Abstract
Increasing evidence indicates that smoking cues contribute to nicotine self-administration and attenuating conditioned reactivity to smoking cues may aid abstinence of smoking and prevention of smoking relapse in individuals with nicotine dependence. Based on prior studies showing that the partial N-methyl-d-aspartate (NMDA) agonist d-cycloserine (DCS) facilitates extinction of learned fear during behavioral exposure therapy in humans and facilitates extinction of cocaine-induced conditioned place preference in animals, we evaluated whether DCS would have potential for reducing reactivity to smoking cues when combined with cue exposure treatment in humans with nicotine dependence. In this double-blind placebo controlled pilot laboratory study, twenty-five smokers were recruited from the general community and randomized to DCS or placebo, plus cue exposure therapy. DCS significantly attenuated smoking cue reactivity in response to in-vivo smoking cues based on physiological reactivity and subjective urge-to-smoke ratings and led to a significantly smaller expired carbon monoxide (CO) level at the 1-week follow-up compared to placebo, although exploratory analyses indicated no effect on smoking behavior overall. These findings provide promising support for DCS combined with cue exposure therapy in attenuating conditioned reactivity to smoking cues.
Keywords: d-cycloserine, cue exposure, nicotine addiction, cue exposure therapy, smoking cues, smoking cue reactivity
1. Introduction
Although there is strong empirical support for first line medications such as nicotine replacement therapy (NRT), bupropion, and varenicline in the aid of smoking cessation (Fiore et al., 2008), the abstinence rate among smokers using these interventions is only 20% to 33% six months after quitting (Fiore et al., 2008), suggesting that there is significant need to continue working to both improve current interventions and to develop new treatments for smoking cessation. Several investigators have highlighted the need for more studies targeting effective behavioral mechanisms for smoking cessation process as well as behavioral methods for informing improved treatment design (Piasecki and Baker, 2001). While prior studies have attended to the relationship between cue reactivity, craving, and the maintenance of smoking and relapse (Drummond et al., 1995; Niaura et al., 1988; Niaura et al., 1998; Abrams et al. 1988), fewer studies have targeted the effect of non-nicotine factors, including non-pharmacological cues associated with smoking (Niaura et al., 1999; Brandon et al. 1995; Perkins et al. 2001, 2006; Caggiula et al., 2002). Such cues, including the sight and smell of cigarettes, among other conditioned reinforcers, may provide clues for enhancing the efficacy of interventions designed to extinguish conditioned reactivity to smoking cues (Caggiula et al., 2002; Perkins et al., 2001; Field and Duka, 2004), as evidence indicates that smoking cues contribute to nicotine self-administration (Abrams et al. 1988; Niaura et al., 1988; Caggiula et al., 2001, 2002; Perkins et al., 1994).
Cue exposure therapy (CET) attempts to reduce conditioned reactions to substance cues and enhance skills training. Once seen as a promising technique for the treatment of addiction (Hammersley, 1992; Heather and Bradley, 1990; O’Brien et al., 1990; Marlatt, 1990), a recent meta-analysis showed no consistent evidence for CET’s efficacy (Conklin and Tiffany, 2002). However, Conklin and Tiffany (2002) pointed out that CET may prove to have merit if greater attention is paid to incorporating theories based on more recent research on learning and motivation (Havermans and Jansen, 2003).
Pharmacological interventions that target the extinction of fear in anxiety disorders may have some applicability to cue-exposure extinction paradigms in addiction. The partial N-methyl-d-aspartate (NMDA) agonists, such as d-cycloserine (DCS), facilitate extinction of learned fear during behavioral exposure therapy (Ressler et al., 2004; Hofmann et al., 2006; Kushner et al., 2007; Wilhelm et al., 2008). Activation of the NMDA receptor appears to be involved in extinction (Myers and Davis, 2002; Santini et al., 2001) and induces long-term potentiation (LTP), an important neuronal substrate of learning (Rioult-Pedotti et al., 2000; Watanabe et al., 1992). DCS may improve cognitive functioning by enhancing the efficacy of glutamatergic transmission via stabilization and strengthening of NMDA receptors during learning processes (Davis et al., 2006).
Ressler and colleagues (2004) found that 50 mg of DCS acutely administered to patients diagnosed with acrophobia prior to two 35-to 45-minute virtual reality exposure sessions to heights resulted in larger reductions of acrophobic symptoms on several fear outcomes in a virtual reality environment and a generalized reduction of acrophobic symptoms in real-life situations 3-months after therapy compared to placebo. Hofmann and colleagues (2006) investigated whether DCS would enhance the effects of exposure therapy for individuals with social phobia. They found that 50 mg of DCS provided 1 hour prior to five 90-minute sessions of individual or group exposure therapy performance situations resulted in a greater reduction in general social anxiety symptoms compared to their counterparts who received placebo.
A recent study (Botreau et al., 2006) showed that extinction of a cocaine-induced conditioned place preference occurred when rats were injected directly into the basolateral amygdala with DCS, indicating that extinction of appetitive conditioned responses (Botreau et al., 2006) may be regulated by the NMDA receptor system that regulates consolidation of extinction memories to aversive conditioned responses (Ledgerwood et al., 2003; Walker et al., 2002). Thus, the mechanism by which DCS attenuates reactivity to drug cues in addiction may involve common neural underpinnings of the NMDA receptor system that is critically involved in facilitating extinction of conditioned fear (Davis and Myers, 2002; Vervliet, 2008) on which DCS acts as a partial agonist, although this association will require further evidence to support its validity.
Given studies demonstrating that DCS facilitates extinction of learned fear in rats (Ledgerwood et al., 2003) and humans (Ressler et al., 2004; Hofmann et al., 2006; Kushner et al., 2007) and that it facilitates extinction of a cocaine-induced conditioned place preference task in animal studies (Botreau et al., 2006), DCS may have potential for reducing reactivity to substance-related contextual stimuli when combined with cue exposure treatment in humans. To date, there are no published studies that have examined the effect of DCS combined with behavioral interventions for extinguishing reactivity to drug cues in addiction.
The purpose of the present study was to conduct a randomized, placebo-controlled, double-blind pilot investigation to evaluate whether DCS would facilitate attenuation of smoking cue reactivity in participants with nicotine dependence. Since it is unknown whether DCS increases substance use when combined with drug cues and because participants were not explicitly requested to stop smoking in this laboratory study, exploratory analyses were performed to evaluate the potential effect of DCS on smoking behavior at follow-up. We incorporated various suggestions for improving cue exposure interventions (Niaura et al., 1999; Havermans and Jansen, 2003) and addressed issues relevant to threats to extinction (Conkin and Tiffany, 2002) by administering repeated cue exposure treatment sessions, extending the length of cue exposure treatment, using in-vivo cues, assessing reactivity during each cue exposure trial, and repeating cue exposure treatment procedures at a later exposure session. We predicted that participants who received DCS 1 hour before each of two cue exposure-extinction treatment sessions would show greater reductions in smoking cue reactivity and urges to smoke than those receiving placebo and identical CET, particularly by the second cue exposure treatment session. Exploratory analyses evaluated whether participants who received DCS would reduce their smoking as measured by biochemical markers for tobacco use (urinary cotinine, carbon monoxide) and self-reported smoking at the 1- and 4-week follow-up.
Methods
2.1 Participants
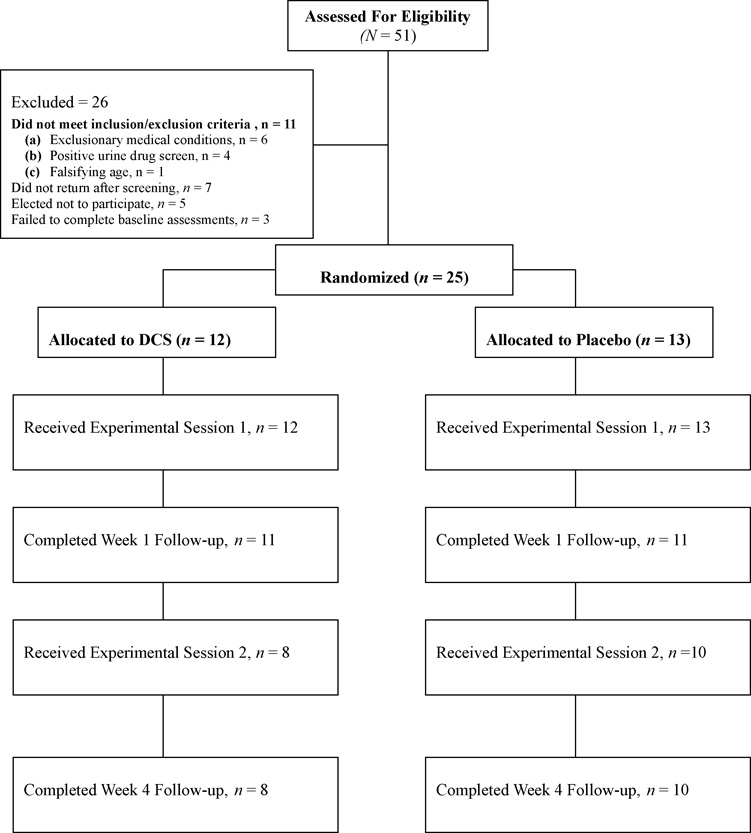
Twenty-five volunteer participants were recruited from the general community through newspaper, television, flyer advertisements and referrals. Participants who appeared eligible based on an initial telephone screen were invited to meet with study staff to obtain written informed consent (approved by the Yale University School of Medicine’s Human Investigation Committee and the VA Connecticut Healthcare System’s Human Studies Subcommittee) and to complete diagnostic intake assessments. Participants were informed that they would not be required to quit smoking, medications for smoking cessation would not be provided during the 4-week study period, and that a smoking quit date would not be implemented during that time. Eligible individuals were required to be between the ages of 18 and 55, speak English, report a history of smoking ≥ 10 cigarettes/day for the past 12 months, and provide a carbon monoxide (CO) level ≥10 ppm. Women were not eligible for the study if they were pregnant, breast feeding, or not using a reliable form of birth control. Participants were excluded for psychiatric or medical illness, serious neurological or seizure disorder, use of psychoactive medication, drug or alcohol dependence, known allergy to DCS, or current use of smokeless tobacco, pipes, cigars, or medications for smoking cessation, such as nicotine replacement, bupropion, or varenicline. Primary reasons for ineligibility were (1) failure to return for scheduled appointments or return calls (n=7), (2) medical conditions meeting study exclusion (n=6), (3) falsifying age in attempt to avoid exclusion (n=1), (4) not interested in participating in the study (n=5), (5) failure to complete baseline assessments (n=3), (6) positive urine drug screen (n=4).
Demographic and sample characteristics of study participants are presented in Table 1. The sample was split approximately evenly between males (n = 12) and females (n = 13), with an average age of 41.3 (sd = 9.1). Participants reported having smoked cigarettes for an average of 24.2 (sd = 9.5) years with a baseline smoking average of 19.9 (sd = 6.5) cigarettes/day and a past history of 3.2 (sd = 3.4) quit attempts. Baseline Fagerström Test for nicotine dependence (FTND) scores, expired CO levels, and urinary cotinine levels were 6.9 (sd = 1.4), 21.4 ppm (sd = 11.4), and 1970.3 ng/mL (sd = 972.5), respectively.
Table 1.
Baseline demographic and clinical characteristics by treatment group
| Characteristic | DCS* (n = 12) |
Placebo (n = 13) |
Total (n = 25) |
x2, F | p-value |
|---|---|---|---|---|---|
| Mean Age (SD) | 41.1 (6.2) | 41.5 (11.5) | 41.3 (9.1) | .01 | .92 |
| Female N (%) | 6 (50) | 7 (53.8) | 13 (52) | .04 | .85 |
| Ethnicity (n, %) | 4.3 | .23 | |||
| European American | 2 (16.7) | 6 (46.2) | 8 (32) | ||
| African American | 6 (50) | 6 (46.2) | 12 (48) | ||
| Latin American | 2 (16.7) | 0 (0) | 2 (8) | ||
| Native American | 2 (16.7) | 1 (7.7) | 3 (12) | ||
| Education (n, %) | .42 | .51 | |||
| ≤12 years | 8 (66.7) | 7 (53.8) | 15 (60) | ||
| >12 years | 4 (33.3) | 6 (46.2) | 10 (40) | ||
| Employed, full or part time (n, %) | 4 (33.3) | 6 (46.2) | 10 (40) | .43 | .51 |
| Married or in stable relationship (n, %) | 4 (33.3) | 2 (15.4) | 6 (24) | 1.1 | .30 |
| Cigarettes smoked per day (past 28 days), Mean (SD) |
18.1 (4.5) | 21.6 (7.6) | 19.9 (6.5) | 1.9 | .18 |
| Years having smoked, Mean (SD) | 24.4 (6.5) | 24.1 (11.9) | 24.2 (9.5) | .01 | .93 |
| Previous quit attempts, Mean (SD) | 2.1 (2.1) | 4.2 (4.1) | 3.2 (3.4) | 2.3 | .14 |
| Fagerström score, Mean (SD) | 6.9 (1.3) | 6.9 (1.6) | 6.9 (1.4) | .00 | .99 |
| Expired Carbon Monoxide, Mean (SD) | 18.8 (10.8) | 23.5 (12.0) | 21.2 (11.4) | 1.0 | .32 |
| Urinary Cotinine, Mean (SD) | 1578.5 (653.3) | 2332.0 (1097.8) | 1970.3 (972.5) | 4.3 | .05 |
| QSU-brief, Mean (SD) | |||||
| Factor 1† | 27.1 (8.3) | 26.5 (8.7) | 26.8 (8.3) | .00 | .86 |
| Factor 2 | 10.0 (5.3) | 9.8 (3.5) | 9.9 (4.3) | .00 | .93 |
DCS: d-Cycloserine treatment group
Factor 1 of the Questionnaire on Smoking Urges (QSU-brief) represents smoking as the result of the ‘desire to smoke’, while Factor 2 represents smoking to ‘relieve negative affect’.
2.2 Procedure
All randomized participants were administered intake screening measures that included a medical and smoking history, EKG, vital signs, and skin conductance assessment. Carbon monoxide was evaluated and participants provided urine samples for cotinine analysis and drug screening. Participants received a medical and psychiatric evaluation by a study physician with whom blood chemistry, illicit drug status, and pregnancy results were reviewed. Participants meeting intake requirements were invited to return for two 4.5-h experimental sessions scheduled two weeks apart and a 1- and 4-week follow-up appointment scheduled 1-week after each of the two experimental sessions. Figure 1 shows the progress of participants in the study.
Figure 1.
Beginning at 10 pm the night prior to each of the two experimental sessions, participants were required to adhere to overnight abstinence (11-hr) from smoking that was confirmed using expired carbon monoxide (CO) assessment during the morning of experimental sessions. The criterion for overnight abstinence was individually determined using a previously validated formula based on each smoker's baseline afternoon CO level (Rose and Behm, 2004). Participants were requested to refrain from consumption of a high fat meal the morning of experimental sessions to avoid slowing DCS absorption (Min et al., 2001). Participants abstained from smoking during each of the two experimental sessions and were requested to abstain from smoking at least 4 hours after leaving the test facility. Prior to cue exposure treatment, participants were administered baseline and skin conductance assessments and were requested to provide a urine sample. Urge to smoke was evaluated at approximately 40 minute intervals and at each cue exposure trial. Smoking behavior was evaluated at each of the two experimental sessions and at the 1 and 4-week follow-up periods using quantitative urinary cotinine analysis (gas chromatographic techniques; Graham Massey Analytical Lab, Shelton, CT), expired CO levels (Vitalograph, Inc., Lenexa, KS, USA) and smoking diaries collected at the beginning of each visit.
2.3 Measures
2.3.1 Skin Conductance Response
Electrodermal activity (EDA) was acquired using the Biopac MP100A-CE workstation (BIOPAC Systems, Inc., Goleta, CA) and the Biopac electrodermal activity amplifier module (GSR 100c) at a channel sampling rate of 50 Hz and a gain of 5 µ℧ per volt (resulting in a resolution of 0.0015 µ℧). Skin conductance EDA electrodes (BIOPAC-EL507) were filled with an isotonic electrode gel and placed on the middle phalanges of the third and fourth digits of the non-dominant hand (the hand not normally used to hold a cigarette when smoking) (Fowles et al., 1981). EDA was recorded for 5 minutes prior to smoking cue exposure trials at baseline and during each of the six cue exposure trials while participants were handling smoking objects. To remove high frequency noise artifacts and obtain a Phasic EDA signal, the EDA data were filtered using a low pass FIR filter at 1.00 Hz (Blackman – 61 dB window with 200 coefficients) and a 0.05 Hz High Pass IIR filter (Q = 0.707). Phasic EDA data were reduced by computing skin conductance response rate at baseline and each of the six cue exposure trials. A skin conductance response was defined as an increase in Phasic EDA greater than 0.05 µ℧. Previous studies have shown increases in skin conductance response to smoking cues (Tong et al., 2007; Field and Duka, 2004; Drobes and Tiffany, 1997).
2.3.2 Self-reported cue exposure urge-to-smoke rating
Urge-to-smoke during smoking cue reactivity exposure trials was evaluated at each of the six cue exposure time points within each of the two experimental sessions. Participants were asked to rate their urge to smoke using a 10-point Likert-scale from 1 (no urge to smoke) to 10 (extreme urge to smoke) at three time intervals: 0 seconds, at 8 seconds, and at 38 seconds while they were handling smoking objects (i.e. cigarette pack, cigarettes, lighter, and ashtray). Cue exposure urge-to-smoke rating procedures were adapted and modified from the standard procedures of Sayette and Hufford (1994) and urge ratings have been shown in previous studies to increase following cue exposure with other substances at pretreatment (Monti et al., 1993).
2.3.3 Quantitative urinary cotinine
At each of the two experimental sessions and at the 1- and 4-week follow-up periods, 10 mls of urine were collected and analyzed with an enzyme immunoassay (EIA) specific for cotinine and having a sensitivity of 25 ng/mL (Graham-Massey Analytical Laboratories Inc. Shelton, CT). Values obtained were not adjusted ("normalized") to the concentration of creatinine.
2.3.4 Expired carbon monoxide
Participants’ expired carbon monoxide levels were measured at intake, at each of the experimental sessions to verify overnight abstinence, and at the 1- and 4-week follow-up to determine tobacco use exposure using a portable CO monitor (Vitalograph, Inc., Lenexa, Kansas).
2.3.5 Nicotine dependence
Participants were administered the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) at screening. The FTND, a self-report measure with demonstrated reliability and validity, assesses the degree of nicotine dependence and has been used widely in smoking studies (Heatherton et al., 1991).
2.3.6 Self-reported cigarette smoking
Average cigarette smoking per day for the 28 days prior to intake was evaluated using the Substance Use Calendar (SUC). The SUC is an interview assessment of self-reported substance use completed at each contact by a research assistant. The SUC was adapted from the Time Line Follow-Back interview (Sobell and Sobell, 1992), which has been shown to be a reliable and valid instrument for monitoring substance use (Fals-Stewart, et al., 2000), and allows for a continuous and reliable evaluation of daily substance use (Carroll et al., 2004). Participants were also asked to report the number of cigarettes smoked per day after their intake and once they were enrolled into the study at the two experimental sessions and at the 1 and 4-week follow-up.
2.3.7 Questionnaire on Smoking Urges –Brief (QSU-brief)
Participants were administered the QSU-brief at 40-minute intervals during experimental sessions and at 1- and 4-week follow-up. The QSU-brief is a 10-item self-report questionnaire that is designed to assess the urge to smoke (Cox et al., 2001). Responses are categorized into two factors. Factor 1 corresponds to the ‘desire to smoke’ while Factor 2 corresponds to the ‘desire to smoke to relieve negative affect’ (Toll et al., 2006).
2.4 Medication
d-Cycloserine (Seromycin®; Eli Lilly and Co, Indianapolis, Ind), is an FDA-approved broad-spectrum antibiotic for the treatment of tuberculosis. The 50 mg oral DCS dose provided acutely 1 hour prior to each of the two experimental sessions in this study was based on prior clinical trials in which the same dose was found effective for enhancing extinction to fear of heights (Ressler et al., 2004) and fear in performance situations (Hofmann et al., 2006). A nurse dispensed the 50 mg DCS capsules, which were identical to placebo. The study pharmacist randomized participants to conditions and maintained the study blind. Adverse effects to the medication were systematically assessed at the beginning and end of each experimental session using an Adverse Events Form. No participants in this study reported adverse effects to the experimental medication.
2.5 Treatment
2.5.1 Smoking cue reactivity intervention
Participants in the DCS and placebo groups were administered a manualized intervention developed for the study (manual available upon request) that provided a series of cognitive behavioral, relaxation, decisional balance, and guided imagery interventions adapted from several sources (Abrams et al., 2003; Miller and Rollnick, 2002; Wynd, 2005). Similar to Monti et al. (1993), coping skills were taught in a graduated fashion to participants in between the six cue exposure trials. While seated in a comfortable chair, participants listened to six audio-cassette guided visualization scripts describing abstinence from smoking scenarios (average running time of 1.5 minutes) presented over headphones that described scenes of personal states of health and wellness while abstaining from smoking (e.g., emphasizing feelings of high energy, smelling fresh, feeling healthier, positive affirmations about health, and visualization about health promoting activities), such as living life as a non-smoker, good nutrition, breathing freely without difficulty, and imagining positive consequences as a non-smoker (Wynd, 2005). Three of the guided imagery scripts were modeled after Wynd (2005), while the remaining three scripts were designed by the first author and based on principles of covert sensitization (Cautela and Kearney, 1986) for smoking cessation.
2.5.2 Smoking cue exposure procedures
Cue exposure procedures were adapted and modified from the standard procedures of Sayette and Hufford (1994). Participants were presented with a covered tray containing a pack of their favored brand of cigarettes, a lighter, and an ashtray. Following the instruction to remove the cover and look at the smoking objects in the tray, participants were asked to provide an initial urge-to-smoke rating (conducted at 0 seconds) on a 10-point Likert scale. Participants were instructed to remove a cigarette from the pack and hold the cigarette the way they normally would using their dominant hand when smoking, after which time skin conductance measures were assessed using the non-dominant hand for 5 minutes. After requesting participants to smell the cigarette, they were instructed to place the cigarette in the ashtray, flick the lighter until they saw the flame, put down the lighter and hold the cigarette again. At 8 and 38 seconds, participants were again requested to rate their urge-to-smoke on the 10-point Likert scale. At 45 seconds, participants were requested to rehearse the strategy of urge-surfing (e.g., coping with the urge to smoke) while they were holding the cigarette in their hand. At 75 seconds, participants were requested to place the cigarette in the ashtray, at which time they were requested to practice relaxation, visualization as a non-smoker, and coping skills for smoking triggers until the end of the skin conductance assessment. Altogether, participants received a series of six smoking cue exposure trials provided at 30–40 minute intervals within each of the two experimental sessions.
2.6 Data analysis
Pretreatment and demographic variables were analyzed using chi square and analysis of variance (ANOVA). Because we were interested in evaluating change over time (i.e. the changing structure of abilities) in the smoking reactivity of participants in both the DCS and placebo conditions, the primary outcomes, skin conductance response rate, co-varying for skin conductance level, and the average of the 0, 8, and 38-second assessment time-points of the self-reported cue exposure urge-to-smoke ratings over the six cue exposure trials, were analyzed using random effects regression models for experimental sessions 1 and 2 (Bryk and Raudenbush, 1987). Rates of change over time were analyzed between groups on the QSU-brief using random effects regression models for data collected in experimental sessions 1 and 2. QSU-brief measures at the 1-and 4-week follow-up were analyzed using ANOVA. The exploratory outcomes, smoking behavior posttreatment variables at the 1 and 4-week follow-up (urinary cotinine, expired carbon monoxide, and self-reported cigarette smoking), were analyzed using analysis of covariance (ANCOVA) using the baseline value for each variable as the covariate. Because nicotine possesses a short half-life (Benowitz and Jacob, 1994) and participants were required to maintain abstinence from smoking overnight, we did not expect differences between groups at experimental sessions on urinary cotinine, carbon monoxide, and self-reported smoking although we reported these values at experimental sessions.
3. Results
3.1 Participant flow
Of the 208 interested individuals who contacted study staff for a phone screening, 51 were eligible to receive the diagnostic intake assessment, of which twenty-six were not eligible for study participation. The remaining 25 participants were randomly assigned, in a double-blind fashion, to DCS plus cue exposure therapy (n = 13) or matching pill placebo plus cue exposure therapy (n = 12).
3.2 Skin conductance response and self-reported cue exposure urge-to-smoke ratings by experimental session
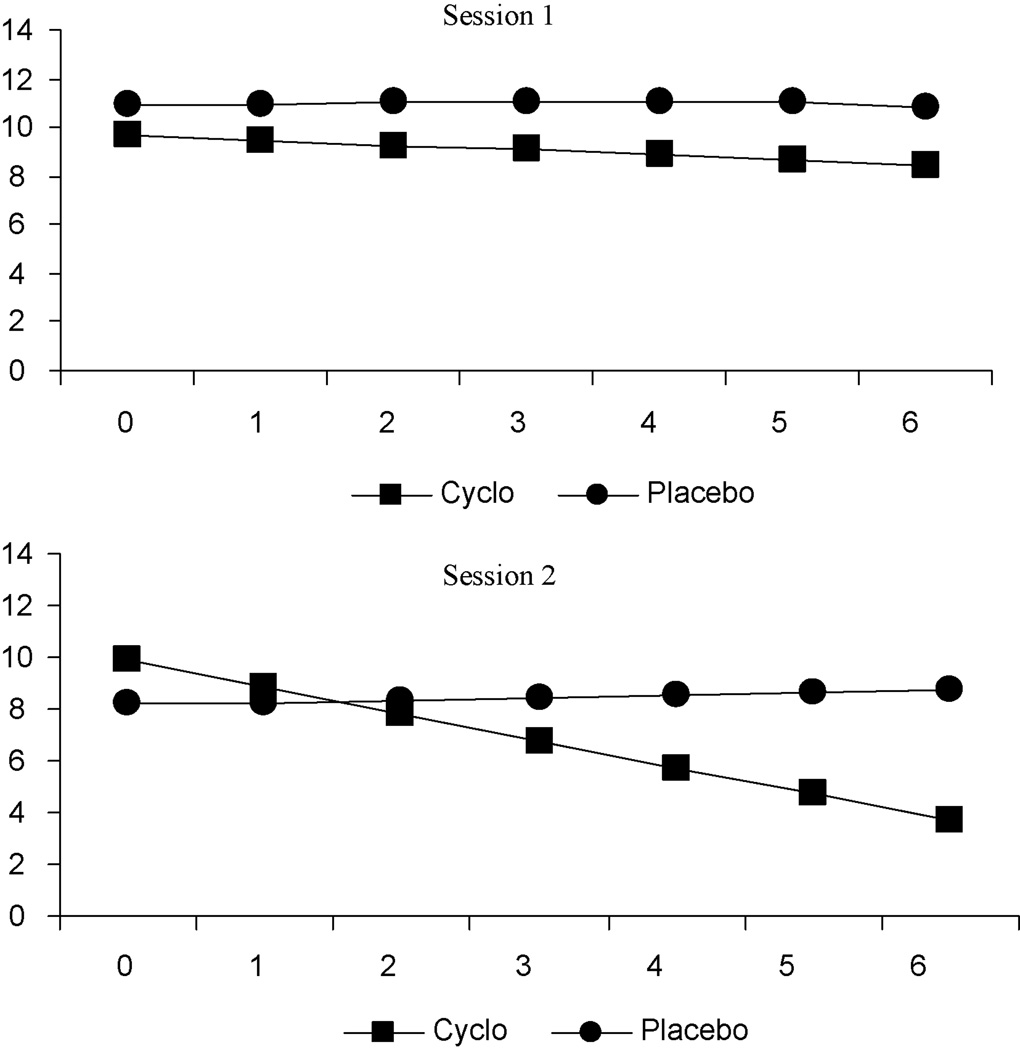
Baseline skin conductance response rate at experimental session 1 and 2 was not significantly different between treatment groups. Random effects regression analysis of repeated skin conductance response rate indicated no significant main effects for group at experimental sessions 1 and 2 (experimental session 1: t = 0.2, p = .81; experimental session 2: t = −0.9, p = .39). Although at experimental session 1 skin conductance response rate was lower at each of the six cue exposure trials for participants in the DCS group compared to participants in the placebo group, there were no significant group-by-time differences. By experimental session 2, a significant time (t = −2.1, p = .04) and group-by-time effect (t = 2.5, p = .01) was found, indicating a reduction over time for both groups, with a greater reduction for the DCS group.
Figure 2 presents the estimated means for skin conductance response rate across the six cue exposure trials at experimental session 1 and 2, respectively. By experimental session 2, participants who received DCS plus cue exposure therapy exhibited a significantly greater decrease in skin conductance response rate across the 6 cue exposure trials, while participants who received placebo exhibited a slight linear increase in skin conductance response rate.
Figure 2.
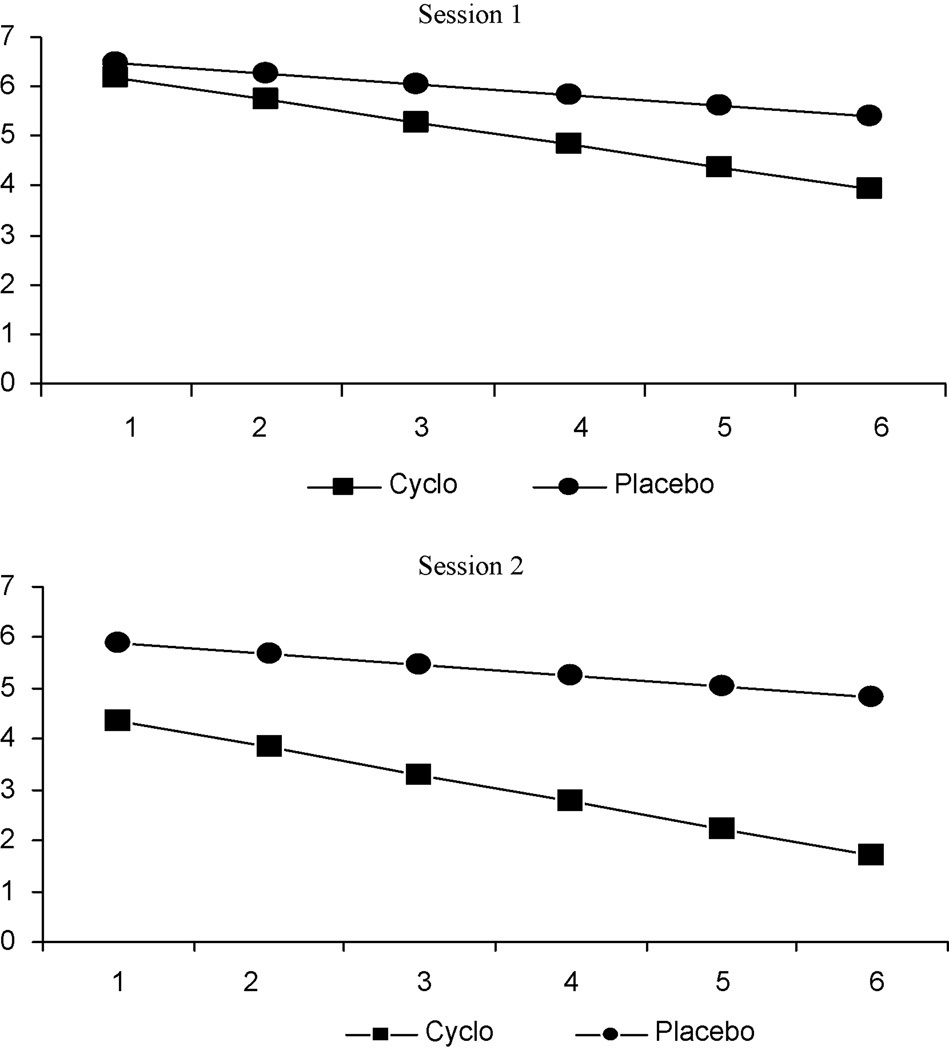
As shown in figure 3, self-reported urge-to-smoke ratings dropped over time in both experimental sessions 1 and 2 (experimental session 1: t = −5.3, p < .001; experimental session 2: t = −7.6, p <.001). The random effects regression model also indicated that there were differences in the rates of change by treatment group (experimental session 1: t = −2.0, p = .05; experimental session 2: t = −2.8, p < .01), revealing a greater reduction over time in urge-to-smoke for participants in DCS compared to placebo. Consistent with objective findings from the skin conductance response rate variable, subjective urge-to-smoke ratings decreased in both treatment groups at experimental sessions 1 and 2, with participants in the DCS group reporting a significantly greater reduction in urge-to-smoke ratings across cue exposure trials in both experimental sessions compared to participants in the placebo group.
Figure 3.
3.3 Smoking behavior as measured by urinary cotinine, expired carbon monoxide, and self-reported cigarette smoking at 1- and 4-week follow-up
As shown in Table 2, there were no significant differences by treatment group for urinary cotinine and self-reported cigarette smoking, covarying for the baseline value of each of these variables at either the 1- or 4-week follow-up. The ANCOVA examining expired carbon monoxide, covarying for baseline, revealed a significant difference at the 1-week follow-up, (F [1,18] = 8.3, p = .01; Cohen’s d = 1.1), with participants in the DCS group exhibiting a lower mean expired carbon monoxide level compared to participants in the placebo group. Although expired carbon monoxide levels were lower for participants in the DCS group compared to participants in the placebo group at the 4-week follow-up, ANCOVA analyses did not indicate a significant difference at this time period. Random effects regression analyses of mean QSU-brief ratings indicated that although mean QSU-brief estimated means were lower for participants in the DCS group compared to participants in the placebo group on factor 1 (‘desire to smoke’) and factor 2 (‘desire to smoke to relieve negative affect’) in both experimental sessions, there were no significant group or group-by-time effects. For experimental session 1, there was a significant difference for time on both QSU-brief factors (factor 1: t = −7.1, p < .001; factor 2: t = −5.4, p < .001), revealing a greater reduction in QSU-brief ratings over time in both treatment conditions. For experimental session 2, there was a significant difference for time on the QSU-brief factor 1 only (t = −4.9, p < .001). In addition, we analyzed the QSU-brief at the 1-and 4-week follow-up and these analyses indicated no significant differences between treatment groups.
Table 2.
Urinary cotinine, expired carbon monoxide, self-reported smoking, and Questionnaire on Smoking Urges (QSU-brief) outcomes, covarying for baseline, by treatment group
| DCS Mean (SD) |
Placebo Mean (SD) |
Total Mean (SD) |
F | p-value | |
|---|---|---|---|---|---|
| Urinary Cotinine (ng/mL) | |||||
| Experimental session 1* | 1492.6 (677.2) | 2530.3 (1830.3) | 2032.2 (1471.7) | .36 | .55 |
| 1-week follow-up | 1509.1 (1026.1) | 2135.2 (1353.1) | 1822.1 (1214.8) | .36 | .55 |
| Experimental session 2 | 1602.5 (680.5) | 1441.2 (937.0) | 1512.8 (813.8) | 3.1 | .10 |
| 4-week follow-up | 1645.5 (1180.8) | 2062.6 (1091.1) | 1877.6 (1117.9) | .02 | .89 |
| Expired Carbon Monoxide (CO) | |||||
| Experimental session 1 | 8.2 (5.3) | 12.6 (5.6) | 10.6 (5.8) | 2.8 | .11 |
| 1-week follow-up | 10.2 (3.9) | 16.8 (8.8) | 13.7 (7.6) | 8.3 | .01 |
| Experimental session 2 | 6.4 (3.5) | 10.9 (7.1) | 8.9 (6.1) | 2.6 | .13 |
| 4-week follow-up | 11.1 (4.8) | 17.6 (9.9) | 14.7 (8.5) | 4.2 | .06 |
| # cigarettes smoked per day | |||||
| 1-week follow-up | 10.3 (6.3) | 13.9 (12.7) | 12.0 (9.7) | .06 | .80 |
| 4-week follow-up | 9.9 (7.7) | 11.0 (9.2) | 10.4 (8.2) | .35 | .56 |
| QSU-brief | |||||
| 1-week follow-up | n = 10 | n = 11 | |||
| Factor 1† | 12.9 (9.1) | 16.9 (7.4) | 15.0 (8.3) | 1.2 | .28 |
| Factor 2 | 5.0 (5.6) | 5.4 (2.1) | 5.2 (4.1) | .04 | .84 |
| 4-week follow-up | n = 7 | n = 10 | |||
| Factor 1 | 10.3 (6.3) | 12.5 (5.9) | 11.6 (6.0) | .55 | .47 |
| Factor 2 | 4.0 (2.6) | 5.0 (2.6) | 4.5 (2.6) | .48 | .50 |
| QSU-brief§ | DCS | Placebo | Group t (p-value) |
Time t (p-value) |
Group × Time t (p-value) |
| Experimental session #1 | n = 12 | n = 13 | |||
| Factor 1† | −.66 (p = .51) | −7.1 (p < .001) | −1.8 (p = .08) | ||
| Time point 1 | 23.6 | 26.2 | |||
| Time point 11 | 13.1 | 19.9 | |||
| Experimental session #1 | |||||
| Factor 2 | −.36 (p = .72) | −5.4 (p < .001) | −.90 (p = .37) | ||
| Time point 1 | 7.1 | 7.6 | |||
| Time point 11 | 3.6 | 5.1 | |||
| Experimental session #2 | n = 8 | n = 10 | |||
| Factor 1 | −.98 (p = .34) | −4.9 (p < .001) | .25 (p = .80) | ||
| Time point 1 | 17.5 | 21.7 | |||
| Time point 11 | 12.6 | 16.2 | |||
| Experimental session #2 | |||||
| Factor 2 | −1.6 (p = .14) | −.86 (p = .39) | 1.9 (p = .06) | ||
| Time point 1 | 3.9 | 5.7 | |||
| Time point 11 | 4.3 | 4.7 |
At experimental session 1, n = 12 in the DCS group and n = 13 in the placebo group. At experimental session 2, n = 8 in the DCS group and n = 10 in the placebo group. At 1 week follow-up, n = 11 (total n = 22) in each treatment group, and at 4 week follow-up, n = 8 in the DCS group and n = 10 in the placebo group.
QSU-brief at experimental session 1 and 2 is the estimated self-reported urge-to-smoke mean ratings of eleven QSU-brief measures administered every 40 minutes throughout each experimental session. We presented only the estimated means for the QSU-brief delivered at time point 1 and time point 11 to show differences between scores at the beginning and end of the QSU-brief administration.
Factor 1 of the QSU-brief represents smoking as the result of the ‘desire to smoke’, while Factor 2 represents smoking to ‘relieve negative affect’.
4. Discussion
This randomized, double-blind, placebo-controlled laboratory pilot investigation is the first to our knowledge to evaluate the effect of DCS combined with a behavioral intervention in humans for reducing reactivity to drug cues in addiction. The results from our primary analyses provided some evidence that DCS plus cue exposure therapy attenuated smoking cue reactivity in response to in-vivo smoking cues based on a measure of objective physiological reactivity (i.e. skin conductance) and subjective urge-to-smoke ratings, while exploratory analyses indicated no significant overall differences by condition on measures of smoking behavior.
Based on prior investigations that showed increases in skin conductance to smoking cues (Tong et al., 2007; Field and Duka, 2004; Drobes and Tiffany, 1997), we hypothesized that participants who received DCS plus cue exposure therapy would demonstrate a reduction in their skin conductance response rate, compared with participants who received placebo. Our findings are consistent with this hypothesis, as random effects regression analysis suggested that participants who received DCS plus cue exposure therapy 1 hour prior to each of the two 4.5 hour experimental sessions exhibited reductions in skin conductance response rate with each successive cue exposure trial at both experimental sessions, while participants who received placebo plus cue exposure therapy exhibited no such decrease. This difference was not statistically significant at the first experimental session, but was found to be significant by the second experimental session. Similarly, random effects regression analysis of self-reported urge-to-smoke ratings indicated that although participants in both groups reduced their urge-to-smoke ratings with each successive cue exposure trial across both experimental sessions, participants who received DCS exhibited a significantly larger reduction in their average urge-to-smoke ratings in experimental sessions 1 and 2.
Our exploratory analyses indicated only one statistically significant difference between groups on posttreatment smoking behavior at follow-up. Participants who received DCS produced a significantly lower expired CO level at the 1-week follow-up after the first experimental session compared to their counterparts who received placebo (a large Cohen’s d effect size of 1.1), although this difference was not significant by the 4-week follow-up. Similarly, there were no significant differences by treatment group on urinary cotinine levels, self-reported cigarette smoking, or Questionnaire on Smoking Urge (QSU-brief) mean scores assessed at experimental sessions and at the 1- and 4-week follow-up.
It seems unlikely that the attenuation in smoking cue reactivity based on physiological and subjective measures in the DCS group was the result of pretreatment differences in smoking behavior or pretreatment level of nicotine dependence since there were no statistically significant pretreatment differences on level of tobacco dependence, expired carbon monoxide, cotinine level, number of cigarettes smoked per day, or urges-to-smoke, thus supporting the role of DCS in attenuating reactivity to smoking cues. Adequate filtering procedures on skin conductance measurement were applied to remove high-frequency noise artifacts and baseline in the waveforms.
While promising, the results of this pilot study should be considered with caution because of the small sample size. Given that the passage of 7 days is the recommended amount of time from the smoking quit date in order to adequately measure non-smoking compliance using cotinine biochemical verification in most studies (Benowitz et al., 2002), and the fact that participants were not specifically requested to stop smoking in this study, it was not surprising that few differences in smoking behavior outcome were found on post-treatment smoking behavior at follow-up. Also given the relatively long half life of cotinine and the fact that cotinine is specific and sensitive when used as a biomarker for smoking cessation (Benowitz et al, 2002; Gariti et al., 2002), the assessment of expired CO may have been a more appropriate measure relative to cotinine in this study because it possesses a shorter half life and is most useful for determining smoking status (Benowitz et al, 2002). Our finding that expired CO was significantly lower in the DCS group compared to the placebo group at the 1-week follow-up beyond experimental session 1 may reflect consolidation of the extinction process in NMDA receptors for participants who received DCS during the post-acquisition period between sessions. However, because participants continued to be exposed to cigarette smoke at varying levels throughout the 4-week trial, we were unable to distinguish the effect of DCS on smoking behavior that may have occurred during the postacquisition period versus the impact of other potential influences in CO outcome, including differences in environmental sources of CO exposure or differences in smoking and pulmonary ventilation rates prior to CO assessment. It is unclear why we found differences between treatment groups on the one-item urge-to-smoke measure provided during the cue exposure trials while no differences between groups were found on the QSU-brief, the later which was assessed several times throughout the experimental sessions. The inconsistent findings between these two self-report measures may reflect an issue of power or the possibility that the effects of the DCS plus cue exposure treatment may be more specific to influencing reactivity to drug cues as opposed to smoking expectancies (e.g., “smoking would make me less depressed”), beliefs (e.g. “I could control things better right now if I could smoke”) and values (e.g. “nothing would be better than smoking a cigarette right now”) which are inherent in some items of the QSU-brief.
Another limitation of the study is that we did not collect blood samples to confirm whether the DCS reached criterion therapeutic levels during experimental sessions. Reporting blood level estimates in future studies may assist to make outcome comparisons between studies based on rapidity of absorption and time to reach peak plasma levels. Finally, we did not evaluate smoking cue reactivity at 1 and 4-week follow-up to determine whether attenuated reactivity would have occurred at these time points outside of experimental sessions.
Although previous studies have shown the beneficial clinical effects of DCS in augmenting exposure therapy and thus facilitating the extinction of clinical anxiety in humans (Ressler et al., 2004; Hoffman et al., 2006), it is unknown whether DCS exhibits a similar and parallel augmentation of exposure therapy to drug cues by exerting the undesirable effect of increasing substance use upon exposure to drug cues. Our exploratory analyses, however, did not indicate that participants in the DCS group increased their smoking based on self-reported number of cigarettes smoked as a result of their exposure to smoking cues.
In conclusion, the results of this pilot investigation indicate that while DCS resulted in reduced reactivity to smoking cues based on physiological reactivity and subjective urge-to-smoke ratings, our findings provided no evidence that DCS was associated with change in smoking behavior. Nevertheless, attenuation in reactivity to smoking cues suggests that the partial agonist DCS, which acts on the NMDA receptor complex and which has been shown to facilitate extinction of conditioned fear in humans and extinction of cocaine-induced conditioned place preference in animals, may be potentially extended to the facilitation of extinction to drug cues in addiction.
References
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav Res Ther. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstein MG, Monti PM. The tobacco dependence treatment handbook: A guide to best practices. New York: The Guilford Press; 2003. [Google Scholar]
- Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., III Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;34:604–611. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Piasecki TM, Quinn EP, Baker TB. Cue exposure treatment in nicotine dependence. In: Drummond DC, Tiffany ST, Glautier S, Remington B, editors. Addictive behavior: Cue exposure theory and practice. New York: Wiley; 1995. [Google Scholar]
- Botreau F, Paolone G, Stewart J. D-cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychol Bull. 1987;101:147–158. [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Cautela JR, Kearney AJ. The Covert Conditioning Handbook. New York: Springer; 1986. [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: A randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Davis M, Meyers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: Clinical implications for exposure therapy. Biol Psychiatry. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: Translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Drobes D, Tiffany S. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. J Abnorm Psychol. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Tiffany ST, Glautier S, Remington B. Cue exposure in understanding and treating addictive behavior. In: Drummond DC, Tiffany ST, Glautier S, Remington B, editors. Addictive Behaviors: Cue Exposure Theory and Practice. London: John Wiley & Sons; 1995. pp. 1–17. [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78:647–652. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME, Murray EW, Bennett G, Heishman S, Husten C, Morgan G, Williams C, Christiansen BA, Piper ME, Hasselblad V, Fraser D, Theobald W, Connell M, Leitzke C. Rockville, MD: Public Health Service; Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. U.S. Department of Health and Human Services. 2008
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18:232–239. doi: 10.1111/j.1469-8986.1981.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Gariti P, Alterman AI, Ehrman R, Mulvaney FD, O’Brien CP. Detecting smoking following smoking cessation treatment. Drug Alcohol Depend. 2002;65:191–196. doi: 10.1016/s0376-8716(01)00162-4. [DOI] [PubMed] [Google Scholar]
- Hammersley R. Cue exposure and learning theory. Addict Behav. 1992;17:297–300. doi: 10.1016/0306-4603(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Havermans RC, Jansen ATM. Increasing the efficacy of cue exposure treatment in preventing relapse of addictive behavior. Addict Behav. 2003;28:989–994. doi: 10.1016/s0306-4603(01)00289-1. [DOI] [PubMed] [Google Scholar]
- Heather N, Bradley BP. Cue exposure as a practical treatment for addictive disorders: why are we waiting? Addict Behav. 1990;15:335–337. doi: 10.1016/0306-4603(90)90043-w. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shickh M, Otto MV. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. d-cycloserine facilitates extinction of conditioned fear as assessed by freezing in rats. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. Vol. 2. New York: Guilford Press; 2002. [Google Scholar]
- Min Z, Nix DE, Adam RD, Chjlds JM, Peloquin CA. Pharmacokinetics of cycloserine under fasting conditions and with high-fat meal, orange juice, and antacids. Pharmacotherapy. 2001;21:891–897. doi: 10.1592/phco.21.11.891.34524. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota A, Colby SM, Goddard P, Abrams DB. Cue exposure with coping skills treatment for male alcoholics: A preliminary investigation. J Consult Clin Psychol. 1993;61:1011–1019. doi: 10.1037//0022-006x.61.6.1011. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction. 1999;94:685–695. doi: 10.1046/j.1360-0443.1999.9456856.x. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;2:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addict Behav. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systematic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmcol Biochem Behav. 1994;47:107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo Conklin C, Sayett M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology. 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res. 2001;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Baker TB. Any further progress in smoking cessation treatment? Nicotine Tob. Res. 2001;3:311–323. doi: 10.1080/14622200110050484. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy. Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: Pharmacological and behavioral treatments. Nicotine Tob. Res. 2004;6:523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological methods. New Jersey: Humana; 1992. pp. 41–72. [Google Scholar]
- Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. J Abnorm Psychol. 1994;103:812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Toll BA, Katulak NA, McKee SA. Investigating the factor structure of the questionnaire on smoking urges-brief (QSU-brief) Addict. Behav. 2006;31:1231–1239. doi: 10.1016/j.addbeh.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C, Bovbjerg DH, Erblich J. Smoking-related videos for use in cue-induced craving paradigms. Addict Behav. 2007;32:3034–3044. doi: 10.1016/j.addbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B. Learning and memory in conditioned fear extinction: Effects of D-cycloserine. Acta Psychol. 2008;127:601–613. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Masayoshi M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Dev Neurosci. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of conditioned fear extinction by systematic administration or intra-amygdala infusions of d-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- Wynd CA. Guided health imagery for smoking cessation and long-term abstinence. J Nurs Sch. 2005:245–250. doi: 10.1111/j.1547-5069.2005.00042.x. 3rd Quarter. [DOI] [PubMed] [Google Scholar]