Extra-adrenal paragangliomas are neoplasms of the paraganglia located within the paravertebral sympathetic and parasympathetic chains. Thus paragangliomas may arise anywhere along these tracts and common sites of occurrence include abdomen, retroperitoneum, chest and mediastinum and various head and neck locations such as jugulotympanic membrane, orbit, nasopharynx, larynx, vagal body and carotid body. Recent literature suggests a molecular basis for the development of some paragangliomas, i.e. germline mutations. Six genes have been identified and are thought to contribute to the development of pheochromocytoma/paraganglioma. These include RET, VHL, NF1 and SDH subunits SDHB, SDHC, and SDHD. SDHD and SDHB mutations account for a significant percentage of head and neck paragangliomas. It is well know that paragangliomas may be hereditary and may be part of genetic syndromes such as Von Hippel-Lindau syndrome, neurofibromatosis type I (von Recklinghausen disease), MEN 2A and MEN 2B. When features of these more commonly known syndromes are not present, many familial cases, often associated with the above mentioned germline mutations, go unrecognized.
In the head and neck region the normal paraganglia are associated with the parasympathetic nervous system and paragangliomas arising from these parasympathetic sites account for up to 70% of extra-adrenal paragangliomas. The most common site is the carotid body. Carotid body paragangliomas arise at the bifurcation of the internal and external carotid arteries and have classic radiographic features.
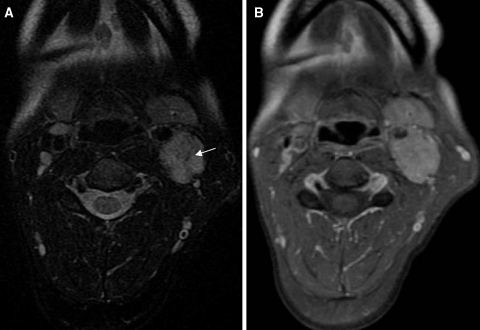
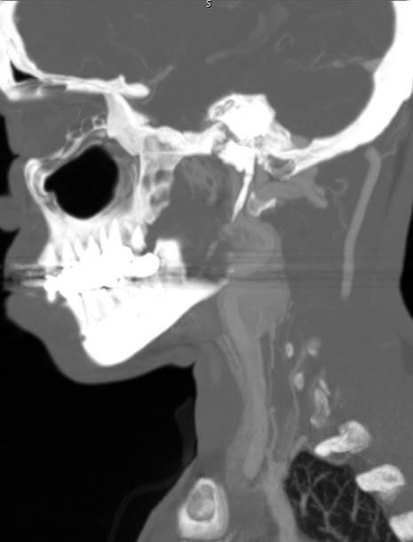
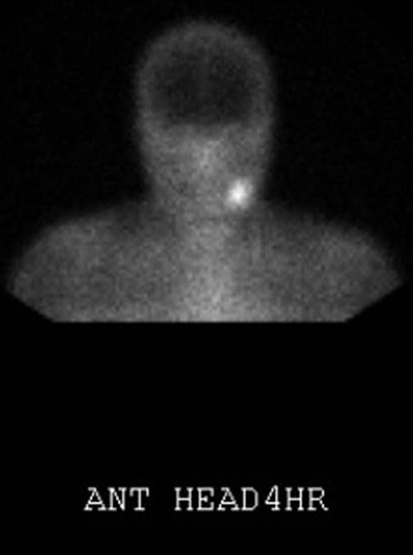
Carotid body paragangliomas are vascular lesions, and this is reflected in their imaging appearance. These lesions splay apart the internal (ICA) and external carotid arteries (ECA), and as it enlarges, it will encase, but not narrow the ICA and ECA. Upon contrast administration the lesions avidly enhance reflecting their vascular nature. Flow voids from the numerous vessels are typically seen on MR imaging, and this finding is part of the classic “salt and pepper” imaging appearance of these lesions seen on T2 weighted images. The “pepper” refers to the low signal flow voids, and the “salt” refers to high signal foci of hemorrhage and/or slow flow. The lesions tend to be isointense relative to muscle on T1 weighted imaging and hyperintense on T2. Avid enhancement is seen on post-contrast imaging (Fig. 1a, b). Cather angiography and CT angiography reveal a hypervascular mass (Fig. 2) with enlarged feeding arteries (typically the ascending pharyngeal or ascending cervical artery), intense tumor blush and early draining veins. Indium-111 octreotide, which is a somatostatin analog, is nuclear medicine imaging study that is useful in the evaluation of paragangliomas, since these are neuroendocrine neoplasms that have surface receptors for somatostatin. A focal area of early intense radiotracer uptake will be seen in the region of the paraganglioma (Fig. 3), and is sensitive for detecting tumors greater than 1.5 cm. Octreotide scanning is useful for detecting the presence of multicentric or metastatic paragangliomas, and for distinguishing scar from residual tumor after surgery.
Fig. 1.
a Axial T2 weighted image demonstrates punctuate areas of low signal consistent with flow voids (arrow). b The post contrast T1 weighted image demonstrates avid contrast enhancement
Fig. 2.
Sagittal reformation from a CT angiogram demonstrates intense enhancement at the left carotid bifurcation with splaying of the internal and external carotid arteries
Fig. 3.
Indium-111 octreotide scan demonstrates avid uptake of radiotracer in the region of the left carotid bifurcation on the 4 h delay scan
Surgical resection is the treatment of choice, but these neoplasms are very vascular making resection challenging. A surgical classification system was developed by Shamblin et al. in order to better predict surgical morbidity, which is related to the relationship of the tumor with the carotid vessels. The Shamblin classification divides the tumors into three groups based on operative notes and gross examination. Group 1 tumors are minimally attached to vessels and easily removed. Group 2 lesions partially surround the vessels and are more adherent to the adventitia, and group 3 lesions were adherent to the entire surface of the carotid bifurcation making surgical resection impossible. Arya et al. demonstrated that preoperative MR imaging can predict the Shamblin group based on the degree of circumferential contact of the paraganglioma with the internal carotid artery on axial imaging.
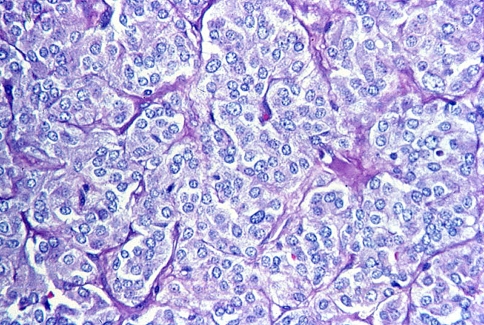
On gross examination the tumors are usually well-circumscribed and may have a pseudocapusle. The cut surface is typically solid with a smooth, rubbery texture but may display some areas of hemorrhage. The actual size of the tumors may vary greatly. Some have reported tumors as large as 10 cm. Paragangliomas that are found in difficult to access locations such as jugulotympanic paragangliomas more often will be fragmented and histologic features may be difficult to decipher. Histologically, carotid body tumors, and paragangliomas in general, have a characteristic growth pattern often referred to as a “zellballen” growth pattern. This refers to a well-developed nested or organoid growth pattern of the tumor cells with an intervening stromal component of delicate fibrovascular tissue and supporting cells or “sustentacular” cells at the periphery of the zellballen or cell nests. The tumor cells (i.e. paraganglioma cells) are predominantly chief cells with round, hyperchromatic nuclei, a dispersed chromatin and abundant granular cytoplasm which may range from eosinophilic to basophilic in color (Fig. 4). Occasionally neuromelanin pigment may be seen and amyloid deposition has also been described. Like the pheochromocytes of adrenal pheochromocytomas, the tumor cells show reactivity with chromogranin and synaptophysin stains by immunohistochemical techniques (Fig. 5a) along with other markers of neuroendocrine differentiation such as CD56 and neuron specific enolase. The sustentacular cell population can usually be identified at the periphery of the nests and are thought to be modified Schwann cells; they are spindle shaped and can be highlighted with S-100 protein staining (Fig. 5b).
Fig. 4.
High power hematoxylin and eosin (H&E) stain showing a well-developed “zellballen” growth pattern. The neoplastic cells demonstrate a basophilic granular cytoplasm and round hyperchromatic nuclei with dispersed chromatin
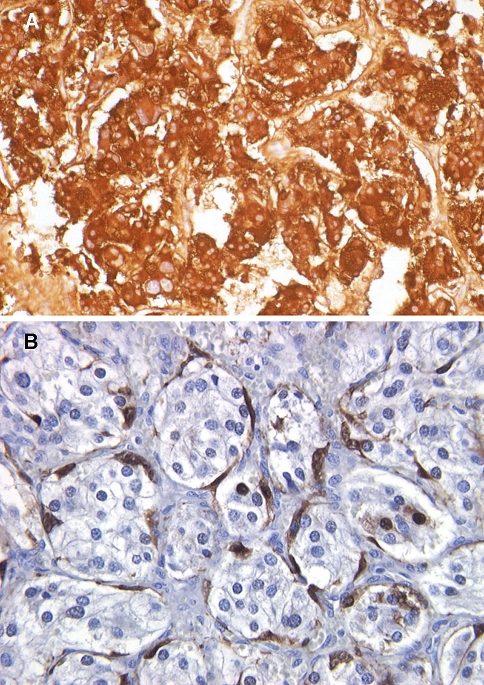
Fig. 5.
a Strong reactivity with chromogranin staining by immunohistochemical technique is shown. b The sutentacular cell population at the periphery of the cell nests (“zellballen”) is highlighted by S-100 protein immunohistochemical staining
Carotid body tumors and paragangliomas of the head and neck are typically painless, slow growing tumors that are often present for years prior to the patient seeking medical attention. They may attain large size and infiltrative growth and local recurrence may lead to death. Although it is estimated that less than 10% of paragangliomas are malignant, in some studies malignancy rates are as high as 50%. It is important to remember that all have malignant potential and it is not always possible to predict malignant behavior based on histologic features alone. There are some studies that have undertaken the task of identifying histologic criteria for malignancy, some of which are helpful, but further studies need to be performed. Some worrisome histologic features include necrosis, extensive capsular or vascular invasion, increased mitotic activity, atypical mitotic figures, loss of a well-differentiated zellballen pattern with loss of the S-100 positive sustentacular cell population and tumor cell spindling. At this point however there are no definitive, well-accepted, reproducible histologic criteria for malignancy in extra-adrenal paraganglioma.
The differential diagnosis of head and neck paragangliomas depends greatly on the actual location of the lesion. The differential diagnoses in tumors arising in the jugulotympanic area include middle ear adenoma, meningioma, and schwannoma, among others. The tumors in this location may be quite fragmented and histologic differentiation may be quite difficult and immunohistochemistry often plays a big role. In carotid body tumors the histologic features are more readily identified and the differential diagnosis includes other neuroendocrine tumors such as medullary thyroid carcinoma and neuroendocrine carcinoma. Hyalinizing trabecular adenoma of the thyroid gland should also be considered but typically does not display neuroendocrine features on immunohistochemistry.
The treatment of choice for carotid body tumor paragangliomas is surgical resection. Pre-operative adrenergic blockade should be considered and due to the high vascularity of these tumors pre-operative embolization is prudent. Overall prognosis is quite good with complete surgical resection. Continued follow-up is necessary, however, as recurrence and metastasis may occur years later. It is estimated that malignant paragangliomas have less than a 50% 10 year survival rate. Surgery remains the treatment choice for these malignant tumors since chemotherapy and radiation do not appear to be of significant benefit.
Bibliography
- Arya S, Rao V, Juvekar S, et al. Carotid body tumors: objective criteria to predict the Shamblin group on MR imaging. AJNR. 2008;29:1349–1354. doi: 10.3174/ajnr.A1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal BE, et al. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J Med Genet. 2002;39(3):178–183. doi: 10.1136/jmg.39.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstrom Elder E, Hjelm Skog AL, Hoog A, Hamburger B. The management of benign and malignant pheochromocytomas and abdominal paraganglioma. Eur J Surg Oncol. 2003;29:278–283. doi: 10.1053/ejso.2002.1413. [DOI] [PubMed] [Google Scholar]
- Hunt J. Diseases of the paraganglia system. In: Thompson LDR, editor. Endocrine pathology, foundations in diagnostic pathology. Philadelphia: Churchill Livingstone/Elsevier; 2006. p. 157–164.
- Neumann HP, et al. New genetic causes of pheochromocytoma: current concepts and the clinical relevance. Keio J Med. 2005;54:15–21. doi: 10.2302/kjm.54.15. [DOI] [PubMed] [Google Scholar]
- Rao AB, et al. Paragangliomas of the head and neck: radiologic-pathologic correlation. Radiographics. 1999;19:1605–1632. doi: 10.1148/radiographics.19.6.g99no251605. [DOI] [PubMed] [Google Scholar]
- Shamblin WR, ReMine WH, Sheps SG, et al. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122:732–739. doi: 10.1016/0002-9610(71)90436-3. [DOI] [PubMed] [Google Scholar]
- Thompson LDR. Pheochromocytoma of the adrenal gland scaled score (PASS) to separate benign form malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551–566. doi: 10.1097/00000478-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Zhang WC, Cheng JP, Li Q, et al. Clinical and pathological analysis of malignant carotid body tumour: a report of nine cases. Acta Otolaryngol. 2009;19:106. doi: 10.3109/00016480802660510. [DOI] [PubMed] [Google Scholar]