Abstract
Human embryonic stem (hES) cells are routinely cultured under atmospheric, 20% oxygen tensions but are derived from embryos which reside in a 3–5% oxygen (hypoxic) environment. Maintenance of oxygen homeostasis is critical to ensure sufficient levels for oxygen-dependent processes. This study investigates the importance of specific hypoxia inducible factors (HIFs) in regulating the hypoxic responses of hES cells. We report that culture at 20% oxygen decreased hES cell proliferation and resulted in a significantly reduced expression of SOX2, NANOG and POU5F1 (OCT4) mRNA as well as POU5F1 protein compared with hypoxic conditions. HIF1A protein was not expressed at 20% oxygen and displayed only a transient, nuclear localisation at 5% oxygen. HIF2A (EPAS1) and HIF3A displayed a cytoplasmic localisation during initial hypoxic culture but translocated to the nucleus following long-term culture at 5% oxygen and were significantly upregulated compared with cells cultured at 20% oxygen. Silencing of HIF2A resulted in a significant decrease in both hES cell proliferation and POU5F1, SOX2 and NANOG protein expression while the early differentiation marker, SSEA1, was concomitantly increased. HIF3A upregulated HIF2A and prevented HIF1A expression with the knockdown of HIF3A resulting in the reappearance of HIF1A protein. In summary, these data demonstrate that a low oxygen tension is preferential for the maintenance of a highly proliferative, pluripotent population of hES cells. While HIF3A was found to regulate the expression of both HIF1A and HIF2A, it is HIF2A which regulates hES cell pluripotency as well as proliferation under hypoxic conditions.
Introduction
Human embryonic stem (hES) cells, typically derived from the inner cell mass (ICM) of the blastocyst, proliferate by self renewal and have the potential to differentiate into all cells of the body (Thomson et al. 1998). Thus, hES cells provide an excellent model to investigate developmental mechanisms and are a potentially unlimited source of cells for transplantation in degenerative disease. However, hES cells are notoriously difficult to maintain in culture due to their propensity for spontaneous differentiation, an effect likely caused by a suboptimal culture environment. Clues to improve the culture of hES cells may be gained by investigating the environment from which these cells are derived. In vivo, preimplantation embryos develop in the secretions of the reproductive tract which are characterised by low, 1.5–8% oxygen tensions (Fischer & Bavister 1993), an environment which has been shown to improve the in vitro embryo development of several species including human (Dumoulin et al. 1999, Petersen et al. 2005, Kovacic & Vlaisavljevic 2008), mouse (Orsi & Leese 2001) and bovine (Thompson et al. 1990, Olson & Seidel 2000). Moreover, mouse embryos cultured at 5% oxygen rather than 20% oxygen displayed a global gene expression pattern which more closely resembled their in vivo counterparts (Rinaudo et al. 2006). Harvey et al. (2004), also observed that culture under 2% oxygen rather than atmospheric oxygen significantly increased the proportion of ICM cells compared with trophectoderm cells, findings consistent with pluripotent cells displaying increased proliferation when cultured under reduced oxygen tensions. Thus, mimicking the in vivo, physiological oxygen concentration may be beneficial for the propagation of hES cells in vitro. This is supported by data from rat mesenchymal and neural crest stem cells, where culture under physiological oxygen tensions showed an increase in the rate of proliferation compared with those maintained at 20% oxygen (Morrison et al. 2000, Lennon et al. 2001). These combined data highlight the benefit that culture under reduced oxygen tension has on the ability to expand scarce stem/precursor cell populations.
In terms of hES cells, atmospheric oxygen remains the culture environment of routine use. There is emerging evidence to suggest that reducing the oxygen concentration towards physiological levels is beneficial for the in vitro maintenance of hES cells in terms of decreasing the amount of spontaneous differentiation, supporting self-renewal (Ezashi et al. 2005, Ludwig et al. 2006, Westfall et al. 2008), and reducing spontaneous chromosomal aberrations (Forsyth et al. 2006). Thus, while hES cells can be maintained under atmospheric oxygen tensions, lowering the oxygen tension to 2–5% appears beneficial for the propagation of a highly proliferative, pluripotent population of cells. However, controversy still remains as a recent report suggests that there are no significant advantages of culturing hES cells under reduced oxygen tension (Chen et al. 2009).
Upon exposure to hypoxic conditions, cells mount a physiological response to ensure sufficient levels for oxygen-dependent processes. This response is regulated by hypoxia inducible factors (HIFs) which regulate the expression of over 200 genes including those involved in erythropoiesis, apoptosis and proliferation (Semenza 2000). HIFs are transcription factors consisting of three oxygen-dependent α subunits: HIF1A, HIF2A (also known as EPAS1) and HIF3A, and a constitutively expressed β subunit, HIF1B (also known as ARNT). Under atmospheric oxygen tensions, HIF1A protein is rapidly degraded due to hydroxylation by prolyl hydroxylase proteins (PHDs). These hydroxylated proteins are then recognised by the Von Hippel–Lindau (VHL) protein which targets them for proteosomal degradation. Under hypoxic conditions, PHDs are unable to hydroxylate HIF1A which therefore cannot be targeted for degradation by the VHL protein (Semenza 2003). Stabilised HIF1A subunits translocate from the cytoplasm to the nucleus where they bind with HIF1B to activate target genes (Wenger 2002). HIF1A, the first HIF-α subunit described, is thought to be the global regulator of the hypoxic response (Semenza & Wang 1992). However, the contribution of HIF2A and HIF3A, which are believed to be regulated in a similar manner (Ivan et al. 2001, Masson et al. 2001), remain to be fully characterised. All three α subunits share many sequence similarities (reviewed by Lee et al. (2004)); they all possess the Per, Arnt/HIF1B and Sim domain essential for binding to HIF1B and the oxygen-dependent degradation domain, the target for degradation under normoxic conditions. HIF3A differs from HIF1A and HIF2A as it lacks the C-terminal activation domain required for co-activator binding. Thus, HIF3A is unable to recruit co-transcriptional regulators and basal transcriptional machinery to gene targets. HIF-α subunits have been found to activate genes that contain the hypoxia response element sequence located in the promoter region of hypoxia responsive genes (Wenger 2002). HIF2A can target this sequence independently of HIF1A, suggesting that they have functionally diverse roles. For example, HIF1A, originally thought to be the main oxygen sensing subunit, predominantly regulates glycolytic genes (Hu et al. 2003), whereas HIF2A is the main regulator of hypoxia-induced erythropoietin in tissues which express both HIF1A and HIF2A (Warnecke et al. 2004, Rankin et al. 2007). HIF2A has also been shown to promote cell cycle progression in hypoxic renal clear cell carcinoma cells (Gordan et al. 2007). To date, little is known about which genes are targeted by HIF3A.
The functional significance of HIF genes has been highlighted using targeted gene inactivation. HIF1A−/− mice are non-viable, displaying developmental arrest by E9.0 with significant mesenchymal cell death and impaired vascular development (Kotch et al. 1999). HIF1B null mice are embryonic lethal by E10.5, displaying yolk sac and placental deficiencies and decreased numbers of haematopoietic progenitors (Nishi et al. 2004). HIF2A−/− mice develop severe vascular defects and show developmental arrest between E9.5 and E12.5 with variability depending on the genetic background (Compernolle et al. 2002, Nishi et al. 2004).
In mouse ES cells, both HIF1A and HIF2A are expressed but HIF1A appears to be central to regulating hypoxic responses, since it targets many oxygen-dependent genes that are not regulated by HIF2A (Hu et al. 2006). However, HIF2A has been found to be a direct upstream regulator of POU5F1 (OCT4) in mouse ES cells, suggesting that HIF2A is involved in the regulation of stem cell maintanence (Covello et al. 2006). In hES cells, the mechanism of hypoxic regulation appears to differ since HIF1A protein is only transiently expressed for ∼48 h following exposure to low oxygen tension (Cameron et al. 2008).
While there has been controversy in the literature, this study aims to prove that hES cell culture is improved under physiological oxygen concentrations compared with ambient, atmospheric oxygen tension. We investigate the effect of oxygen tension on hES cell morphology, pluripotency and proliferation and determine the functional significance and potential hierarchy of HIFs in regulating these hypoxic responses.
Results
Cell morphology
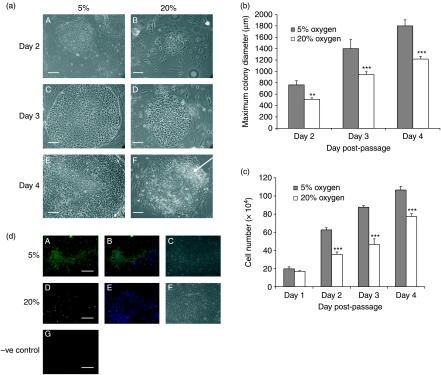
Under hypoxic conditions, morphology was altered (Fig. 1a); colonies appeared more compact with clearly defined borders. At 20% oxygen, the cells began to show peripheral, spontaneous differentiation by day 4 post-passage, characterised by enlarged, flattened cells with translucent cytoplasm (Fig. 1aF). As a consequence, hES cells cultured at 20% oxygen required more frequent passaging to limit spontaneous differentiation. In contrast, hES cells maintained at 5% oxygen were passaged due to space constraints, rather than to remove differentiated areas. Thus, colony diameter was ∼50% larger at 5% oxygen than those cultured under 20% oxygen on each day post-passage (P<0.01–P<0.001; Fig. 1b). In total, hES cells were maintained under both 5% and 20% oxygen for ∼10–12 months, or 36–40 passages and the same morphological differences were maintained throughout this period. To further quantify the increase in colony size, equal numbers of cells were seeded on Matrigel coated plates on day 0 under both oxygen tensions and total hES cell number determined on each subsequent day post-passage. A significant increase in cell number was apparent by 48 h post-passage under 5% oxygen compared with 20% oxygen and was maintained on days 3 and 4 (P<0.001; Fig. 1c). Using Ki67 labelling of hES cells on Matrigel on day 3 post-passage, there was a dramatic decrease in the number of proliferating cells at 20% oxygen compared with 5% oxygen with ∼50% of hES cells cultured under 20% oxygen being Ki67 positive compared with virtually all cells at 5% oxygen (Fig. 1d). Similar results were obtained for hES cells cultured on mouse embryonic fibroblasts (MEFs; data not shown).
Figure 1.
(a) Phase contrast images of Hues 7 hES colonies cultured on MEFs under 5% (A, C and E) and 20% (B, D and F) oxygen on days 2 (A and B), 3 (C and D) and 4 (E and F) post-passage. Area of differentiation highlighted with arrow. Scale bar, 500 μm. (b) Average maximum hES cell colony diameter at 5 and 20% oxygen on days 2–4 post-passage **P<0.01, ***P<0.001 significantly different from 5% oxygen (n=6 for each day). (c) Average hES cell number at 5% and 20% oxygen on days 1–4 post-passage. Cell numbers were normalised on day 0 by passaging equal numbers of cells under both oxygen tensions. ***P<0.001 significantly different from 5% oxygen (n=21 for each day). (d) Ki67 (green) and DAPI (blue) labelling of Hues 7 hES cells at 5% (A–C) and 20% (D–F) oxygen on day 3 post-passage, phase contrast (C and F). Negative control, secondary antibody only (G). Scale bar, 250 μm.
Pluripotency marker expression
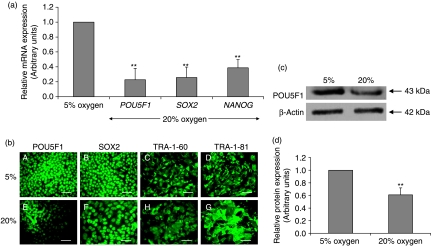
To investigate the effect of oxygen tension on the expression of pluripotency markers, hES cells cultured under either 20% or 5% oxygen were obtained on day 3 post-passage, prior to the onset of any overt differentiation observed in cells cultured at 20% oxygen. There was a significant (P<0.05) reduction in POU5F1 (80±20%), SOX2 (75±18%) and NANOG (60±10%) mRNA expression in cells maintained at 20% oxygen compared with those cultured at 5% oxygen (Fig. 2a). Although immunocytochemistry detected similar immunoreactivity for POU5F1, SOX2, TRA-1-60 and TRA-1-81 (Fig. 2b), using western blotting, POU5F1 was significantly decreased by ∼40% in hES cells cultured at 20% oxygen compared with 5% oxygen (P<0.01; Fig. 2c and d).
Figure 2.
(a) mRNA expression of POU5F1, SOX2 and NANOG cultured under 5% and 20% oxygen using relative quantification real-time RT-PCR. All data have been normalised to UBC and to 1 for 5% oxygen **P<0.01 (n=6). (b) Immunocytochemistry of POU5F1 (A and E), SOX2 (B and F), TRA-1-60 (C and H) and TRA-1-81 (D and G) of hES cells cultured under 5% (A–D) and 20% (E–H) oxygen. Scale bar=100 μm. (c) Representative western blot of POU5F1 expression in hES cells cultured under 5% and 20% oxygen. (d) Quantification of POU5F1 western blots. Data were normalised to β-actin and to 1 for 5% oxygen. **P<0.01 significantly different to 5% oxygen (n=3).
HIF expression
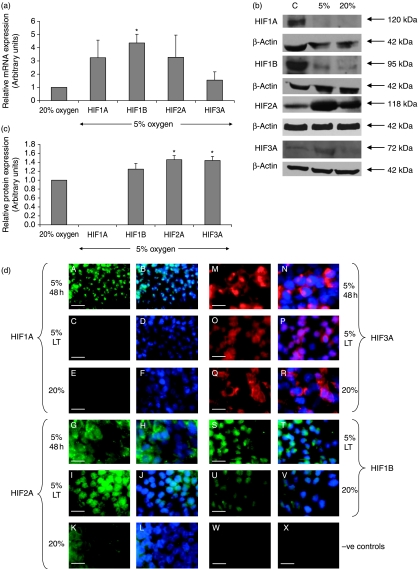
Real-time RT-PCR was used to investigate the effect of environmental oxygen tension on the differential expression of HIF1A, HIF1B, HIF2A and HIF3A subunits. All HIFs were expressed in hES cells cultured under both 20% and 5% oxygen, but there was no significant difference in the mRNA expression of the α subunits with respect to oxygen tension. Surprisingly, HIF1B was significantly upregulated under hypoxic conditions (Fig. 3a).
Figure 3.
(a) mRNA expression of HIFs in hES cells at 5% and 20% oxygen. All data have been normalised to UBC and to 1 for 20% oxygen. *P<0.05 significantly different to 20% oxygen (n=3). (b) Representative western blots of HIFs after long-term culture at 5% or 20% oxygen (C) represents positive control protein. (c) Quantification of HIF western blots. Data were normalised to β-actin and to 1 for 20% oxygen. *P<0.05 significantly different to 20% oxygen (n=3). (d) Protein expression of HIFs by immunocytochemistry in hES cells cultured under 5% oxygen for 48 h (5% 48 h; A and B, G and H, M and N), 5% oxygen for long-term (5% LT=more than three passages; C and D, I and J, O and P, S and T) and 20% oxygen (E and F, K and L, Q and R, U and V). HIF1A (green; A–F), HIF2A (green; G–L), HIF3 (red; M–R) and HIF1A (green; S–V). DAPI (blue). Negative controls=FITC secondary only (W) and Texas Red secondary only (X). Scale bar=40 μm.
Western blotting was performed on day 3 post-passage to quantify the level of HIF protein expression in hES cells (Fig. 3b). HIF1A was not expressed following long-term culture under 5% or 20% oxygen tension and there was no significant difference in the expression of HIF1B. However, HIF2A and HIF3A were significantly upregulated under 5% oxygen compared with 20% oxygen (P<0.05; Fig. 3c).
Using immunocytochemistry, HIF1A was found to be only transiently expressed being present in the nucleus of hES cells cultured under 5% oxygen for 48 h but absent after long-term culture under hypoxic conditions (Fig. 3d). HIF1A was also not expressed in hES cells cultured under 20% oxygen. HIF2A and HIF3A both displayed a largely cytoplasmic expression following 48 h of culture at 5% oxygen. However, following long-term hypoxic culture they both displayed a predominantly nuclear localisation. Under 20% oxygen, HIF2A and HIF3A remained cytoplasmic. HIF1B, which is known to be constitutively expressed, was present in the nuclei of hES cells when cultured under both 20% and 5% oxygen tensions. It was therefore not necessary to investigate the localisation of HIF1B in hES cells cultured in 5% oxygen for 48 h.
HIF knockdown
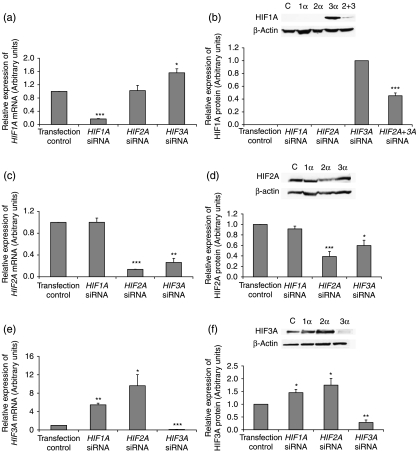
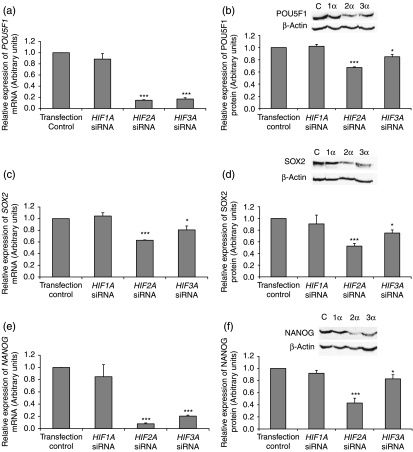
Following siRNA real-time RT-PCR confirmed an 83%, 86% and 90% knockdown of HIF1A, HIF2A and HIF3A respectively compared with transfection control siRNA (Fig. 4a, c and e). Initial studies examined the effect of knocking down individual HIF-α subunits for 48 h. When HIF2A was silenced HIF1A mRNA expression was not affected (Fig. 4a). However, the knockdown of HIF3A significantly upregulated HIF1A mRNA expression (P<0.05, Fig. 4a). As expected, HIF1A protein was not expressed in hES cells maintained at 5% oxygen and was not induced by silencing HIF2A. Interestingly, HIF1A protein was induced when HIF3A was silenced (Fig. 4b). When both HIF2A and HIF3A were silenced, HIF1A was expressed but at a significantly reduced level compared with when HIF3A alone was knocked down (P<0.001, Fig. 4b).
Figure 4.
mRNA expression levels of (a) HIF1A, (c) HIF2A and (e) HIF3A when each HIF-α isoform was silenced in hES cells cultured under 5% oxygen for 48 h. All data has been normalised to UBC and to 1 for the transfection control. *P<0.05, **P<0.01, ***P<0.001 significantly different to transfection control (n=3). (b) Representative HIF1A protein expression and quantification when HIF2A and HIF3A were silenced separately and simultaneously. *** P<0.001 significantly different to HIF3A siRNA. Representative (d) HIF2A and (f) HIF3A protein expression and quantification when each HIF-α isoform was silenced *P<0.05, **P<0.01, *P<0.001 significantly different to transfection control (n=3). Protein was collected for all samples 48 h post-transfection.
When HIF1A was silenced, HIF2A mRNA expression were unaffected. However, there was a significant reduction of HIF2A mRNA (P<0.01) and protein (P<0.05) expression observed when HIF3A was silenced (Fig. 4c and d).
HIF3A mRNA (Fig. 4e) and protein (Fig. 4f) expression were found to be significantly upregulated when HIF1A and HIF2A were knocked down independently.
Effect of HIFs on pluripotency marker expression
Using real-time RT-PCR there was a significant reduction in POU5F1 (Fig. 5a; P<0.001), SOX2 (Fig 5c; P<0.001, P<0.05) and NANOG (Fig. 5e; P<0.001) when HIF2A and HIF3A were silenced independently. As expected, silencing of HIF1A did not alter the mRNA expression of POU5F1, SOX2 and NANOG compared with transfection control siRNA (Fig. 5a, c and e). At the protein level, POU5F1, SOX2 and NANOG were significantly reduced when HIF2A (P<0.001) and HIF3A (P<0.05) were silenced (Fig. 5b, d and f).
Figure 5.
mRNA expression levels of (a) POU5F1, (c) SOX2 and (e) NANOG when HIF-α subunits were silenced in hES cells cultured under 5% oxygen for 48 h. Data normalised to UBC and to 1 for the transfection control. *P<0.05, ***P<0.001 significantly different to transfection control (n=3). Representative (b) POU5F1, (d) SOX2 and (f) NANOG protein expression and quantification by western blotting when HIF-α subunits were silenced. *P<0.05, ***P<0.001 significantly different to transfection control (n=3). Protein was collected for all samples 48 h post-transfection.
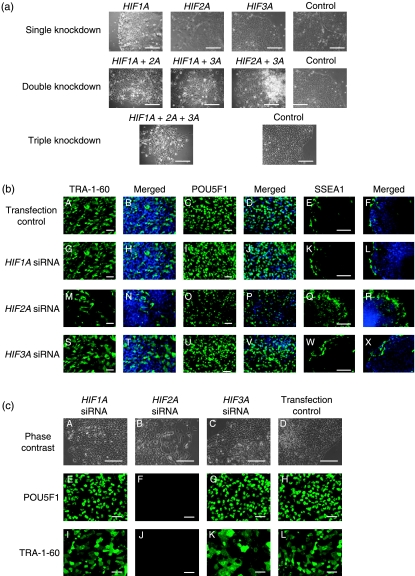
Effect of HIF expression on hES cells morphology
Silencing HIF1A or HIF3A did not affect hES cell morphology (Fig. 6a) and following knockdown these cells could be maintained in culture, remaining TRA-1-60 and POU5F1 positive 48 h (Fig. 6b) and two passages (Fig. 6c) post transfection. Moreover, HIF1A and HIF3A silenced colonies contained similar levels of SSEA1 expression as the transfection controls (Fig. 6b). However, when HIF2A expression was knocked down colonies appeared to have less clearly defined borders and large areas of differentiation (Fig. 6a). These cells failed to maintain pluripotency, being SSEA1 positive and displaying large areas that were TRA-1-60 and POU5F1 negative (Fig. 6b) and failed to proliferate during culture. Consequently, HIF2A silenced hES cells that were cultured for two passages post transfection were negative for TRA-1-60 and POU5F1 (Fig. 6c). Double knockdowns combining HIF1A and either HIF2A or HIF3A showed significant areas of differentiation and the cells failed to form colonies (Fig. 6a). When HIF2A and HIF3A were both silenced simultaneously, hES cells were capable of forming colonies but possessed large areas of differentiation. In contrast, triple HIF-α knockdown hES cells did not form colonies and did not survive in culture.
Figure 6.
(a) Representative phase contrast images of hES cells cultured under 5% oxygen 48 h after transfection with HIF-α siRNA. Controls contain the same volume and concentration of transfection reagent and AllStars control siRNA as each of the knockdowns. Scale bar=100 μm. (b) Protein expression of pluripotency markers TRA-1-60 (A, G, M and S), merged with DAPI (B, H, N and T), POU5F1 (C, I, O and U), merged with DAPI (D, J, P and V) and differentiation marker SSEA1 (E, K, Q and W), merged with DAPI (F, L, R and X) in hES cells 48 h following siRNA transfection of HIF-α subunits. Scale bar=100 μm. (c) Phase contrast and protein expression of POU5F1 and TRA-1-60 in hES cells two passages following HIF-α gene silencing. Scale bar=100 μm.
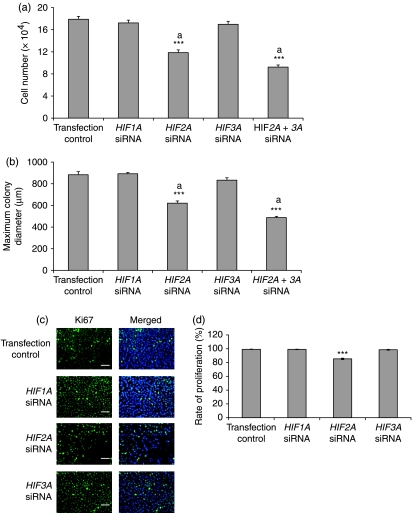
Effect of HIF expression on hES cells proliferation
At 48 h post-transfection, hES cell number (Fig. 7a) and colony size (Fig. 7b) were significantly (P<0.001) reduced by ∼30% when HIF2A expression was knocked down and displayed a further decrease (P<0.01) when HIF2A and HIF3A were silenced simultaneously. There was no additional increase in the diameter of HIF2A silenced cells 72 h post-transfection (data not shown). Colony size and cell number were unaffected by HIF1A or HIF3A knockdown. It was not possible to measure the colony size of double knockdown combinations of HIF1A and HIF2A or HIF1A and HIF3A or the triple knockdown due to the absence of colony formation occurring in these populations. Virtually all cells were positive for Ki67 when either HIF1A or HIF3A expression was silenced whereas Ki67 expression decreased to ∼85% when HIF2A was knocked down (P<0.001, Fig. 7c and d).
Figure 7.
Effect of silencing HIF-α subunits in hES cells cultured under 5% oxygen 48 h following siRNA transfection on average cell number (n=3). Cell numbers were normalised on day 0 by passaging an equal number of cells. (b) Effect of HIF-α silencing 48 h following siRNA transfection on average maximum colony diameter (n=6). ***P<0.001 significantly different from transfection control. Bars with the same superscript are significantly different; aP<0.01. (c) Ki67 (green) and DAPI (blue) labelling of HIF-α silenced hES cells and transfection control. Scale bar=100 μm. (d) Percentage of HIF-α silenced Ki67 positive hES cells. ***P<0.001 significantly different from transfection control.
Discussion
There is emerging evidence to suggest that culture under more physiological oxygen conditions (2–5% oxygen) decreases the amount of spontaneous hES cell differentiation (Ezashi et al. 2005, Westfall et al. 2008). Similarly, we found that spontaneous differentiation was apparent by day 4 post-passage in hES cells cultured under atmospheric conditions, whereas cells cultured at 5% oxygen maintained pluripotency. As a result of this, hES cells cultured under 20% oxygen required more frequent passaging prior to confluency to remove differentiated areas, whereas hES cells maintained at 5% oxygen were only passaged due to space constraints on the plate. These findings suggest that environmental oxygen tension has a critical role in the maintenance of hES cell pluripotency. To investigate differences between the two oxygen concentrations, results were obtained on day 3 post-passage, prior to overt cell differentiation.
Culture at 5% oxygen, whether on MEFs or feeder-free on Matrigel, increased the rate of cell proliferation which agrees with data from Ludwig et al. (2006), producing significantly larger hES cell colonies. This may be due to the observed increase in NANOG expression levels, since it has recently been shown that NANOG regulates S-phase entry in hES cells and that overexpression of NANOG significantly increases proliferation by binding to regulatory regions of CDK6 and CDC25A, two important cell cycle regulators (Neganova et al. 2009, Zhang et al. 2009). Similar increased rates of proliferation have also been observed in many other primitive populations including mesenchymal stem cells and neural progenitor cells when cultured under low oxygen tensions (Grayson et al. 2007, Zhao et al. 2008). Data from mesenchymal stem cells suggest the involvement of HIFs, specifically HIF2A (Grayson et al. 2007). This is supported in hypoxic carcinoma cells where HIF2A has been shown to promote cell cycle progression (Gordan et al. 2007).
In agreement with Forsyth et al. (2008) and Westfall et al. (2008), there was no significant increase in the mRNA expression of the HIF-α subunits under hypoxia suggesting that altered protein levels are due to post-transcriptional regulation as found in other cell types (Huang et al. 1998, Lang et al. 2002). However, in contrast to Westfall et al. (2008), HIF1B mRNA expression was upregulated under hypoxia which may reflect differences in both the methodology and cell lines used. To date, most research has focused on the HIF1A subunit which is thought to be the master regulator of the hypoxic response (Mazure et al. 2004). However, our data suggests this is not the case, at least in hES cells since HIF1A was only transiently expressed in the nucleus for ∼48 h following exposure to hypoxia. These findings are in agreement with recent data from Cameron et al. (2008) using a 2% environmental oxygen tension, although contrary to those of Westfall et al. (2008), who found HIF1A protein to be expressed in hES cells after 10 days of culture under both 5 and 20% oxygen. The reason for this discrepancy is unknown although HIF1A would be expected to be rapidly degraded by PHDs under atmospheric conditions. Moreover, the HIF1A staining observed by the latter investigators was cytoplasmic and therefore would not be expected to regulate the expression of hypoxia regulated genes. Our study suggests that HIF1A may play a role in the initial adaptation of hES cells to hypoxia but some other factor(s) must be responsible for maintaining the hypoxic response. We propose that HIF2A or HIF3A, both of which are translocated from the cytoplasm to the nucleus and are upregulated following culture under hypoxic conditions, may take over the initial, transient role of HIF1A. This is the first time that HIF3A and HIF1B have been demonstrated in hES cells.
hES cell colonies cultured at 5% or 20% oxygen were both positive for pluripotency markers albeit at a significantly decreased level at 20% oxygen. These results were not apparent using immunocytochemistry and highlight the importance of performing quantitative techniques when assessing pluripotency. The current results suggest that although hES cells are capable of being maintained under atmospheric conditions pluripotency is reduced before the appearance of morphological differentiation. This shows that the manual removal of differentiated cells at the time of passage, a method often used during routine culture at 20% oxygen is not effective in ensuring the maintenance of a highly pluripotent population. However, culturing hES cells at 5% oxygen does not require the routine removal of differentiated areas and can therefore be considered a much more effective method of maintaining pluripotency during long term culture. Interestingly, our data shows that hES cells cultured at 5% oxygen have an increased expression of SOX2, NANOG and POU5F1 compared with cells cultured under 20% oxygen even on day 3 post-passage, before these cells appear to be morphologically differentiated. These data are in agreement with similar POU5F1 results obtained by Ludwig et al. (2006). However, they are contrary to those of Forsyth et al. (2008) and Westfall et al. (2008), who both observed no difference in the expression SOX2, POU5F1 and NANOG between hES cells cultured at 2 and 4% oxygen respectively compared with atmospheric oxygen tensions. However, the latter investigators did show that POU5F1-regulated genes such as left-right determination factor 2 and fibroblast growth factor receptor 2 (FGFR2) were down-regulated under atmospheric oxygen tensions, suggesting that although mRNA expression of pluripotency markers was not reduced under 20% oxygen, their downstream targets display decreased expression.
Taken together, our data suggest that either low oxygen tension is beneficial for the maintenance of pluripotency or that cultures at 5% oxygen contain a greater proportion of undifferentiated cells (or both). In favour of the former, an increase in POU5F1 mRNA and protein has also been observed in mouse ES cells when HIF2A expression was increased using a genetic knock-in strategy (Covello et al. 2006). It is tempting to speculate that HIF2A regulates the long-term response of hES cells to reduced oxygen but this should be interpreted with caution as the little studied HIF3A is also differentially regulated and may also be fundamental to the hypoxic responses.
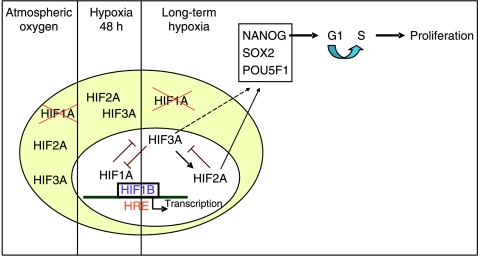
Our concept is illustrated in Fig. 8, which summarises our data on HIF expression and localisation in hES cells. We suggest that HIF1A and HIF3A are able to regulate each other's expression because when HIF1A is knocked down HIF3A is upregulated and when HIF3A is silenced HIF1A expression increases. Thus, HIF1A may be key in the initial adaptation of hES to the hypoxic environment, but when this expression is lost after ∼48 h in hypoxic conditions, HIF3A is upregulated, translocates to the nucleus where it is transcriptionally active, and takes over from HIF1A in maintaining the long-term response to hypoxia. In addition, HIF3A appears to be an upstream regulator of HIF2A since when HIF3A expression is lost HIF2A expression significantly decreases. Thus, it appears that HIF3A regulates the expression of the other HIF-α isoforms in hES cells. The precise role of HIF3A has previously been unknown but data from the human kidney suggests that it may be a negative regulator of HIF-α gene expression (Hara et al. 2001) and therefore might have a role in the downregulation of HIF1A in hES cells exposed to long-term hypoxia. This is supported by data from mouse ES cells where hypoxic expression of HIF1A was shown to suppress LIF–STAT signalling leading to the inhibition of self renewal and the promotion of cell differentiation (Jeong et al. 2007).
Figure 8.
A schematic representation of the HIF-α subunit localisation, regulation and effect on proliferation in hES cells cultured under atmospheric and hypoxic oxygen tensions.
HIF3A is also able to regulate downstream targets through the upregulation of HIF2A in hES cells. Our data show that HIF2A is an upstream regulator of POU5F1 expression in hES cells, which agrees with data from the mouse (Covello et al. 2006). Moreover, we believe this is the first description of HIF2A regulating the expression of SOX2 and NANOG and highlights the importance of HIF2A in the maintenance of hES cells under hypoxic conditions. Since HIF3A positively regulates HIF2A, a decrease in HIF3A expression results in a decrease in HIF2A expression. Thus, the decrease in POU5F1, SOX2 and NANOG mRNA and protein expression observed in HIF3A knockdowns may be a result of loss of HIF2A, not HIF3A. HIF1A does not appear to affect hES cell pluripotency or morphology.
As well as maintaining pluripotency, HIF2A was found to regulate proliferation in hES cells with a significant decrease in both cell number and colony size occurring when HIF2A expression was silenced. This may be due to the reduced NANOG expression observed when HIF2A was silenced since a decrease in NANOG has been found to delay entry into S-phase of the cell cycle, thus decreasing proliferation (Zhang et al. 2009).
Colony morphology was severely affected when two HIF-α subunits were silenced simultaneously, with a combination of HIF1A and either HIF2A or HIF3A being non-viable in culture. This may be due to HIF2A being instrumental in controlling pluripotency and HIF3A in regulating the expression of HIF2A. Not surprisingly, therefore, the simultaneous loss of HIF1A, HIF2A and HIF3A results in these cells being unable to survive hypoxia. hES cells in which both HIF2A and HIF3A were silenced formed viable colonies but contained large areas of differentiation. The survival of these cells may be due to compensation by HIF1A, which was switched back on at the protein level. This highlights the importance of HIF1A in maintaining the hypoxic response when the other α subunits are not active as observed in the initial adaptation of hES cells to hypoxia.
In conclusion, these studies demonstrate that the culture of hES cells is preferential under a reduced oxygen environment. In contrast to other cell types, HIF1A is only responsible for the initial adaptation of cells to hypoxia, whereas HIF2A regulates the long-term hypoxic response by controlling hES cell pluripotency and proliferation. HIF3A acts to regulate the expression level of both HIF1A and HIF2A. These data provide a greater understanding of the mechanisms which regulate hES cell function and the pluripotent state.
Materials and Methods
Culture of hES cells
Hues-7 hES cells (D Melton, Howard Hughes Medical Institute/Harvard University) were maintained on γ-irradiated MEFs in knockout (KO) DMEM (Invitrogen) supplemented with 10% KO serum replacement (Invitrogen), 1 mM l-glutamine, 50 mM β-mercaptoethanol, 1% non-essential amino acids, 10 ng/ml basic FGF factor (Peprotech Ltd, London, UK) and 100 μg/ml penicillin/streptomycin before being transferred on to plates pre-coated with Matrigel (BD Biosciences, San Diego, CA, USA), in medium that had been cultured over-night on γ-irradiated MEFs. hES cells were initially cultured under atmospheric (∼20%) oxygen before half were transferred into 5% O2, 5% CO2 and balanced nitrogen. Unless stated, cells were cultured at 5% oxygen for a minimum of three passages prior to use.
Quantitative real-time RT-PCR
mRNA was isolated from hES cells cultured on Matrigel on day 3 post-passage using TriReagent (Sigma) and RNA (2 μg) was reverse transcribed into cDNA using Moloney murine leukaemia virus reverse transcriptase (Promega). The standard curve method of relative quantification real-time PCR was performed using Applied Biosystems reagents in 20 μl reactions containing 4 μg of cDNA, 10 μl 2× Taqman Universal PCR Master Mix, 1 μl Probes and Primer Mix (HIF1A: Hs00936368_m1; HIF1B: Hs00231048_m1; HIF2A: Hs01026142_m1; HIF3A: Hs00541709_m1; POU5F1: Hs01895061_u1; NANOG: Hs02387400_g1; SOX2: Hs00602736_s1; ubiquitin C (UBC): Hs00824723_m1) using a 7500 Real-Time PCR System. The following conditions were used; 50 °C for 2 min, 95 °C for 10 min, 45 cycles at 95 °C for 15 s and 60 °C for 1 min. Placental cDNA (0–10 ng) was used to create a standard curve for each gene of interest as well as for UBC, used as an endogenous control. All target transcripts were analysed in duplicate and normalised to UBC.
Western blotting
hES cells cultured on Matrigel were rinsed with ice-cold PBS and treated with ice-cold radio immunoprecipitation assay lysis buffer (50 mM Tris–HCl (pH 7.4), 1% nonyl phenoxylpolyethoxylethanol, 0.25% Na-deoxycholate and complete protease inhibitors (Roche) supplemented with 1 mM phenylmethylsulphonyl fluoride, 1 mM Na3VO4 and 1 mM NaF) for 30 min with gentle agitation. Protein concentration was quantified using the Bradford assay (Bradford 1976) and lysates (75 μg) were resolved on an 8% SDS bisacrylamide gel. Positive controls were run for each protein of interest; hypoxic colorectal carcinoma cell protein for HIF1A, foetal heart (obtained following ethical approval from the Southampton & South West Hampshire Local Research Ethics Committee, under guidelines issued by the Polkinghorne Committee) for HIF1B and NT2 cells treated with 130 μM cobalt chloride for 4 h for HIF2A and HIF3A. The protein was electro-transferred to a nitrocellulose membrane and blocked in PBS containing 5% non-fat powdered milk and 0.1% Tween-20 for 1 h at room temperature. Primary antibodies were diluted in blocking buffer and incubated with the membrane overnight at 4 °C; HIF1A (BD Biosciences) 1:250, HIF1B (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) 1:250, HIF2A (Novus Biologicals, Cambridge, UK) 1:500, HIF3A (Santa Cruz) 1:1000, POU5F1 (Santa Cruz) 1:1000, SOX2 (Millipore, Billerica, MA, USA) 1:000 and NANOG (Abcam, Cambridge, UK) 1:1000. Membranes were washed and incubated with either peroxidase labelled anti-mouse antibody (Amersham) 1:50 000, or anti-goat antibody (Sigma) 1:200 000, in blocking buffer for 1 h at room temperature. Protein expression was quantified relative to β-actin expression which was detected with mouse anti-β-actin peroxidase-conjugated antibody (1:50 000; Sigma). Membranes were developed using ECL advanced western blotting detection kit (Amersham).
Immunocytochemistry
hES cells cultured on Matrigel were fixed in 4% paraformaldehyde for 20 min. Non-specific antibody binding was blocked with 3% donkey serum and where necessary cells were permeabilised with 0.1% tritonX-100 for 1 h before the addition of primary antibodies diluted in PBS and 3% donkey serum. Primary antibodies used were TRA-1-60 (Santa Cruz) 1:100, TRA-1-81 (gift from P Andrews) 1:50, SSEA1 (Santa Cruz) 1:100, POU5F1 1:100, SOX2 (Chemicon) 1:150, HIF1A 1:250, HIF1B 1:100, HIF2A 1:100, HIF3A 1:50, Ki67 (Novocastra Laboratories, Newcastle upon Tyne, UK) 1:100 and incubated overnight in a humidified chamber at 4 °C. Secondary antibody staining was performed with anti-mouse IgG conjugated-FITC 1:100, anti-mouse IgM conjugated-FITC 1:200 (Sigma) or anti-goat IgG Alexa Fluor 594 1:100 (Sigma) for 1 h in a humidified chamber. Nuclei were labelled with DAPI (Vecta Laboratories, Peterborough, UK).
siRNA
siRNA experiments were carried out on hES cells cultured on Matrigel under 5% oxygen for a minimum of three passages. hES cells cultured under 5% oxygen were passaged and incubated overnight. For each transfection 50 nM siRNA (HIF1A: Hs_HIF1A_5; HIF2A: Hs_EPAS1_5_HP; HIF3A: HsHIF3A_1_HP; Qiagen) along with 12 μl HiPerfect transfection reagent (Qiagen) were mixed into 200 μl of KO-DMEM and added in a dropwise manner to 1-well of a 6-well plate. At 48 h after transfection, cells were harvested and extracts were prepared for mRNA and protein. For HIF3A, siRNA targeting exon 5 was used which is present in all the major known HIF3A isoforms (Maynard et al. 2003). AllStars Negative Control (Transfection control, Qiagen) siRNA that has no homology to any known mammalian gene was used as a negative control for each transfection. For double and triple knockdowns 50 nM siRNA and 12 μl HiPerfect transfection reagent were added in 600 μl of KO-DMEM. Twice or three times the volume of Allstars Negative control was added to controls for double and triple knockdowns respectively. hES cells were analysed for knockdown using relative quantification real-time RT-PCR.
Statistical analysis
All data were analysed to determine whether they were normally distributed, using the Anderson–Darling normality test. Differences between oxygen tension and maximum colony diameter and cell number were analysed using a Student's t-test. Differences in relative gene and protein expression between cells cultured at 5 and 20% oxygen were analysed using a 1-sample t-test. Differences between negative control and knockdown results were analysed using a Student's t-test. All data represent at least three independent experiments. A value of P<0.05 was considered significant. Data are presented as mean±s.e.m.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was funded by the Gerald Kerkut Charitable Trust, a Wellome Trust Research Career Development Fellowship (WT066492MA), the MRC (G0701153), the Wellcome Trust in partnership with JDRF (WT074320MA). N A H receives support from Manchester NIHR BRC.
Footnotes
N A Hanley is now at Endocrine Sciences Research Group, Manchester Academic Health Science Centre, University of Manchester, AV Hill Building, Oxford Road, Manchester, M13 9PT, UK
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cameron CM, Harding F, Hu WS, Kaufman DS. Activation of hypoxic response in human embryonic stem cell-derived embryoid bodies. Experimental Biology and Medicine. 2008;233:1044–1057. doi: 10.3181/0709-RM-263. [DOI] [PubMed] [Google Scholar]
- Chen HF, Kuo HC, Chen W, Wu FC, Yang YS, Ho HN. A reduced oxygen tension (5%) is not beneficial for maintaining human embryonic stem cells in the undifferentiated state with short splitting intervals. Human Reproduction. 2009;1:71–80. doi: 10.1093/humrep/den345. [DOI] [PubMed] [Google Scholar]
- Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, et al. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nature Medicine. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes and Development. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin JC, Meijers CJ, Bras M, Coonen E, Geraedts JP, Evers JL. Effect of oxygen concentration on human in vitro fertilisation and embryo culture. Human Reproduction. 1999;14:465–469. doi: 10.1093/humrep/14.2.465. [DOI] [PubMed] [Google Scholar]
- Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. PNAS. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Journal of Reproduction and Fertility. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- Forsyth NR, Musio A, Vezzoni P, Simpson AH, Noble BS, McWhir J. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning and Stem Cells. 2006;8:16–23. doi: 10.1089/clo.2006.8.16. [DOI] [PubMed] [Google Scholar]
- Forsyth NR, Kay A, Hampson K, Downing A, Talbot R, McWhir J. Transcriptome alterations due to physiological normoxic (2% O2) culture of human embryonic stem cells. Regenerative Medicine. 2008;3:817–833. doi: 10.2217/17460751.3.6.817. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu C-J, Diehl JA, Simon MC. HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Hara S, Hamada J, Kobayashi C, Kondo Y, Imura N. Expression and characterization of hypoxia-inducible factor (HIF)-3α in human kidney: suppression of HIF-mediated gene expression by HIF-3α. Biochemical and Biophysical Research Communications. 2001;287:808–813. doi: 10.1006/bbrc.2001.5659. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, Thompson JG. Oxygen-regulated gene expression in bovine blastocysts. Biology of Reproduction. 2004;71:1108–1119. doi: 10.1095/biolreprod.104.028639. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1a (HIF-1α) and HIF-2α in hypoxic gene regulation. Molecular and Cellular Biology. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, Iyer S, Sataur S, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 α (HIF-1α) and HIF-2α in stem cells. Molecular Cell Biology. 2006;26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. PNAS. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jeong CH, Lee HJ, Cha JH, Kim JH, Kim KR, Kim JH, Yoon DK, Kim KW. Hypoxia-inducible factor-1 alpha inhibits self-renewal of mouse embryonic stem cells in Vitro via negative regulation of the leukemia inhibitory factor-STAT3 pathway. Journal of Biological Chemistry. 2007;282:13672–13679. doi: 10.1074/jbc.M700534200. [DOI] [PubMed] [Google Scholar]
- Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1α-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Developmental Biology. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- Kovacic B, Vlaisavljevic V. Influence of atmospheric versus reduced oxygen concentration on development of human blastocysts in vitro: a prospective study on sibling oocytes. Reproductive Biomedicine Online. 2008;17:229–236. doi: 10.1016/s1472-6483(10)60199-x. [DOI] [PubMed] [Google Scholar]
- Lang KJD, Kappel A, Goodall GJ. Hypoxia-inducible factor-1α mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Molecular Biology of the Cell. 2002;13:1792–1801. doi: 10.1091/mbc.02-02-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-W, Bae S-H, Jeong J-W, Kim S-H, Kim K-W. Hypoxia-inducible factore (HIF-1)α: its protein stability and biological functions. Experimental and Molecular Medicine. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. Journal of Cellular Physiology. 2001;187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, et al. Derivation of human embryonic stem cells in defined conditions. Nature Biotechnology. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO Journal. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard MA, Qi H, Chung J, Lee EH, Kondon Y, Hara S, Conaway RC, Conaway JW, Ohh M. Multiple splice variants of the human HIF-3α locus are targets of the von Hippel–Lindau E3 ubiquitin ligase complex. Journal of Biological Chemistry. 2003;278:11032–11040. doi: 10.1074/jbc.M208681200. [DOI] [PubMed] [Google Scholar]
- Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, Dayan F, Ginouves A, Berra E, Pouyssegur J. HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochemical Pharmacology. 2004;68:971–980. doi: 10.1016/j.bcp.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. Journal of Neuroscience. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neganova I, Zhang X, Atkinson S, Lako M. Expression and functional analysis of G1 to S regulatory components reveals an important role for CDK2 in cell cycle regulation in human embryonic stem cells. Oncogene. 2009;28:20–30. doi: 10.1038/onc.2008.358. [DOI] [PubMed] [Google Scholar]
- Nishi H, Nakada T, Kyo S, Inoue M, Shay JW, Isaka K. Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT) Molecular Cell Biology. 2004;24:6076–6083. doi: 10.1128/MCB.24.13.6076-6083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SE, Seidel GE. Reduced oxygen tension and EDTA improve bovine zygote development in a chemically defined medium. Journal of Animal Science. 2000;78:152–157. doi: 10.2527/2000.781152x. [DOI] [PubMed] [Google Scholar]
- Orsi NM, Leese HJ. Protection against reactive oxygen species during mouse preimplantation embryo development: role of EDTA, oxygen tension, catalase, superoxide dismutase and pyruvate. Molecular Reproduction and Development. 2001;59:44–53. doi: 10.1002/mrd.1006. [DOI] [PubMed] [Google Scholar]
- Petersen A, Mikkelsen AL, Lindenberg S. The impact of oxygen tension on developmental competence of post-thaw human embryos. Acta Obstetrica et Gynecologica Scandinavica. 2005;84:1181–1184. doi: 10.1111/j.0001-6349.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. Journal of Clinical Investigation. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertility and Sterility. 2006;86:1252–1265. doi: 10.1016/j.fertnstert.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Schöler HR, Balling R, Hatzopoulos AK, Suzuki N, Gruss P. Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO Journal. 1989;8:2551–2557. doi: 10.1002/j.1460-2075.1989.tb08393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes and Development. 2000;14:1983–1991. [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Molecular and Cellular Biology. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thompson JG, Simpson AC, Pugh PA, Donnelly PE, Tervit HR. Effect of oxygen concentration on in vitro development of preimplantation sheep and cattle embryos. Journal of Reproduction and Fertility. 1990;89:573–578. doi: 10.1530/jrf.0.0890573. [DOI] [PubMed] [Google Scholar]
- Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU. Differentiating the functional role of hypoxia-inducible factor (HIF)-1α and HIF-2α (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2α target gene in Hep3B and Kelly cells. FASEB Journal. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB Journal. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- Westfall SD, Sachdev S, Das P, Hearne LB, Hannink M, Roberts RM, Ezashi T. Identification of oxygen-sensitive transcriptional programs in human embryonic stem cells. Stem Cells and Development. 2008;17:869–882. doi: 10.1089/scd.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Neganova I, Przyborski S, Yang C, Cooke M, Atkinson SP, Anyfantis G, Fenyk S, Keith WN, Hoare SF, et al. A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. Journal of Cell Biology. 2009;184:67–82. doi: 10.1083/jcb.200801009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang CP, Liu ZH, Wu LY, Huang X, Wu HT, Xiong L, Wang X, Wang XM, Zhu LL, et al. Hypoxia-driven proliferation of embryonic neural stem/progenitor cells-role of hypoxia-inducible transcription factor-1α. FEBS Journal. 2008;275:1824–1834. doi: 10.1111/j.1742-4658.2008.06340.x. [DOI] [PubMed] [Google Scholar]