Abstract
Manipulating dopamine and/or adenosine 3′,5′ monophosphate regulated phosphoprotein of 32 kD (DARPP-32) can influence sexual behavior of rodents. The ventral tegmental area (VTA) is an important brain site for progestogens to facilitate sexual behavior of rodents. We hypothesized that, in the VTA, dopamine type 1-like receptor (D1)-mediated increases in progesterone (P4)-facilitated lordosis involve DARPP-32. To investigate this, ovariectomized hamsters and rats, primed with estradiol (E2; 10 μg), received infusions to the VTA of saline vehicle or sense or anti-sense oligonucleotides targeted against DARPP-32 (4 nM). Subjects were then administered P4 via subcutaneous injection (hamsters: 200 μg; rats: 0 or 100 μg). Hamsters and rats were pre-tested for lordosis 3.5 hours post-P4 injections, and then infused with the D1 agonist SKF38393 (100 ng) or vehicle to the VTA, and re-tested for sexual behavior 30 mins later. Anti-sense oligonucleotides targeted against DARPP-32, but not infusions of sense oligonucleotides, to the VTA blocked the ability of systemic P4 to enhance receptive behavior of hamsters and rats. Similarly, SKF38393-mediated increases in P4-facilitated sexual behaviors were blocked by DARPP-32 anti-sense oligonucleotides to the VTA. The same pattern of effects was not observed in rats that were primed with E2-alone. Together, these findings suggest that, in the midbrain VTA, P4’s actions to facilitate sexual behavior of female rodents, involving D1 receptors, may require DARPP-32.
Keywords: mating, sexual behavior, 5α-pregnan-3α-ol-20-one, allopregnanolone, neurosteroids
1. Introduction
The ventral tegmental area (VTA) is a site of novel, or non-genomic, actions of progestogens. We, and others, have investigated this using lordosis, or the characteristic posture that female rodents assume to allow mating to occur, as a behavioral index, or bioassay, of the effects of steroids and their downstream molecular mechanisms [39] and [40]. The data in support of the VTA as an important site for progestogens’ membrane-mediated actions for lordosis of estradiol (E2)–primed rodents is as follows [19]. Destruction of the VTA with electrolytic lesions attenuates progesterone (P4)-facilitated lordosis of E2-primed hamsters and rats [31], [42], and [58], suggesting that P4’s actions in the VTA are a necessary component of lordosis. Furthermore, application of progestogens, P4 or P4’s 5α-reduced metabolite and neurosteroid, 5α-pregnan-3α-ol-20-one (3α,5α-THP), to the VTA rapidly enhances lordosis of E2-primed hamsters and rats [6], [12], [51], and [52]. Moreover, limiting P4’s actions to cellular membranes does not reduce its effectiveness at rapidly enhancing lordosis of E2-primed rodents. For example, P4 conjugated to bovine serum albumin is too large and hydrophobic to cross cellular membranes [30], but is as effective as unconjugated P4 in facilitating lordosis of E2-primed hamsters and rats, when applied directly to the VTA [11], [12], and [13]. Thus, one way in which progestogens modulate lordosis of E2-primed rodents is via rapid, membrane-mediated actions in the midbrain VTA.
A potential membrane target of progestogens in the VTA for these rapid effects on lordosis may be dopamine type 1-like receptors (D1). In support, pharmacological manipulation of D1, or downstream components of D1-initiated intracellular signaling pathways, modulates lordosis of hamsters and rats. Systemic or intracerebroventricular (ICV) administration of D1 agonists (e.g. apomorphine, SKF38393) increase, and a D1 antagonist (e.g. SCH23390) decreases, lordosis of E2- or E2+ P4-primed rodents [1], [10], [24], [25], and [34]. Immunohistochemistry and autoradiography studies have localized D1, albeit low levels, to the VTA [5], [18], [26], and [56]. However, a small number of receptors may be sufficient to induce a behavioral response. Activating D1 in the VTA with infusions of SKF38393 enhances progestogen-facilitated lordosis of E2-primed hamsters and rats [22], [47], and [54]. Furthermore, in the midbrain VTA, progestogens’ actions for lordosis involve D1 -mediated intracellular signaling cascades. Activation of D1 increases adenosine 3′,5′ monophosphate (cAMP) synthetic enzyme adenylyl cyclase, cAMP and the cAMP associated protein kinase, protein kinase A (PKA) [53]. There is a positive relationship between changes in cAMP, and/or PKA activity, and lordosis of rats, such that increases in cAMP or PKA signaling facilitate, and decreases in cAMP or PKA signaling attenuate, lordosis of E2- or E2 + P4-primed rats [3], [23], [35], and [46]. D1-mediated increases in progesogen-facilitated lordosis of E2-primed rats and hamsters can be blocked by infusions of inhibitors of G-proteins, adenylyl cyclase, or PKA to the midbrain VTA [20], [21], [46], [47], [48], and [49]. Together, these studies provide support that, in the VTA, progestogens may have actions for facilitating lordosis of E2-primed rats and hamsters that include D1 and/or D1-initiated intracellular signaling cascades.
Phosphoprotein of 32 kD (DARPP-32) may be one of the downstream targets for progestogens’ actions involving D1-initiated intracellular signaling cascades in the midbrain VTA for lordosis. DARPP-32 was initially recognized as a target for dopamine and adenylyl cyclase/PKA signaling in the striatum, but has since been characterized as being important for a variety of other neurotransmitters and neuromodulators in other brain regions in its phosphorylated state [55]. There is some evidence to support involvement of DARPP-32 in P4’s actions for lordosis, such that ICV administration of P4 increases phospho-DARPP-32 levels in the hypothalamus of ovariectomized rats in the presence or absence of E2-priming [35]. As well, blocking DARPP-32 synthesis, with ICV infusions of anti-sense oligonucleotides for DARPP-32, reduces P4 facilitated lordosis of E2-primed rats [35]. Given that expression of DARPP-32 has been documented in the VTA [44], a question is whether DARPP-32 in this region is a downstream target for progestogens’ actions involving D1, and/or other signaling pathways. As such, the present study tests the hypothesis that, in the midbrain VTA, D1-mediated increases in P4-facilitated lordosis of rats and hamsters involve DARPP-32. To investigate this, ovariectomized, hormone-primed rats and hamsters were administered infusions to the VTA of vehicle or a D1 agonist, co-administered with vehicle or sense or antisense oligonucleotides targeted against DARPP-32. A cross-species approach was utilized in this study to begin to be able to parse out the specific effects of progestogens versus E2. Because rats are less reliant on progestogens’ actions for lordosis than hamsters, and demonstrate lordosis with E2-priming alone [9], comparing the effects of manipulations of DARPP-32 in E2 or E2+P4-primed rats will suggest whether the effects are specific to progestogens.
2. Materials and Methods
2.1. Animals and housing
All procedures were approved by The University at Albany-SUNY Institution Animal Care and Use Committee and conform to the animal care and welfare guidelines in “Principles of Laboratory Animal Care” (National Institutes of Health publication number 85-23, rev. 1996). Female LVG hamsters (N=30) were bred from those obtained from Harlan Laboratories (Indianapolis, IN, USA). Female Long-Evans rats (N=85) were bred from stock from Taconic Farms (Germantown, NY, USA). All animals were raised in the temperature-controlled (21 ± 1°C) University at Albany-SUNY Laboratory Animal Care Facility. Hamsters and rats were group-housed (3–5 per cage) in separate, species-specific colony rooms in the Laboratory Animal Care Facility in the Social Sciences Building, or in the Life Sciences Research Building, and were maintained on reverse lighting schedules (hamsters: lights on between 2200 and 0800 hrs; rats: lights on between 2000 and 0800 hrs). Animals were housed in polycarbonate cages (38 cm × 33 cm × 17 cm for hamsters; 45 cm × 24 cm × 21 cm for rats) with woodchips for bedding. Food and water were available ad libitum to subjects when they were in their homecages.
2.2. Hormone extirpation and hormone replacement
2.2.1. Ovariectomy
At approximately 55 days of age, ovaries were excised, via lower back (hamster) or bilateral flank (rats) incisions, when experimental subjects were anesthetized. Anesthesia for surgical procedures was accomplished by intraperitoneal injections of pentobarbital sodium (75 mg/kg; Abbott Laboratories, North Chicago, IL, USA) to hamsters or a cocktail of xylazine (12 mg/kg; Bayer Corp., Shawnee Mission, KS, USA) and ketamine hydrochloride (80 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA, USA) to rats.
2.2.2. Hormone-priming of experimental hamsters and rats
Crystalline 17β-E2 and P4 were obtained from Sigma Chemical Co. (St Louis, MO, USA) and dissolved in corn oil (E2: 10 μg/0.2 ml; P4: 100 or 200 μg/0.2 ml) for subcutaneous (SC) injection. For hamsters, E2 was administered in pulsed (5 μg each) SC injections at hrs 0 and 24. For rats, E2 priming consisted of a single SC injection (10 μg) at hour 0. All animals received a single injection of P4, or vehicle, 3.5 hrs prior to testing. These regimen are considered threshold dosing so that there would not a ceiling or basement effect for the pharmacological manipulations utilized, described below. In order for there to be ample time for circulating steroids to clear, there was a 7-day washout period after ovariectomy and prior to initial hormone priming and between E2 injections.
2.2.3. Stimulus Animals
Stimulus males, used during evaluation of female sexual behavior, consisted of gonadally-intact, sexually-experienced hamsters and rats. Stimulus females, used to verify and/or maintain sexual vigor of stimulus males, were ovariectomized hamsters and rats, with subcutaneous 2.5 cm silastic implants filled with E2. Stimulus female hamsters were also administered 500 μg P4 (SC) 3.5 hrs prior to use.
2.3. Intra-VTA Pharmacological Manipulations
2.3.1. Stereotaxic implantation of cannulae
While under anesthesia, hamsters and rats were implanted with guide cannuale, aimed at the left and right VTA. Guide cannulae were modified 23-gauge, thin-walled stainless steel needles with 30-gauge removable inserts that protruded 1 mm past the guide cannulae. Coordinates were initially obtained from the stereotaxic atlases of Morin and Wood [41] and Paxinos and Watson [45] and were modified according to past hit rates (for hamsters, from bregma: AP = −2.7, ML = ±0.3, DV = −7.0; for rats, from bregma: AP: −5.3; ML: ±0.4; DV: −7.0). After surgery, hamsters and rats were assessed for their neurological function (according to methods described in Marshall and Teitlebaum [37]). Briefly, subjects were monitored to determine whether they were neurologically intact (i.e. could cage climb, right themselves when placed on their side, and did not show abnormal motor responses or turning/rotating behavior). Subjects were also monitored for weight gain. If these criteria were met, subjects were included in the present experiments. There was one hamster and one rat that did not meet these criteria.
2.3.2. Infusions
During infusions animals were awake and immobilized with minimal hand-held restraint [14] and [16]. The infusion apparatus consisted of a 30-gauge needle that was attached, with PE-20 tubing, to a 5 μl Hamilton syringe. An infusion volume of 1 μl was delivered to the left and right VTA (total volume 2 μl) at a rate of 1 μl/1 min. Following delivery of the infusate, the infusion needle remained in place for 60 secs. Although diffusion of these infusate into nearby brain regions may have occurred [4] and [36], we were not able to determine this in the present study. We have found that similar volumes typically spread about 1–2 mm from infusion site [17]. Furthermore, infusions of 3α,5α-THP to nearby sites, such as the substantia nigra or central gray do not produce the same behavioral effects [17] and [21]. In the present study, there were only 3 hamsters and 5 rats that had cannulae placement that was not bilaterally directed at the VTA (i.e. at the substantia nigra), which precluded statistical analyses of comparisons between VTA infusions and those outside the VTA in nearby regions that infusates may have spread to.
2.4. Pharmacological Agents
2.4.1. Oligonucleotides
The anti-sense and sense oligonucleotides used in the present experiments correspond to the rat mRNA sequence 5′CCGCCATGGACCCCAAGG-3′ (GenBank accession # NM_138521) [7] and [35]. Full phosphorothioate HPLC-purified DARPP-32 sense and anti-sense oligonucleotides were synthesized, such that S-oligonucleotides were capped and remaining links were unmodified (Genomechanix; Gainesville, FL, USA). For intra-VTA administration, oligonucleotides were suspended in sterile saline at a concentration of 4 nM/μl. Previous in vitro findings demonstrate that DARPP-32 expression, in rat cultured cerebellum neurons, is knocked down 65 ± 6% following incubation in anti-sense, but not mis-sense, oligonucleotides for DARPP-32 [29]. Previous reports involving behavioral measures demonstrate that ICV infusions of 4 nM of anti-sense oligonucleotides for DARPP-32 at hrs 0 and 24 effectively block the ability of similar infusions of P4 or SKF38393, but not serotonin, to facilitate lordosis of E2-primed rats [35]. Pilot data from our laboratory suggested that administration of anti-sense oligonucleotides to the VTA using this regimen to rats produced modest reduction of P4-faciltiated lordosis [14]. Therefore, in the present experiment, hamsters and rats received infusions of saline vehicle, sense (control) oligonucleotides, or anti-sense oligonucleotides to the VTA (4 nM) at hrs 0, 12 and 24. It was not possible to have anti-sense and sense oligonucleotides targeted against the hamster mRNA because, to our knowledge, this has not been fully characterized in this species. Another limitation is that we were not able to determine whether protein levels of DARPP-32 were reduced in hamster subjects in the present study, or analyze this with immunocytochemistry in the tissues that were generated from hamsters or rats in the present study. We verified that there was an apparent reduction in DARPP-32 in dissected out midbrain tissue in a separate pilot group of rats administered these manipulations to the VTA with western blot analyses (done at the University at Albany, Center for Functional Genomics; data not shown). The behavioral effects of these manipulations were robust in both hamsters and rats in the present experiment.
2.4.2. D1 Agonist
The D1 receptor agonist (±)-SKF38393 hydrochloride was obtained from Sigma (St. Louis, MO, USA) and dissolved in sterile saline to a concentration of 100 ng/μl for intra-VTA administration. We have previously shown that this dosing regimen significantly increases P4-, but not E2-, facilitated lordosis of hamsters and rats [22], [46], [48], and [54].
2.5. Procedure and Experimental Design
A timeline depicting when hamsters and rats were injected with steroids, infused with compounds to the VTA, and behaviorally assessed is included (See Figure 1).
Fig. 1.
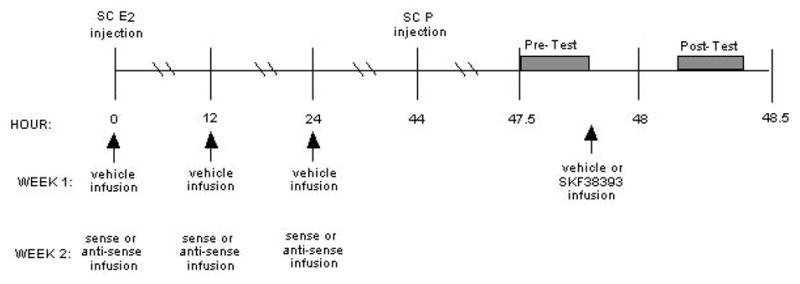
A timeline demonstrating when hamsters and rats were hormone-primed, infused with the DARPP-32 vehicle, sense, or anti-sense oligonucleotides, and infused with the D1 agonist or vehicle, and behaviorally tested.
2.5.1. Experiment 1: Effects of anti-sense oligonucleotides against DARPP-32 on lordosis of hamsters
Hamsters are heavily reliant on P4’s central actions for exhibiting lordosis. For example, E2-primed hamsters require P4 administration to both the ventromedial hypothalamus and the VTA in order to display lordosis [6] and [52]. For this reason, we initially examined the effects of VTA infusions of anti-sense oligonucleotides for DARPP-32 on E2- and P4-facilitated lordosis in hamsters. At hours 0 and 24, hamsters received SC injections of E2 (5 μg). At hours 0, 12, and 24, hamsters were infused bilaterally to the VTA saline on Week 1 and anti-sense or sense oligonucleotides for DARPP-32 (4 nM/side for a total of 8 nM) on Week 2. Although order effects may be a concern, this design was utilized to avoid any possible carryover effects from the oligonucleotide preparations. At hour 44, hamsters were administered SC injections of P4 (200 μg). Pre-testing for sexual behavior took place 3.5 hrs later (at hour 47.5). Immediately after pre-testing, subjects received bilateral infusions of the D1 agonist SKF38393 (100 ng/side) or saline vehicle aimed at the VTA. Post-testing for lordosis took place 30 mins after infusions of SKF38393 or saline vehicle. Hamsters were randomly- assigned to the following between-subjects conditions: anti-sense oligonucleotides and SKF38393 (n=6), sense oligonucleotides and SKF38393 (n=7), anti-sense oligonucleotides and SKF38393 vehicle (n=5) or sense and SKF38393 vehicle (n=8).
2.5.2. Experiment 2: Effects of anti-sense oligonucleotides for DARPP-32 on lordosis of rats
The first experiment examined the effects of VTA infusions of anti-sense oligonucleotide infusions on sexual behavior, lordosis, of hamsters. Because hamsters do not display appreciable lordosis in the absence of P4, rats were used to examine the effects of VTA infusions of anti-sense oligonucleotides on E2+P4- and/or E2-mediated sexual behaviors in Experiments 2a and 2b, respectively. At hour 0, rats received SC injections of E2 (10 μg) and bilateral VTA infusions of saline vehicle on Week 1 and anti-sense or sense oligonucleotides targeted against DARPP-32 (4 nM/side for a total of 8 nM) on Week 2. Although order effects may be a concern in Experiments 2a and 2b, this experimental design was utilized to avoid any possible carryover effects from the oligonucleotide preparations. Indeed, clear differences were not noted between rats that were administered oligonucleotides in week 2 of Experiment 2a or during week 1 in our pilot study (data not shown). Saline or oligonucleotide infusions were repeated at hrs 12 and 24. At hour 44, rats received SC injections of P4 (100 μg) in Experiment 2a, or oil vehicle in Experiment 2b. Pre-testing for receptive sexual behaviors took place 3.5 hrs later. Immediately after the pre-test, rats received bilateral infusions of the D1 agonist SKF38393 (100 ng/side) or saline vehicle aimed at the VTA. Post-tests for sex behavior took place 30 mins later. During sexual behavior testing, experimental female rats were vaginally-masked to minimize mating-induced changes in sexual receptivity [50]. In Experiment 2a, E2+P4-primed rats were randomly-assigned to receive anti-sense oligonucleotides and SKF38393 (n=11), sense oligonucleotides and SKF38393 (n=9), anti-sense oligonucleotides and vehicle (n=10) or sense oligonucleotides and vehicle (n=9). In Experiment 2b, E2-primed rats were randomly-assigned to receive anti-sense oligonucleotides for DARPP-32 and SKF38393 (n=10), sense oligonucleotides for DARPP-32 and SKF38393 (n=10), anti-sense oligonucleotides and vehicle (n=11) or sense oligonucleotides and vehicle (n=9).
2.6. Behavioral Testing
2.6.1. Sexual behavior of hamsters
During lordosis testing of hamsters, experimental females were placed in a glass chamber (50 × 25 × 30 cm) with a sexually-responsive stimulus male for 10-min. An observer timed the intervals during which experimental females exhibited lordosis. Total lordosis durations (TLDs) were determined by summing the total amount of time that females spent in lordosis following mounting by a sexually-experienced male [16] and [18]. During lordosis testing, hamsters were vaginally-masked to minimize mating-induced changes in sexual receptivity [27] and [28].
2.6.2. Sexual behavior of rats
Sexual behavior testing of rats consisted of placing an experimental female in a Plexiglas chamber (50 × 25 × 30 cm) until the female received 10 mounts or 10 mins had passed. During this time, the frequency of lordosis was recorded by an observer. Lordosis quotients (LQs), the number of times that a female exhibited lordosis in response to male mounting divided by the total number of mounts received were calculated ([instances of lordosis/mounts by male] × 100), and used to quantify receptive behavior of rats [10] and [15].
2.7. Tissue Collection and analyses
2.7.1. Fixation and histology
Following testing, hamsters and rats from Experiments 1 and 2 were deeply anesthetized (with an overdose of sodium pentobarbital; 150 mg/kg or to effect), exsanguinated with saline, and then perfused with 10% formalin. Fixed brains were removed from the skull and stored in 10% formalin for 3–5 days and then a 30% sucrose-saline solution until processed. For processing, brains were first frozen. Frozen sections were sliced at 40 μm, mounted and stained with cresyl violet. Light microscopy was used to verify infusion sites of rats and hamsters. Infusions located medial to the substantia nigra, lateral to the interfascicular nucleus, dorsal to the mammillary peduncle, and ventral to the red nucleus were considered to be within the VTA. Three hamsters and 5 rats had cannulae placement that was not bilaterally directed at the VTA (i.e. at the substantia nigra). The data from these subjects are not included in the final data analyses because the missed placement of the cannulae obviated interpretation of their data.
2.8. Statistical Analyses
In Experiment 1, data from hamsters were analyzed with repeated measures analyses of variance (ANOVAs) with oligonucleotide (anti-sense or sense oligonucleotides) and D1 agonist (SKF38393 or vehicle) as between-subject variables and testing occasion (Week 1- saline or Week 2- oligonucleotides) and test time (pre- or post-test) as the repeated, within-subjects variables. In Experiment 2a and 2b, data from rats were analyzed with repeated-measures ANOVAs with oligonucleotide type (anti-sense or sense) and D1 agonist (SKF38393 or vehicle) as between-subject variables and testing occasion (Week 1 or 2) and test time (pre- or post-test) as within-subjects, repeated variables. In each experiment, an α level of 0.05 was used to determine statistical significance and an α of less than 0.10 was utilized to determine a statistical trend. When appropriate, significant findings from hamster or rat ANOVAs were further defined using Fisher PLSD comparisons.
3. Results
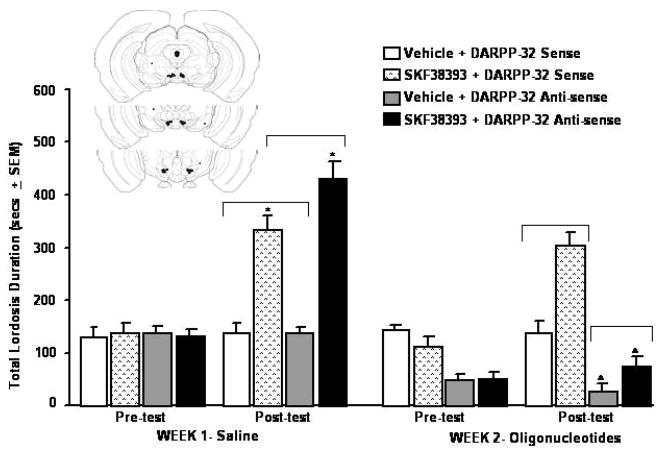
3.1. Experiment 1: Anti-sense oligonucleotides against DARPP-32 to the VTA reduce lordosis of hamsters, particularly after SKF383-administration (Figure 2)
Fig. 2.
Infusions of anti-sense oligonucleotides for DARPP-32 to the VTA reduce P4 and/or SKF38393-facilitated total lordosis durations (TLDs, mean ± SEM) of ovx, E2-primed hamsters. Inset is schematic representation of hamster coronal sections depicting the VTA. Infusion sites within the VTA are designated with closed circles and infusion sites outside of the VTA are indicated with closed squares and a small arrow. * SKF38393 vs. vehicle, p <0.05. ^ Anti-sense vs. sense oligonucleotide, p <0.05
There were significant main effects of the between-subjects variables, oligonucleotide condition, F(1,22) = 13.78, p < 0.01, and SKF38393 condition, F(1,22) = 30.77, p < 0.01. TLDs of hamsters that received DARPP-32 anti-sense oligonucleotides were significantly lower than that of hamsters administered sense oligonucleotide infusions and SKF38393 significantly increased TLDs of hamsters compared to vehicle. There were significant interactions of the testing time with the week hamsters were tested, F(1,22) = 21.15, p < 0.01, and whether they were administered SKF38393 or not, F(1,22) = 5.16, p < 0.03, or their anti-sense oligonucleotide condition, F(1,22) = 14.87, p < 0.01. The interaction between these variables was due to anti-sense oligonucleotides, compared to sense oligonucleotide infusions, reducing P4- and SKF38393-facilitated TLDs on the post-test, but not pre-test on Week 2.
3.2. Experiment 2: Anti-sense oligonucleotides against DARPP-32 to the VTA reduce sexual behavior of E2+P4-primed rats, particularly following SKF38393 infusions (Figure 3)
Fig. 3.
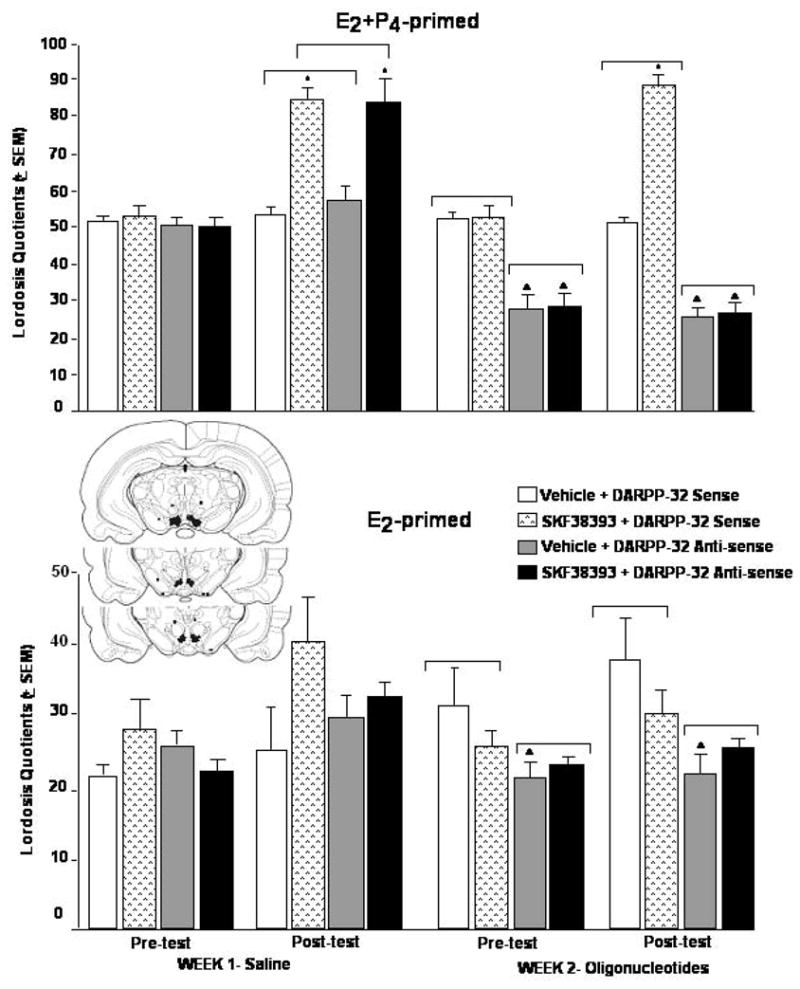
Infusions of anti-sense oligonucleotides for DARPP-32 to the VTA reduce P4 (but not E2-) and/or SKF38393-facilitated sexual behavior of rats. Top panel: Lordosis quotients (LQs) of E2+P4-primed rats (mean ± SEM). Bottom panel: LQs of E2-primed rats (mean ± SEM). Inset is schematic representation of rat coronal sections depicting the VTA. Infusion sites within the VTA are designated with closed circles and infusion sites outside of the VTA are indicated with closed squares and a small arrow. * SKF38393 vs. vehicle, p <0.05. ^ Anti-sense vs. sense oligonucleotide, p <0.05
The results of Experiment 2a for lordosis are similar to those observed in Experiment 1 with E2+P4-primed hamsters (Figure 3, top). Among E2+P4-primed rats, there was a main effect of oligonucleotide infusion, F(1,35) = 20.43, p < 0.01, and SKF38393 condition, F(1,35) = 32.79, p < 0.01, and an interaction between these variables, F(1,35) = 3.67, p < 0.06, to influence lordosis. SKF38393 infusions to the VTA of E2+P4-primed rats increased LQs compared to vehicle infusions. LQs of E2+P4-primed rats that received DARPP-32 anti-sense infusions to the VTA were significantly lower than those of rats that were infused with sense oligonucleotides, and this effect was particular to rats that were co-administered SKF38393.
A slightly different pattern of effects emerged in rats that were primed with E2 alone in Experiment 2b for LQs (Figure 2, bottom). There was no main effect of SKF38393 condition for LQs, but there was a main effect of oligonucleotide condition for LQs, F(1,36) = 5.06, p < 0.03. Rats administered the anti-sense oligonucleotide had lower LQs than rats administered vehicle or sense oligonucleotides.
4. Discussion
The current findings partly support our hypothesis that, in the VTA, P4’s actions for sexual behavior of female hamsters and rats involve D1 and DARPP-32. Following intra-VTA saline or sense oligonucleotide pretreatment, VTA infusions of the D1 agonist, SKF38393, increased sexual behavior of E2+P4-primed hamsters and rats. However; pretreatment with anti-sense oligonucleotides targeted against DARPP-32 reduced the ability of systemic P4, or systemic P4 plus intra-VTA SKF38393, to enhance lordosis of E2-primed hamsters and rats. The D1 manipulations did not produce the same changes in lordosis of rats primed with E2 alone. Thus, as a whole, our findings support the notion that P4’s actions, in the VTA, for facilitating female sexual behavior of hamsters and rats involve D1-initiated cellular responses that include DARPP-32.
Although there is no direct data indicating that P4 has membrane actions at D1 in the VTA, the present findings do suggest that, in the midbrain VTA, P4’s actions for lordosis may include D1 and D1-initated increases in DARPP-32 activity. As well, the observation that VTA infusions of SKF38393 do not increase lordosis of E2-primed rats, as is the case with ICV infusions [34] and [35], further demonstrate that, in the VTA, progestogens may be required for D1-mediated increases in lordosis [22]. However, it cannot be entirely concluded from the present study if these effects were solely due to actions involving D1 because there was similar reductions in lordosis due to knocking down DARPP-32 following pre-treatment with saline or SKF38393. Although this may be due to basement effects of drug manipulations utilized, it is interesting to note that lordosis in rats following knock down of DARPP-32 resembled that of E2-only primed rats, and not lower, which is another suggestion of the role of progestins in this effect. Effects are not likely due to enhancing activity at dopamine type 2-like receptors in the midbrain VTA [22], but may include other signaling pathways (i.e. phospholipase C, protein kinase C) [19], and the interactions of these cascades in this system needs to be investigated. Indeed, DARPP-32 is a downstream target in a number of signaling pathways. Thus, the current findings extend previous knowledge by demonstrating that in the VTA, P4 is likely to modulate the activity of D1-associated processes to facilitate sexual behavior of female hamsters and rats.
The present experiment confirms and extends current knowledge of the modulatory role of DARPP-32 on P4-mediated sexual behavior of rodents from the hypothalamus to the midbrain VTA. Previous reports demonstrate that administration of anti-sense oligonucleotides for DARPP-32 interferes with P4- and D1-facilitation of lordosis of E2-primed rats. ICV infusions of anti-sense, but not sense, oligonucleotides for DARPP-32 reduce the ability of subsequent infusions of P4 or SKF38393 to enhance lordosis of E2-primed rats [35]. Conversely, ICV infusions of P4 increase levels of phospho-DARPP-32 in the hypothalamus and facilitate lordosis of E2-primed rats [35]. These findings suggest that P4’s actions in the hypothalamus for lordosis require DARPP-32. In the present study, we provide converging evidence from 2 rodent species, to demonstrate that VTA infusions of anti-sense oligonucleotides targeted against DARPP-32 reduce P4-facilitated sexual behavior of hamsters and rats. By comparing these rodent species that have differ in their reliance on P4’s actions in the VTA for lordosis, we demonstrate that, although the effects of activating D1 in the VTA for lordosis require P4, there are modest reductions in lordosis following E2-priming alone when rats are administered anti-sense oligonucleotides targeted against DARPP-32. However, these data need to be interpreted with some caution because the modest effect of anti-sense oligonucleotides for DARPP-32 on E2-facilitated lordosis may have been due to typically low basal lordosis responding in rats that were only administered physiological dosing of E2. However, previous reports show that E2-mediated increases in phospho-DARPP-32 occur in brain regions that also express high levels of E2-induced progestin receptors, such as the medial preoptic area, bed nucleus of the stria terminalis or caudal ventromedial hypothalamus [2]. Given that there are remarkably few E2-induced progestin receptors in the VTA [18] and [33] and, therefore, little evidence of co-localization of E2-induced progestin receptors with D1 in the VTA, this suggests that the decrements in sexual behavior observed following anti-sense oligonucleotide treatments to the midbrain VTA in the present report were due to disruptions of P4’s mechanisms, rather than E2’s.
In summary, the current finings demonstrate that, in the VTA, D1-mediated increases in P4-facilitated lordosis require DARPP-32. The current findings are relevant for further understanding dopamine-mediated mechanisms in the midbrain VTA, and its mesolimbic dopamine projections, for reproduction-related motivated/rewarding behaviors, such as mating and motherhood [8], [32], [38], [43], and [57]. The signaling molecules that are downstream of DARPP-32 and are important for progestogens’ actions for lordosis are a current topic of interest in our laboratory.
Acknowledgments
This research was supported in part by grants from The National Science Foundation (IBN03-16083) and The National Institute of Mental Health (MH06769801). Technical assistance provided by Drs. S. Petralia and Q. Lin is appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apostolakis EM, Garai J, Fox C, Smith CL, Watson SJ, Clark JH, O’Malley BW. Dopaminergic regulation of progesterone receptors: brain D5 dopamine receptors mediate induction of lordosis by D1-like agonists in rats. J Neurosci. 1996;16:4823–4834. doi: 10.1523/JNEUROSCI.16-16-04823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auger AP, Meredith JM, Snyder GL, Blaustein JD. Oestradiol increases phosphorylation of a dopamine- and cyclic AMP-regulated phosphoprotein (DARPP-32) in female rat brain. J Neuroendocrinol. 2001;13:761–768. doi: 10.1046/j.1365-2826.2001.00700.x. [DOI] [PubMed] [Google Scholar]
- 3.Beyer C, Canchola E, Larsson K. Facilitation of lordosis behavior in the ovariectomized estrogen primed rat by dibutyryl camp. Physiol Behav. 1981;26:249–51. doi: 10.1016/0031-9384(81)90019-6. [DOI] [PubMed] [Google Scholar]
- 4.Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: an electrophysiological study in cerebral cortex. J Neurosci Methods. 2001;105:133–41. doi: 10.1016/s0165-0270(00)00348-4. [DOI] [PubMed] [Google Scholar]
- 5.Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBold JF, Malsbury CW. Facilitation of sexual receptivity by hypothalamic and midbrain implants of progesterone in female hamsters. Physiol Behav. 1989;46:655–660. doi: 10.1016/0031-9384(89)90347-8. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich ME, Kurihara T, Greengard P. Rat DARPP-32: cloning, sequencing, and characterization of the cDNA. J Mol Neurosci. 1990;2:1–10. doi: 10.1007/BF02896920. [DOI] [PubMed] [Google Scholar]
- 8.Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–19. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm Behav. 2001;40:226–33. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- 10.Frye CA, Bayon LE, Vongher JM. Intravenous progesterone elicits a more rapid induction of lordosis in rats than does SKF38393. Psychobiol. 2000;28:99–109. [Google Scholar]
- 11.Frye CA, DeBold JF. P-3-BSA, but not P-11-BSA, implants in the VTA rapidly facilitate receptivity in hamsters after progesterone priming to the VMH. Behav Brain Res. 1993;53:167–175. doi: 10.1016/s0166-4328(05)80276-1. [DOI] [PubMed] [Google Scholar]
- 12.Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav. 1996;30:682–691. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- 13.Frye CA, Mermelstein PG, DeBold JF. Evidence for a non-genomic action of progestins on sexual receptivity in hamster ventral tegmental area but not hypothalamus. Brain Res. 1992;578:87–93. doi: 10.1016/0006-8993(92)90233-y. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA, Petralia SM. 3α,5α-THP’s actions in the ventral tegmental area for lordosis: a model system for defining function and mechanisms for progestins. In: Smith S, editor. Neurosteroids and the GABAA Receptor. Boca Raton: CRC Press; 2003. pp. 265–290. [Google Scholar]
- 15.Frye CA, Petralia SM. Lordosis of rats is modified by neurosteroidogenic effects of membrane benzodiazepine receptors in the ventral tegmental area. Neuroendocrinology. 2003;77:71–82. doi: 10.1159/000068338. [DOI] [PubMed] [Google Scholar]
- 16.Frye CA, Petralia SM. Mitochondrial benzodiazepine receptors in the ventral tegmental area modulate sexual behaviour of cycling or hormone-primed hamsters. J Neuroendocrinol. 2003;15:677–686. doi: 10.1046/j.1365-2826.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- 17.Frye CA, Rhodes ME. Infusions of 3α,5α-THP to the VTA enhance exploratory, anti-anxiety, social, and sexual behavior and increase levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex of female rats. Behav Brain Res. 2008;187:88–99. doi: 10.1016/j.bbr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frye CA, Vongher JM. GABAA, D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav Brain Res. 1999;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 19.Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–13. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frye CA, Walf AA, Petralia SM. Progestin-facilitation of lordosis in rodents involves adenylyl cyclase activity in the ventral tegmental area. Horm Behav. 2006;50:237–244. doi: 10.1016/j.yhbeh.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Frye CA, Walf AA, Petralia SM. Progestins’ effects on sexual behaviour of female rats and hamsters involving D1 and GABAA receptors in the ventral tegmental area may be G-protein-dependent. Behav Brain Res. 2006;172:286–293. doi: 10.1016/j.bbr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Frye CA, Walf AA, Sumida K. Progestins’ actions in the VTA to facilitate lordosis involve dopamine-like type 1 and 2 receptors. Pharmacol Biochem Behav. 2004;78:405–418. doi: 10.1016/j.pbb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Flores O, Ramirez-Orduna JM, Lima-Hernandez FJ, Garcia-Juarez M, Beyer C. Differential effect of kinase A and C blockers on lordosis facilitation by progesterone and its metabolites in ovariectomized estrogen-primed rats. Horm Behav. 2006;49:398–404. doi: 10.1016/j.yhbeh.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Grierson JP, James MD, Pearson JR, Wilson CA. The effect of selective D1 and D2 dopaminergic agents on sexual receptivity in the female rat. Neuropharmacology. 1988;27:181–189. doi: 10.1016/0028-3908(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 25.Hamburger-Bar R, Rigter H. Apomorphine: facilitation of sexual behaviour in female rats. Eur J Pharmacol. 1975;32:357–360. doi: 10.1016/0014-2999(75)90304-0. [DOI] [PubMed] [Google Scholar]
- 26.Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci USA. 1992;89:11988–11992. doi: 10.1073/pnas.89.24.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huck UW, Lisk RD. Mating-induced inhibition of receptivity in the female golden hamster. I. Short-term and long-term effects. Behav Neural Biol. 1986;45:107–119. doi: 10.1016/s0163-1047(86)80010-3. [DOI] [PubMed] [Google Scholar]
- 28.Huck UW, Lisk RD, Parente EJ, Guyton CL. Mating-induced inhibition of receptivity in the female golden hamster (Mesocricetus auratus): III. Stimuli mediating long-term effects. Physiol Behav. 1987;39:421–428. doi: 10.1016/0031-9384(87)90367-2. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi SY, Hirano T. Signaling cascade regulated long-term potentiation of GABAA receptor responsiveness in cerebellar Purkinje neurons. J Neurosci. 2002;22:3969–3976. doi: 10.1523/JNEUROSCI.22-10-03969.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ke FC, Ramirez VD. Membrane mechanism mediates progesterone stimulatory effect on LHRH release from superfused rat hypothalami in vitro. Neuroendocrinology. 1987;45:514–517. doi: 10.1159/000124784. [DOI] [PubMed] [Google Scholar]
- 31.Lisciotto CA, DeBold JF. Ventral tegmental lesions impair sexual receptivity in female hamsters. Brain Res Bull. 1991;26:877–883. doi: 10.1016/0361-9230(91)90252-f. [DOI] [PubMed] [Google Scholar]
- 32.Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–41. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Lonstein JS, Blaustein JD. Immunocytochemical investigation of nuclear progestin receptor expression within dopaminergic neurones of the female rat brain. J Neuroendocrinol. 2004;16:534–543. doi: 10.1111/j.1365-2826.2004.01198.x. [DOI] [PubMed] [Google Scholar]
- 34.Mani SK, Allen JM, Clark JH, Blaustein JD, O’Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 35.Mani SK, Fienberg AA, O’Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O’Malley BW. Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- 36.Marcus DS, Schuler H, Boccella L, Zivic W, Josimovich JB. New technique of injection of estradiol into preoptic-anterior hypothalamic area of newborn rats: technique limiting diffusion and duration, with preliminary results on postpuberal block of ovulation. Endocrinology. 1977;100:862–72. doi: 10.1210/endo-100-3-862. [DOI] [PubMed] [Google Scholar]
- 37.Marshall JF, Teitlebaum P. Further analysis of sensory inattention following lateral hypothalamic damage in rats. J Comp Physiol Psychol. 1974;86:375–395. doi: 10.1037/h0035941. [DOI] [PubMed] [Google Scholar]
- 38.Miller SM, Lonstein JS. Dopamine d1 and d2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav Neurosci. 2005;119:1072–83. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- 39.Mong J, Easton A, Kow LM, Pfaff D. Neural, hormonal and genetic mechanisms for the activation of brain and behavior. Eur J Pharmacol. 2003;480:229–31. doi: 10.1016/j.ejphar.2003.08.109. [DOI] [PubMed] [Google Scholar]
- 40.Mong JA, Pfaff DW. Hormonal symphony: steroid orchestration of gene modules for sociosexual behaviors. Mol Psychiatry. 2004;9:550–6. doi: 10.1038/sj.mp.4001493. [DOI] [PubMed] [Google Scholar]
- 41.Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. San Diego: Academic Press; 2001. p. 146. [Google Scholar]
- 42.Muntz JA, Rose JD, Shults RC. Disruption of lordosis by dorsal midbrain lesions in the golden hamster. Brain Res Bull. 1980;5:359–364. doi: 10.1016/s0361-9230(80)80005-0. [DOI] [PubMed] [Google Scholar]
- 43.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 44.Ouimet CC, Miller PE, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regualted phosphoprotein enriched in dopamine innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain. New York: Academic Press; 1986. p. 456. [Google Scholar]
- 46.Petralia SM, Frye CA. In the ventral tegmental area, cAMP mediates progesterone’s actions at dopamine type 1 receptors for lordosis of rats and hamsters. J Neuroendocrinol. 2006;18:902–914. doi: 10.1111/j.1365-2826.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- 47.Petralia SM, Frye CA. In the ventral tegmental area, G-proteins and cAMP mediate 3α,5α-THP actions at dopamine type 1 receptors for lordosis of rats. Neuroendocrinology. 2004;80:233–243. doi: 10.1159/000082752. [DOI] [PubMed] [Google Scholar]
- 48.Petralia SM, Frye CA. In the ventral tegmental area, G-proteins mediate progesterone’s actions at dopamine type 1 receptors for lordosis of rats and hamsters. Psychopharmacology. 2006;186:133–142. doi: 10.1007/s00213-006-0311-9. [DOI] [PubMed] [Google Scholar]
- 49.Petralia SM, Walf AA, Frye CA. In the ventral tegmental area, progestins’ membrane-mediated actions for lordosis of hamsters and rats involve protein kinase A. Neuroendocrinology. 2006;84:405–14. doi: 10.1159/000100510. [DOI] [PubMed] [Google Scholar]
- 50.Pfaus JG, Smith WJ, Byrne N, Stephens G. Appetitive and consummatory sexual behaviors of female rats in bilevel chambers. II. Patterns of estrus termination following vaginocervical stimulation. Horm Behav. 2000;37:96–107. doi: 10.1006/hbeh.1999.1562. [DOI] [PubMed] [Google Scholar]
- 51.Pleim ET, Baumann J, Barfield RJ. A contributory role for midbrain progesterone in the facilitation of female sexual behavior in rats. Horm Behav. 1991;25:19–28. doi: 10.1016/0018-506x(91)90036-h. [DOI] [PubMed] [Google Scholar]
- 52.Pleim ET, Lisciotto CA, DeBold JF. Facilitation of sexual receptivity in hamsters by simultaneous progesterone implants into the VMH and ventral mesencephalon. Horm Behav. 1990;24:139–51. doi: 10.1016/0018-506x(90)90001-e. [DOI] [PubMed] [Google Scholar]
- 53.Stoof JC, Kebabian JW. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984;35:2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- 54.Sumida K, Walf AA, Frye CA. Progestin-facilitated lordosis of hamsters may involve dopamine-like type 1 receptors in the ventral tegmental area. Behav Brain Res. 2005;161:1–7. doi: 10.1016/j.bbr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–96. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 56.Tohyama M, Takatsuji K. Atlas of neuroactive substances and their receptors in the rat. USA: Oxford University Press; 1999. p. 356. [Google Scholar]
- 57.Wansaw MP, Pereira M, Morrell JI. Characterization of maternal motivation in the lactating rat: contrasts between early and late postpartum responses. Horm Behav. 2008;54:294–301. doi: 10.1016/j.yhbeh.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamanouchi K, Arai Y. The role of mesencephalic tegmentum in regulating female rat sexual behaviors. Physiol Behav. 1985;35:255–259. doi: 10.1016/0031-9384(85)90346-4. [DOI] [PubMed] [Google Scholar]