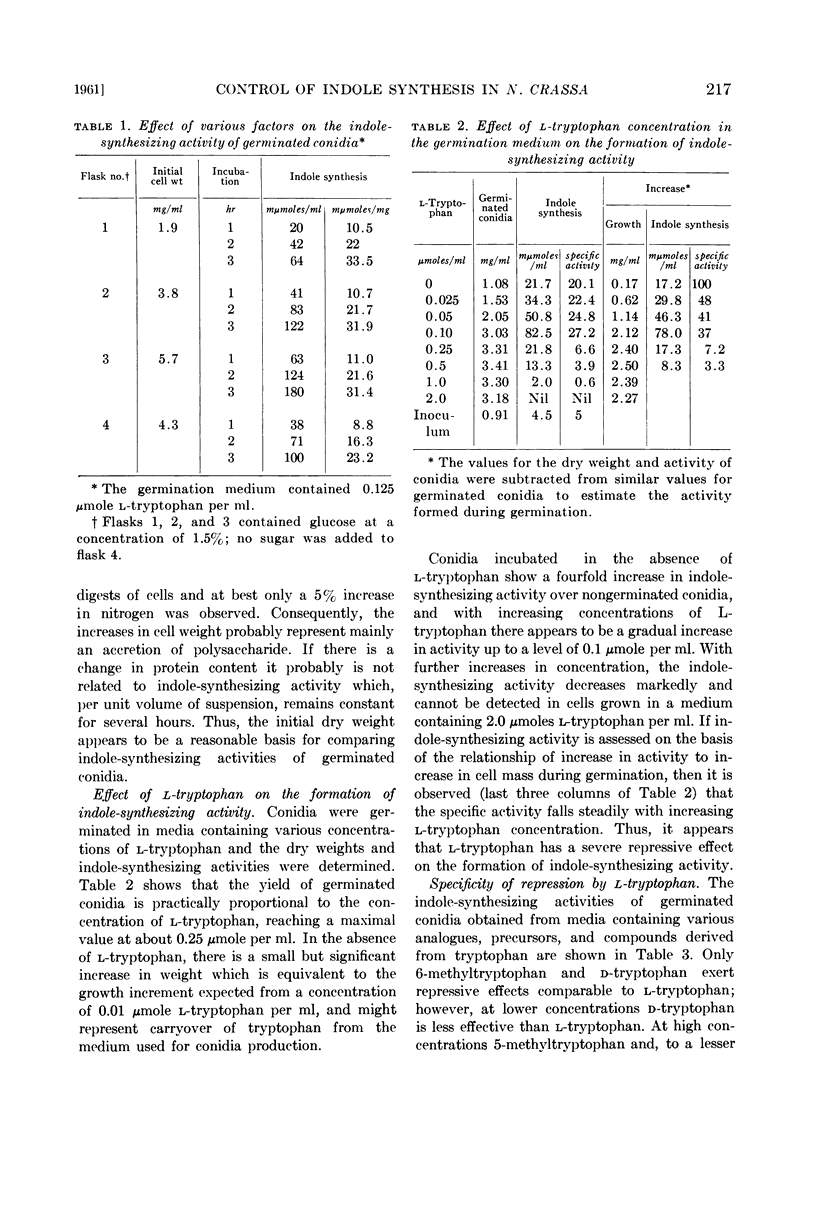
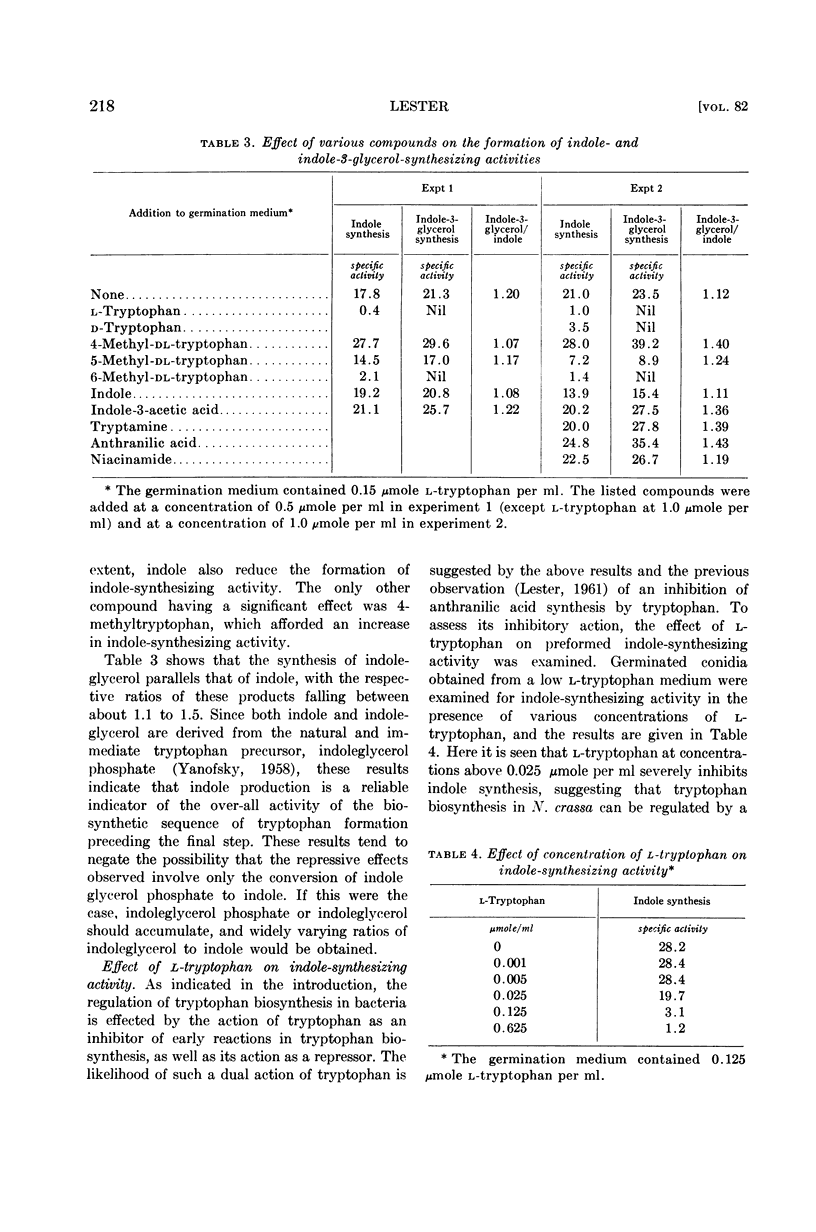
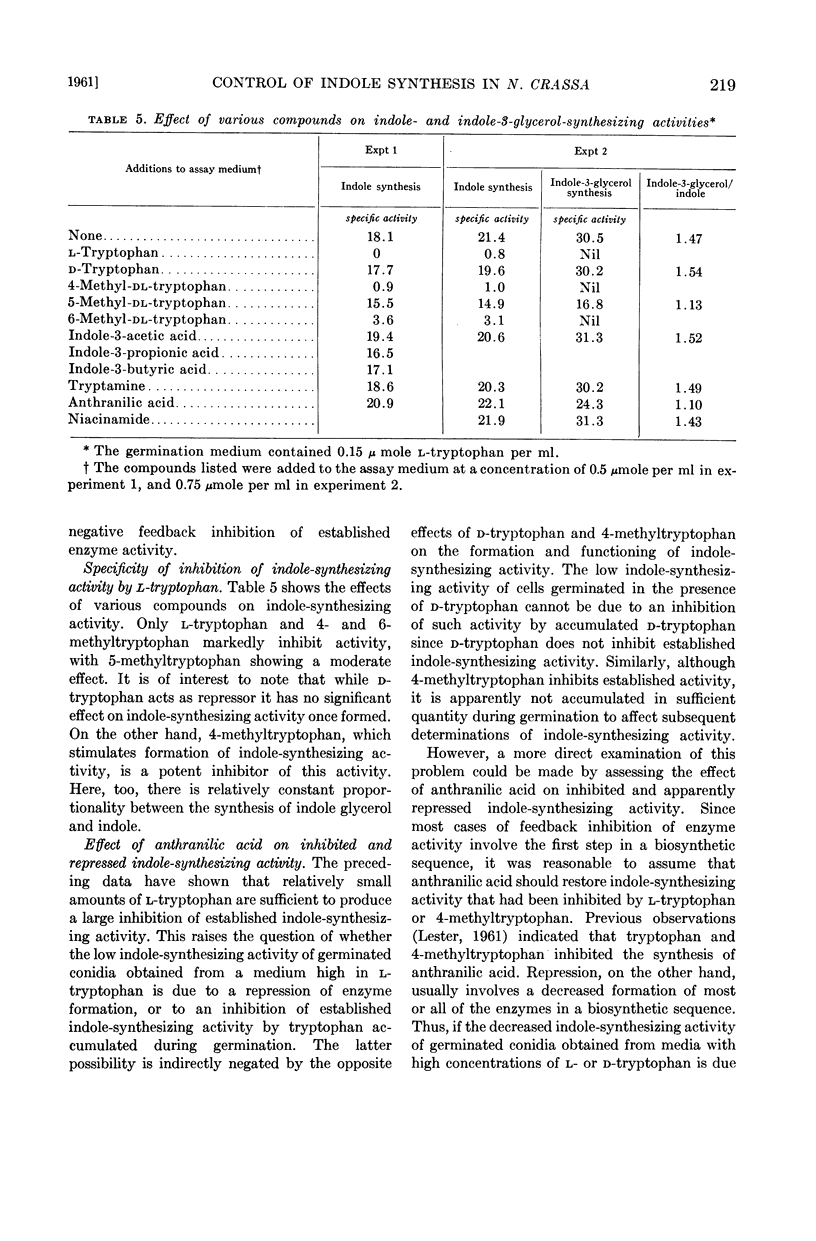
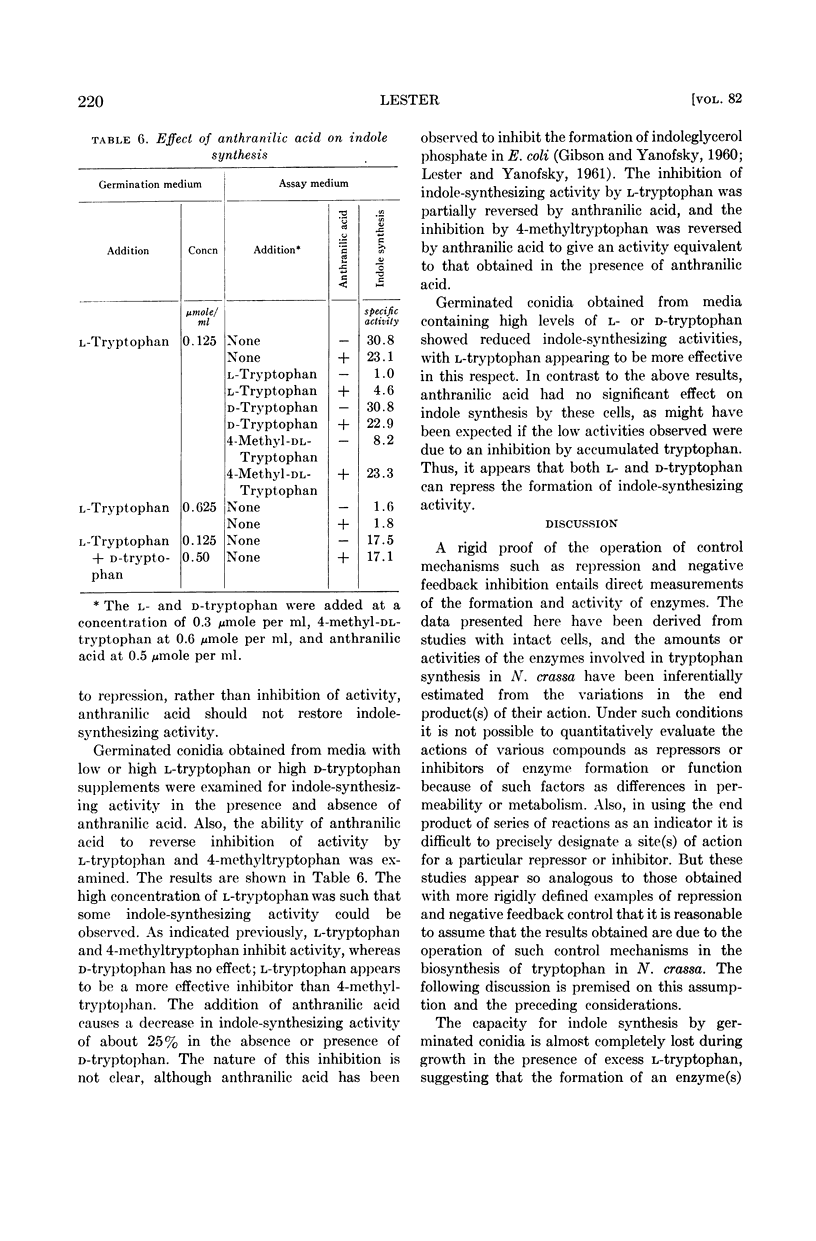
Abstract
Lester, Gabriel (Worcester Foundation for Experimental Biology, Shrewsbury, Mass.). Repression and inhibition of indole-synthesizing activity in Neurospora crassa. J. Bacteriol. 82:215–223. 1961.—The possibility of repression and feedback inhibition as regulating mechanisms for the synthesis of tryptophan by Neurospora crassa has been examined in a tryptophan auxotroph which accumulates indole (and indole-glycerol). Indole-synthesizing activity was determined with germinated conidia suspended in medium lacking tryptophan. This activity was almost absent from cells cultured on germination medium containing more than 1.0 μmole l-tryptophan per ml, and increased with decreasing concentrations of l-tryptophan. A similar depression of the formation of indole synthesizing activity was caused by 6-methyl- and d-tryptophan, and less effectively by 5-methyltryptophan; 4-methyltryptophan was slightly stimulatory. Preformed indole synthesizing activity was inhibited by l-tryptophan, 4- and 6-methyltryptophan, and to a lesser extent by 5-methyltryptophan; d-tryptophan had no effect in this respect. The inhibition of preformed activity was partially reversed by anthranilic acid, which is a precursor of indole. However, anthranilic acid did not increase indole synthesis by cells wherein the formation of indole-synthesizing activity had been depressed by culture in the presence of high concentrations of l- or d-tryptophan. These observations indicate that regulation of tryptophan synthesis in N. crassa might result from the action of tryptophan as a repressor and as a feedback inhibitor. The relation of these results to other regulatory systems is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIVEN M., KNOX W. E. The specificity of tryptophan analogues as inducers, substrates, inhibitors, and stabilizers of liver tryptophan pyrrolase. J Biol Chem. 1960 Jun;235:1716–1718. [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- DOY C. H., PITTARD A. J. Feedback control of tryptophan biosynthesis. Nature. 1960 Mar 26;185:941–942. doi: 10.1038/185941b0. [DOI] [PubMed] [Google Scholar]
- GIBSON F., YANOFSKY C. The partial purification and properties of indole-3-glycerol phosphate synthetase from Escherichia coli. Biochim Biophys Acta. 1960 Oct 7;43:489–500. doi: 10.1016/0006-3002(60)90471-6. [DOI] [PubMed] [Google Scholar]
- Gross S R, Fein A. Linkage and Function in Neurospora. Genetics. 1960 Jul;45(7):885–904. doi: 10.1093/genetics/45.7.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ N. H., SHEN S. C. Neurospora tyrosinase. J Biol Chem. 1952 May;197(2):513–520. [PubMed] [Google Scholar]
- JAKOBY W. B., BONNER D. M. Kynureninase from Neurospora: purification and properties. J Biol Chem. 1953 Dec;205(2):699–707. [PubMed] [Google Scholar]
- LESTER G. Some aspects of tryptophan synthetase formation in Neurospora crassa. J Bacteriol. 1961 Jun;81:964–973. doi: 10.1128/jb.81.6.964-973.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G., YANOFSKY C. Influence of 3-methylanthranilic and anthranilic acids on the formation of tryptophan synthetase in Escherichia coli. J Bacteriol. 1961 Jan;81:81–90. doi: 10.1128/jb.81.1.81-90.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G. L'effet d'inhibition spécifique dans la biosynthèse de la tryptophane-desmase chez Aerobacter aerogenes. C R Hebd Seances Acad Sci. 1953 Feb 2;236(5):530–532. [PubMed] [Google Scholar]
- MOYED H. S., FRIEDMAN M. Interference with feedback control; a mechanism of antimetabolite action. Science. 1959 Apr 10;129(3354):968–969. doi: 10.1126/science.129.3354.968. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Effects of azatryptophan on bacterial enzymes and bacteriophage. Biochim Biophys Acta. 1958 Feb;27(2):330–344. doi: 10.1016/0006-3002(58)90340-8. [DOI] [PubMed] [Google Scholar]
- Suskind S. R., Yanofsky C., Bonner D. M. ALLELIC STRAINS OF Neurospora LACKING TRYPTOPHAN SYNTHETASE: A PRELIMINARY IMMUNOCHEMICAL CHARACTERIZATION. Proc Natl Acad Sci U S A. 1955 Aug 15;41(8):577–582. doi: 10.1073/pnas.41.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUDINGER P. A., COHEN G. N. The effect of 4-methyltryptophan on growth and enzyme systems of Escherichia coli. Biochem J. 1956 Mar;62(3):488–491. doi: 10.1042/bj0620488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H. J. REPRESSED AND INDUCED ENZYME FORMATION: A UNIFIED HYPOTHESIS. Proc Natl Acad Sci U S A. 1957 Jun 15;43(6):491–496. doi: 10.1073/pnas.43.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAINWRIGHT S. D., BONNER D. M. On the induced synthesis of an enzyme required for biosynthesis of an essential metabolite: induced kynureninase synthesis in Neurospora crassa. Can J Biochem Physiol. 1959 Jun;37(6):741–750. [PubMed] [Google Scholar]
- YANOFSKY C., RACHMELER M. The exclusion of free indole as an intermediate in the biosynthesis of tryptophan in Neurospora crassa. Biochim Biophys Acta. 1958 Jun;28(3):640–641. doi: 10.1016/0006-3002(58)90533-x. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The enzymatic conversion of anthranilic acid to indole. J Biol Chem. 1956 Nov;223(1):171–184. [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YURA T., VOGEL H. J. Pyrroline-5-carboxylate reductase of Neurospora crassa; partial purification and some properties. J Biol Chem. 1959 Feb;234(2):335–338. [PubMed] [Google Scholar]



