0.02 | −5.3±0.7 | 0.2±0.02 | −4.4±0.7 | 0.8±0.2 | −0.4±0.1 | 0.02±0.004 | −9.3±1.5 | 0.02±0.002 | −9.7±1.2 |
| BIQPEP | 0.2±0.03 | −4.2±0.5 | 0.1±0.02 | −6.3±1.1 | 0.8±0.2 | −0.5±0.1 | 0.01±0.003 | −10.9±2.1 | 0.01±0.002 | −11.4±1.8 |
The ΔΔGC values derived for the BIQ and BIQPEP complexes are listed in Table 2. As we have previously demonstrated (12), Ca2+ binding only to the C-ter or N-ter EF hand pair in CaM lowers the stability of the BIQ complex, with respective ΔΔGC values of 4.1±0.6 and 6.5±0.9 kJ/mol (Table 2). These are balanced by the ΔΔGC values for Ca2+ binding to the remaining N-ter or C-ter EF hand pair, which are respectively −3.5±0.5 and −5.8±0.8 kJ/mol. As a result, the stabilities of the Ca2+-free and Ca2+-saturated complexes are closely similar, with an overall ΔΔGC value of 0.7±0.1 kJ/mol. As might be inferred from its ΔΔGB values, the situation with the BIQPEP complex is quite different. When Ca2+ is bound only to the C-ter or N-ter EF hand pair in CaM the stability of the complex is increased or remains approximately the same, with respective ΔΔGC values of −4.2±0.5 and −0.5±0.1 kJ/mol. In both cases binding to the unoccupied N-ter or C-ter EF hand pair increases stability, with respective ΔΔGC values of −6.3±1.1 or −10.9±2.1 kJ/mol. The overall ΔΔGC value for the BIQPEP complex is −11.4±1.8 kJ/mol, corresponding with the ~100-fold decrease in the Kd value noted earlier (Table 2). A key aspect of this behavior is its asymmetry with respect to the order of Ca2+-binding: in the C-ter→N-ter order the overall increase in stability is distributed over both steps, while in the in N-ter→C-ter order it occurs entirely at the second step. The ΔΔGC values for the BIQPEP complex are consistent with the equilibrium Ca2+-binding constants reported for the CaM complex with a peptide containing the PEP19 IQ domain (18).
Energy coupling in the CaM complexes with BIQ variants
The overall ΔΔGC value for the BIQK−1A complex is −3.5±0.6 kJ/mol, which is ~4 kJ/mol less than the overall ΔΔGC value for the BIQ complex and ~8 kJ/mol larger than the value for the BIQPEP complex (Table 2). The ΔΔGC values for the individual steps in both the C-ter→N-ter and N-ter→C-ter Ca2+-binding orders appear to be ~2 kJ/mol less than the values for the BIQ complex. However, most of these differences are not quite statistically significant. It is therefore unclear how the K−1A substitution affects stability at the level of the individual Ca2+-binding steps, although the significant decrease in the overall ΔΔGC value is apparently not produced at a single step.
The overall ΔΔGC value for the BIQG6K complex is −7.0±1.1 kJ/mol, which is ~8 kJ/mol less than the overall value for the BIQ complex, and ~4 kJ/mol larger than the value for the BIQPEP complex. The steps in the C-ter→N-ter Ca2+-binding order have respective ΔΔGC values of −3.4±0.6 and −3.6±0.6 kJ/mol, while the respective values for the steps in the N-ter→C-ter order are 1.8±0.3 and −8.5±1.2 kJ/mol. This asymmetric pattern of Ca2+-dependent stability changes closely resembles the one exhibited by the BIQPEP complex (Table 2).
The overall ΔΔGC value for BIQA3Q complex is identical to the value for the BIQ complex (Table 2), and ΔΔGC values for the individual steps in the C-ter→N-ter and N-ter→C-ter Ca2+-binding orders do not differ to a statistically significant degree from the values for the BIQ complex (Table 2). Thus, as suggested by the ΔΔGB values for this complex, the ~5 kJ/mol decrease in stability produced by the A3Q replacement appears to be Ca2+-independent.
The ΔΔGC values for the BIQTr and BIQPEP complexes appear to be similar, and none of the apparent differences between them are statistically significant (Table 2). Since the effect of the A3Q replacement on stability is Ca2+-independent, the sum of the Ca2+-dependent effects of the K−1A and G6K replacements must account for the distinctive patterns of Ca2+-dependent stability changes exhibited by the BIQ and BIQPEP complexes. Indeed, the G6K replacement alone appears to account for most of the Ca2+-dependent differences between the two reporter complexes.
Discussion
As reported previously and confirmed here, the stability of the BIQ reporter complex decreases when Ca2+ is bound to a single EF hand pair, but Ca2+ binding has little or no overall effect on stability (12). This produces a biphasic change in the stability of the complex as the free Ca2+-concentration is increased (12). These characteristics are shared by the CaM complex with full-length neuromodulin (12). In contrast, when Ca2+ is bound to a single EF hand pair in the BIQPEP reporter complex it either increases stability or has little effect, depending upon whether the C-ter or N-ter EF hand pair is occupied, and occupancy of both EF hand pairs substantially increases the stability of this complex, producing a ~100-fold decrease in the Kd value. Our results indicate that a G6K replacement largely accounts for the distinctive patterns of intermediate and overall Ca2+-dependent stability changes exhibited by the BIQ and BIQPEP complexes. The K−1A replacement accounts for the remaining Ca2+-dependent differences. This and the A3Q replacement account for most of the Ca2+-independent differences between the two complexes. An interesting aspect of these results is that under equilibrium conditions, unless there is a Gly at position 6, the distinction between IQ domains and other types of CaM-binding domains does not appear to be clear-cut.
A Gly residue is found at position 6 in about half all known IQ domain sequences (Fig. 8), so our results suggest that the presence or absence of this residue defines two major functional classes of IQ domains. Consistent with this hypothesis, a variety of published data demonstrate that the identity of the amino acid at this position significantly affects the structure of a CaM-IQ domain complex.
FIG 8. Logo representation of the aligned sequences of 3000 IQ domain regions.
The sequence is numbered in relation to the first residue (0) in the 11 residue IQ motif, which has been underlined. Each position is represented by a stack of one-letter amino acid symbols. The total height of a stack is an indication of how well conserved that position is; the height of each amino acid symbol in a stack is an indication of its relative frequency. The arrow indicates a semi-conserved Gly residue that appears to be a major determinant of the pattern of Ca2+-dependent stability changes exhibited by a CaM-IQ domain complex.
Two major structural classes of CaM-IQ domain complexes
Structural and biophysical studies indicate that myosin light chain subunits and Ca2+-free CaM can form two different types of complexes with IQ domains. One is compact, with the N-ter lobe interacting closely with the IQ domain, and the other extended, with the N-ter lobe largely free of the IQ domain (7, 8). Although crystal structures supporting an extended conformation have been determined only for light chain subunits bound to myosin IQ domains, fluorescence energy transfer measurements performed with labeled CaM and peptides indicate that CaM forms similar complexes with these domains (7, 8). Crystal structures supporting a compact conformation have been determined for both CaM and light chain subunit complexes with myosin IQ domains containing the semi-conserved Gly (7–9). Structural and biophysical investigations of native and specifically altered IQ domains indicate that for the compact structure to be formed Gly or another small amino acid (Ala, Ser, Thr, Val) must be present at position 6 in the IQ domain, and a basic residue must also be present at position 10, which is well-conserved (see Fig. 8). The presence of a bulky amino acid (Lys, Arg, Met) at position 6 appears to result in the extended conformation (7, 8). Structural studies suggest that the N-ter CaM lobe interacts extensively with the IQ domain in Ca2+-saturated complexes, regardless of the identity of the residue at position 6, and this is presumably also the case for the N-ter lobes in the Ca2+-saturated BIQ and BIQPEP complexes (10, 11). In both Ca2+-free and Ca2+-saturated CaM-IQ domain complexes the C-ter CaM lobe appears to interact in the same orientation with the N-ter half of the IQ domain (7, 8, 10, 11). This is consistent with the significant Ca2+-independent effects on stability produced by the K−1A and A3Q replacements, which are in this region. The semi-conserved nature of the Gly at position 6 (Fig. 8) suggests that its role goes beyond allowing the compact Ca2+-free CaM-IQ domain complex, since this does not appear to specifically require a Gly. We are currently investigating additional BIQ variants in order to determine the specific functional effects of different amino acid replacements at this position.
Relating the functional and probable structural effects of the G6K substitution
Based on the above dissussion, the Ca2+-free CaM complex with the BIQ is likely to be in the compact conformation, while the complexes with BIQPEP and BIQG6K are likely to be in the extended conformation. Although the ~2 kJ/mol drop in stability associated with the G6K substitution is consistent with loss of interactions with the Ca2+-free N-ter lobe, this relatively small effect may be an underestimate of the contribution of these interactions to stability. This is because replacement of the Gly might be also expected to be stabilizing, given an α-helical conformation for the bound IQ domain (7–9).
When Ca2+ is bound only to the N-ter EF hand pair in CaM it has a minimal effect on the stabilities of the BIQPEP and BIQG6K complexes, suggesting that the the N-ter lobe is not only extended in these complexes in the absence of Ca2+, but remains so in this Ca2+-bound state. In contrast, Ca2+-binding only to the N-ter EF hand pair in the BIQ complex produces a ~7 kJ/mol decrease in stability. This may reflect a loss of interactions with the N-ter lobe when Ca2+ is bound and/or formation of an antagonistic interaction between the Ca2+-bound N-ter lobe and the IQ domain.
When Ca2+ is bound only to the C-ter EF hand pair in CaM it decreases the stability of the BIQ complex by ~4 kJ/mol, and has the opposite effect on the BIQPEP and BIQG6K complexes (Table 2). The essentially identical increases in the stabilities of the all the complexes examined produced when Ca2+ is subsequently bound to the N-ter EF hand pair suggests that the N-ter CaM lobe is in a similar state in the intermediate Ca2+-bound complexes (Table 2).
Our results demonstrate that the presence or absence of a Gly at position 6 defines two classes of IQ domains whose complexes with CaM exhibit distinct patterns of Ca2+-dependent stability changes. This is likely to at least in part be a reflection of differences in interactions with the N-ter CaM lobe in these two classes. Additional structural and biophysical investigations are needed to confirm and extend this hypothesis. Structures of the intermediate Ca2+-bound states of CaM-IQ domain complexes are particular important, as major differences between the stabilities these states appears to be a key feature of the two classes.
FIG 5. Binding of Ca2+-saturated CaM to reporters containing mutant neuromodulin IQ domains altered to match the PEP19 sequence at positions -1, 3 and 6.
Data for the following reporter constructs are presented: BIQK−1A (■), BIQA3Q (●), BIQG6K (▲), and BIQTr (◆), which contains all three amino acid changes. The complete amino acid sequences of the IQ domains in these reporters are listed in Fig. 1. Apparent Kd values derived from fits of a hyperbolic binding equation to these (solid lines) and similar data are listed in Table 1. Ca2+ saturation was effected by addition of ~250 µM free Ca2+. Additional experimental details are given in “Experimental Procedures” or in the caption to Fig. 2. Fitted curves for binding of Ca2+-saturated CaM to BIQ (-.-) and BIQPEP (…) are presented for comparative purposes (see Fig. 2).
FIG 6. The effects of Ca2+ binding to the C-terminal EF hand pair in CaM on its affinities for reporters containing mutant neuromodulin IQ domains altered to match the PEP19 sequence at positions -1, 3 and 6.
Data for binding of NxCCaM to the following reporter constructs are presented: BIQK−1A (■), BIQA3Q (●), BIQG6K (▲), and BIQTr (◆), which contains all three amino acid changes. The complete amino acid sequences of the IQ domains in these reporters are listed in Fig. 1. At the ~250 µM free Ca2+ concentration used for these experiments the native EF hand pair in NxCCaM is Ca2+ saturated. Apparent Kd values derived from fits of a hyperbolic binding equation to these (solid lines) and similar data are listed in Table 1. Additional experimental details are given in “Experimental Procedures” or in the caption to Fig. 2. Fitted curves for binding of Ca2+-saturated NxCCaM to BIQ (-.-) and BIQPEP (…) are presented for comparative purposes (see Fig. 2).
Footnotes
This work was supported by NIH Grant DK53863 to A.P.
Abbreviations
BIQ, fluorescent reporter containing an IQ domain sequence derived from neuromodulin; BIQPEP, fluorescent reporter containing an IQ domain sequence derived from PEP19; BIQK−1A, BIQA3Q and BIQG6K, variants of BIQ with the following substitutions: A for K at the -1 position, Q for A at the 3 position and K for G at the 6 position; BIQTr, variant of BIQ containing all three substitutions; NxCCaM (NxC), mutant CaM with E31A and E67A substitutions; NCxCaM (NCx), mutant CaM with E104A and E140A substitutions; N2CCaM (N2C), CaM with Ca2+ bound to both N-ter EF hands; NC2CaM (NC2), CaM with Ca2+ bound to both C-ter EF hands; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid; dibromo-BAPTA, 1,2-bis(2-amino-5,5'-dibromophenoxy)ethane-N,N,N',N'-tetraacetic acid; TPEN, tetrakis-(2-pyridylmethyl)ethylenediamine.
References
- 1.Persechini A, Yano K, Stemmer PM. Ca2+ binding and energy coupling in the calmodulin-myosin light chain kinase complex. J. Biol. Chem. 2000;275:4199–4204. doi: 10.1074/jbc.275.6.4199. [DOI] [PubMed] [Google Scholar]
- 2.Forsén S, Vogel HJ, Drakenberg T. Biophysical studies of calmodulin. In: Cheung WY, editor. Calcium and Cell Function. New York: Academic Press; 1986. pp. 113–157. [Google Scholar]
- 3.Mooseker MS, Cheney RE. Unconventional myosins. Ann. Rev. Cell Dev. Biol. 1995;11:633–675. doi: 10.1146/annurev.cb.11.110195.003221. [DOI] [PubMed] [Google Scholar]
- 4.Jurado LA, Chockalingam PS, Jarrett HW. Apocalmodulin. Physiol. Rev. 1999;79:661–682. doi: 10.1152/physrev.1999.79.3.661. [DOI] [PubMed] [Google Scholar]
- 5.Yus-Najera E, Santana-Castro I, Villarroel A. The identification and characterization of a noncontinuous calmodulin-binding site in noninactivating voltage-dependent KCNQ potassium channels. J. Biol. Chem. 2002;277:28545–28553. doi: 10.1074/jbc.M204130200. [DOI] [PubMed] [Google Scholar]
- 6.Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107–113. doi: 10.1016/s0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- 7.Terrak M, Rebowski G, Lu RC, Grabarek Z, Dominguez R. Structure of the light chain-binding domain of myosin V. Proc. Natl. Acad. Sci. USA. 2005;102:12718–12723. doi: 10.1073/pnas.0503899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrak M, Wu GM, Stafford WF, Lu RC, Dominguez R. Two distinct myosin light chain structures are induced by specific variations within the bound IQ motifs - functional implications. EMBO J. 2003;22:362–371. doi: 10.1093/emboj/cdg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houdusse A, Gaucher JF, Krementsova E, Mui S, Trybus KM, Cohen C. Crystal structure of apo-calmodulin bound to the first two IQ motifs of myosin V reveals essential recognition features. Proc Natl Acad Sci U S A. 2006;103:19326–19331. doi: 10.1073/pnas.0609436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Petegem F, Chatelain FC, Minor DL. Insights into voltage-gated calcium channel regulation from the structure of the Ca(V)1.2 IQ domain-Ca2+/calmodulin complex. Nature Structural & Molecular Biology. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori MX, Vander Kooi CW, Leahy DJ, Yue DT. Crystal structure of the CaV2 IQ domain in complex with Ca2+/calmodulin: high-resolution mechanistic implications for channel regulation by Ca2+ Structure. 2008;16:607–620. doi: 10.1016/j.str.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black DJ, Leonard J, Persechini A. Biphasic Ca2+-dependent switching in a calmodulin-IQ domain complex. Biochemistry. 2006;45:6987–6995. doi: 10.1021/bi052533w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persechini A, Cronk B. The relationship between the free concentrations of Ca2+ and Ca2+-calmodulin in intact cells. J. Biol. Chem. 1999;274:6827–6830. doi: 10.1074/jbc.274.11.6827. [DOI] [PubMed] [Google Scholar]
- 14.Persechini A, Stemmer PM. Calmodulin is a limiting factor in the cell. Trends Cardiovasc. Med. 2002;12:32–37. doi: 10.1016/s1050-1738(01)00144-x. [DOI] [PubMed] [Google Scholar]
- 15.Persechini A. Monitoring the intracellular free Ca(2+)-calmodulin concentration with genetically-encoded fluorescent indicator proteins. Methods Mol. Biol. 2002;173:365–382. doi: 10.1385/1-59259-184-1:365. [DOI] [PubMed] [Google Scholar]
- 16.Fruen BR, Black DJ, Bloomquist RA, Bardy JM, Johnson JD, Louis CF, Balog EM. Regulation of the RYR1 and RYR2 Ca2+ release channel isoforms by Ca2+-insensitive mutants of calmodulin. Biochemistry. 2003;42:2740–2747. doi: 10.1021/bi0267689. [DOI] [PubMed] [Google Scholar]
- 17.Tang W, Halling DB, Black DJ, Pate P, Zhang JZ, Pedersen S, Altschuld RA, Hamilton SL. Apocalmodulin and Ca2+ calmodulin-binding sites on the Ca(V)1.2 channel. Biophys. J. 2003;85:1538–1547. doi: 10.1016/s0006-3495(03)74586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putkey JA, Waxham MN, Gaertner TR, Brewer KJ, Goldsmith M, Kubota Y, Kleerekoper QK. Acidic/IQ motif regulator of calmodulin. J. Biol. Chem. 2008;283:1401–1410. doi: 10.1074/jbc.M703831200. [DOI] [PMC free article] [PubMed] [Google Scholar]