Abstract
The attentional myopia model of behavioral control (Mann & Ward, 2007) was tested in an experiment investigating the relationship between physiological arousal and aggression. Drawing on previous work linking arousal and narrowed attentional focus, the model predicts that arousal will lead to behavior that is relatively disinhibited in situations in which promoting pressures to aggress are highly salient. In situations in which inhibitory pressures are more salient, the model predicts behavior that is relatively restrained. In the experiment, 81 male undergraduates delivered noise-blasts against a provoking confederate while experiencing either high or low levels of physiological arousal and, at the same time, being exposed to cues that served either to promote or inhibit aggression. In addition to supporting the predictions of the model, this experiment provided some of the first evidence for enhanced control of aggression under conditions of heightened physiological arousal. Implications for interventions designed to reduce aggression are discussed.
Keywords: Aggression, Attention, Myopia, Self-control, Self-regulation
Arousal leaves us mind-blind.
Malcolm Gladwell (2005, p. 229)
The costs of aggression are undeniable, ranging from personal injury to societal malaise. It is therefore not surprising that psychologists have long pursued a better understanding of aggression and its causes, as well as methods to enable individuals to better control their aggressive tendencies (for reviews, see Anderson & Bushman, 2002; Berkowitz, 1993). Although aggression, like many psychological phenomena, is multi-faceted and multiply-determined, one factor that has been consistently implicated in aggression is physiological arousal (Lindsay & Anderson, 2000).
According to Anderson and Bushman (2002), arousal can influence aggression in three ways. First, classic research linking arousal to aggression suggests that arousal can amplify aggressive responses. According to Hebb (1955), for example, arousal acts to energize, or strengthen, whatever behavior is most appropriate in the situation (see also Yerkes & Dodson, 1908). Thus, in situations that typically feature some level of aggressive response, an aroused individual would be expected to display exceptionally aggressive behavior (Geen & O’Neal, 1969).
Second, in a process known as excitation transfer (Zillmann, 1988), arousal produced by non-aggressive sources, such as exercise, can be misattributed to sources of provocation, inviting angry retaliation. Research in this area has pointed to the cognitively debilitating nature of arousal as a cause of aggression. According to this view, arousal impairs cognitive processes needed to inhibit aggression, such as recognition of the “true” source of one’s arousal, the retrieval of personal moral standards, consideration of potential negative consequences, and reassessment of aggression-provoking incidents in light of mitigating factors (Zillmann, Bryant, Cantor, & Day, 1975; Zillmann, Johnson, & Day, 1974). Of course, these two accounts are not inconsistent with one another, as both may draw on the fact that arousal can limit cognitive-attentional resources, a finding that has been repeatedly documented in both classic and contemporary research (R. S. Baron, 2000; Bodenhausen, 1993; Chajut & Algom, 2003; Easterbrook, 1959; Eysenck, 1982; Humphreys & Revelle, 1984; Kahneman, 1973; Mandler, 1975; Paulhus & Lim, 1994). Thus, individuals in an aroused state may simply not possess the necessary resources to attend to and/or act upon relatively subtle inhibiting cues intended to restrain aggression.
According to Anderson & Bushman (2002), a final “and as yet untested, possibility is that unusually high and low levels of arousal may be aversive states, and may therefore stimulate aggression in the same way as other aversive or painful stimuli” (p. 39). Indeed, two recent meta-analyses linked low resting heart rates in children and adults with increased levels of aggression and/or antisocial behavior (Lorber, 2004; Ortiz & Raine, 2004). However, each of these reviews was concerned with physiological arousal as an individual difference variable rather than as a target for experimental manipulation, and, as the authors acknowledged, individuals with abnormally low levels of basal arousal may engage in behaviors, including aggression, that are intended to raise their levels of arousal to “normal” levels. At any rate, such findings do not address the relationship, explored in the present study, between brief inductions of high levels of arousal and subsequent aggressive responses.
Although there is now ample evidence that various forms of arousal can enhance aggression (e.g., Anderson, Anderson, & Deuser, 1996; Bandura, 1973; Barclay, 1971; R. A. Baron, 1971; Berkowitz, 1970; Jaffee, Malamuth, Feingold, & Feshbach, 1974; Rule & Nesdale, 1976; Zillmann & Bryant, 1974), we propose that under some circumstances, the effects of enhanced arousal on cognition can actually lead to a relative decrease in aggressive behavior, even when that arousal is not intrinsically pleasurable (cf. R. A. Baron, 1974). Specifically, we propose that arousal can limit attentional focus to highly salient situational cues, resulting in behavior that is under the near-exclusive influence of those cues. To the extent that those salient cues are inhibitory in nature, arousal may be associated with enhanced inhibition. This proposal represents a specific prediction of the attentional myopia model of behavioral control (Mann & Ward, 2004, 2007; see also Steele & Josephs, 1990), which argues that when attentional resources are limited, the most salient internal and external cues will receive a disproportionate share of attention, to the neglect of less salient cues.
For example, in one study restrained eaters (i.e., chronic dieters) were given the opportunity to eat highly salient fatty foods while completing either a cognitive load task designed to limit attentional focus or a less demanding task that required few attentional resources (Ward & Mann, 2000). Participants in the high cognitive load condition consumed more food than did those in the low cognitive load condition. By contrast, in a second study, in which dietary prohibitions were made highly salient, restrained eaters who performed a high cognitive-load task displayed enhanced inhibition, consuming less of a high-fat milkshake than did restrained eaters who performed a low cognitive-load task (Mann & Ward, 2004).
Results similar to those obtained with cognitive load have been obtained with alcohol, another source of attentional limitation (Steele & Josephs, 1990). In one study, for example, intoxicated bar patrons exhibited stronger intentions to engage in risky sexual behavior than did sober patrons, a pattern that reversed when a strong inhibiting cue (i.e., a handstamp that read “AIDS KILLS”) was introduced into the environment (MacDonald, Fong, Zanna, & Martineau, 2000). Similarly, intoxicated participants engaged in more aggressive behavior, in the form of delivering electric shock to a confederate, than did sober individuals, a pattern that also reversed when participants had to perform an additional neutral distracting task (Giancola & Corman, 2007; see also Leonard, 1989).
Like cognitive load and alcohol intoxication, high levels of arousal have been shown to create a state of myopia, focusing attention onto the most central cues in a situation, at the expense of more peripheral cues (Easterbrook, 1959). According, we sought to investigate whether arousal could be associated with either excessively inhibited or disinhibited aggressive behavior, depending on the presence of relevant environmental cues. Our focus was on behavior that has been labeled hostile or reactive aggression, that is, aggression in response to a provocation, with the intent of harming the provocateur (Anderson & Bushman, 2002).
Arousal
Arousal is not a unitary phenomenon, and the effects of different forms of arousal have been explored in recent research (Ariely & Loewenstein, 2006; Hanoch & Vitouch, 2004; McNaughton & Gray, 2000; Robbins, 1997; Shackman et al., 2006; Thayer, 1989). For the investigation detailed here, we made use of exercise-induced arousal, which has been shown in a number of studies to increase attentional vigilance (Adam, Teeken, Ypelaar, Verstappen, & Paas, 1997; see also Smit, Eling, Hopman, & Coenen, 2005). In particular, we examined the effect of short-term increases in markers of sympathetic activation (SA), namely heart-rate and blood-pressure, on aggressive responses.
In sum, the present investigation represented the first attempt to show that elevated arousal could be associated with both increased and decreased levels of aggression in the same situation, depending on relevant salient cues (see Berkowitz, 1993). We hypothesized that in a situation in which cues signaling promoting pressures to aggress are more salient than cues indicating pressures to refrain from aggressing, exercise-induced arousal would serve to promote aggressive behavior. By contrast, in a situation in which cues to refrain from aggressing are more salient than cues to aggress, arousal was hypothesized to result in relatively inhibited aggressive behavior.
STUDY
In this study participants interacted with a rude confederate whose behavior was intended to provoke an aggressive response (as provocation would seem to be a necessary catalyst for aggression in laboratory studies; see Bushman, Baumeister, & Stack, 1999). Participants were further led to believe that they were actually aggressing against the confederate. At the same time, participants were randomly assigned to both experience low versus high levels of physiological arousal and be exposed to salient cues that served either to promote or inhibit aggression. Finally, because males have been consistently shown to exhibit more aggression than females across a wide range of domains, only male participants were recruited for this study (see Cohen, Nisbett, Bowdle, & Schwarz, 1996).
Method
Participants
A total of 85 male students from an introductory psychology class participated in this experiment for course credit. Four participants, distributed across three of the four experimental conditions, provided responses that were more than two standard deviations above the mean and, at the minimum, nearly an entire standard deviation above the next highest score on the primary outcome measure and were thus dropped from the analysis, leaving a final sample size of 81. The omission of these four outliers also conformed to a method based on Tukey’s outlier criterion, in which outliers that were above the third quartile plus 1.5 times the interquartile range were omitted (see Klauer & Teige-Mocigemba, 2007).
Materials and Apparatus
In order to assess physiological arousal, heart rate (HR) was measured using an A1 Polar heart monitor; systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured with a CVS Digital Blood Pressure Monitor. Arousal was manipulated using a Precor 714 standard stairclimber. All participants were asked to step on the stair climber until they elevated their HR to the level determined by their experimental condition, and then to maintain this HR for 2 min. Based on past research using physiological arousal to narrow attention (Tomporowski & Ellis, 1986), participants assigned to the high arousal condition were asked to maintain their HR at 150 beats/min for 2 min; participants assigned to the low arousal condition were asked to maintain their HR at 100 beats/min for 2 min.
Participants were exposed to four different stimuli designed to serve as highly salient aggression-promoting or aggression-inhibiting cues. Participants in the aggression-promoting condition heard music from movie battle scenes while seated directly in front of both a poster of the actor Clint Eastwood holding a large gun and computer “wallpaper” of a water polo player punching an opponent, all while using a computer mouse pad that featured a picture of an angry face. Participants in the aggression-inhibiting condition were exposed to soothing Chinese flute music, a poster of a placid beach scene, computer wallpaper of puppies, and a mouse pad featuring a Monet nature painting.
Procedure
When the participant arrived for the experiment, he completed an informed consent form and then was informed by the experimenter he would be participating in a study of physiological arousal and reaction time with one other participant (the confederate) who had not yet arrived. After making the participant wait for several minutes, the confederate arrived, at which point baseline levels of arousal were assessed. In addition, the participant completed a questionnaire that probed for any health condition that would prevent him from engaging in exercise (none was reported) and asked him to indicate how many times per week he exercised in a manner that increased his heart rate for more than 10 minutes. No condition differences emerged on this latter variable, and it receives no further discussion.
The experimenter then introduced them to a game based on Taylor’s (1967) competitive reaction-time task (adapted from Bushman et al., 1999), which has been shown to be a valid measure of aggression (Carnagey & Anderson, 2005; Giancola & Zeichner, 1995). The object was to press a button as quickly as possible on each trial of the game. Whoever was slower would receive a blast of noise of intensity and duration chosen by the winner at the beginning of that trial. Intensity was adjusted by the participant using a sliding scale on the computer screen that ranged from 0 to 10 and corresponded to noise levels ranging from 0 to approximately 110 decibels, whereas duration, indicated by the length of time the computer mouse was depressed, was unbounded. The experimenter informed the participant and confederate that they would be playing against each other from separate computers in adjoining rooms. The confederate was then escorted into an adjacent room for a series of practice trials. In actuality, all participants played against the computer, which was programmed to allow them to win one trial and lose the other three practice trials. Upon completion of the four trials, the confederate returned and, in a rude manner, asked the participant, “Were you even trying?”
The experimenter then explained that the experiment required one participant to exercise on a stair climber and one participant to sit and relax in a comfortable massage chair. In order to anger the participant, the confederate interrupted the experimenter and demanded the comfortable chair. The experimenter acceded to the confederate and instructed him on how to use the chair. The participant was given directions on how to use the stairclimber and maintain the desired HR for 2 min, according to the assigned arousal condition. After 1 min of exercising, the confederate commented to the participant, “Are you kidding me? I think you’re doing that wrong.” Then, turning to the experimenter, the confederate added, “I could do that so much better!”
After the participant exercised for 2 min under conditions intended to induce either high or low levels of physiological arousal, the confederate and participant were instructed to return to their respective computers to play the reaction-time game. Once the participant was seated in front of the computer, the experimenter took another arousal reading (i.e., Time 1 arousal), during which time the participant filled out a 14-item questionnaire assessing negative mood. The questionnaire consisted of the Negative Affect Schedule from the Positive and Negative Affect Schedule (Watson, Clark, & Tellegen, 1988) with 4 additional items intended to measure “anger-hostility” (i.e., “angry,” “disgusted,” loathing,” and “scornful”; see Rusting & Nolen-Hoeksema, 1998). Each item was rated on a 9-point scale ranging from very slightly or not at all (1) to extremely (9). The experimenter ostensibly collected the same information from the confederate, who was situated in the adjoining room. The experimenter then instructed the participant and confederate to play eight trials of the reaction-time game. As with the practice trials, the participant actually played against the computer during the reaction-time game, with the participant winning half the trials. At the end of the game, the experimenter returned and took a final arousal reading (Time 2 arousal), moved the participant to another empty desk in the room, and inconspicuously obscured the aggression-promoting or inhibiting cues. The participant was then asked to complete a final manipulation check assessing how noticeable he had found each of the relevant promoting or inhibiting cues (1= not at all noticeable; 9 = extremely noticeable), along with a questionnaire assessing how stressful, tiring, difficult, and fun he had found the task (1 = not at all; 7 = extremely). In addition, the participant was asked to indicate how angry he felt toward the other participant (1 = not at all; 7 = extremely). Finally, participants were probed for suspicion, debriefed, and thanked for their participation.
Results
Manipulation Checks
Analysis of our cardiovascular indicators confirmed that we had successfully manipulated arousal. In particular, we computed an Arousal × Aggression Cue Multivariate Analysis of Covariance (MANCOVA) on participants’ HR, SBP, and DBP from the measures taken immediately following exercise (Time 1) and again immediately following the reaction-time game (Time 2), each time with participants’ baseline scores serving as a covariate.1 Whereas there were no main effects or interactions involving aggression cue, the effect of arousal condition was highly significant, both at Time 1, multivariate F(3, 67) = 13.14, p < .001, ηp2 = .37, and again at Time 2, multivariate F(3, 72) = 6.53, p = .001, ηp2 = .21, indicating that participants in the high arousal condition experienced a much greater increase in physiological arousal than did those in the low arousal condition, both at the beginning and at the end of the aggression task (see Table 1).
Table 1.
Mean increase in physiological measures from baseline at Time 1 and Time 2
| Condition | Time 1 | Time 2 | ||||
|---|---|---|---|---|---|---|
| Heart Rate (beats/min) | Systolic Blood Pressure (mm Hg) | Diastolic Blood Pressure (mm Hg) | Heart Rate (beats/min) | Systolic Blood Pressure (mm Hg) | Diastolic Blood Pressure (mm Hg) | |
| Low Arousal | 20.90 | 16.50 | 6.04 | 12.54 | 7.69 | 1.15 |
| SD | 19.40 | 22.70 | 11.39 | 24.00 | 15.14 | 13.75 |
| High Arousal | 60.64 | 21.20 | 10.03 | 31.73 | 14.76 | 2.48 |
| SD | 33.52 | 22.46 | 11.86 | 20.01 | 14.39 | 12.11 |
In addition, participants for whom promoting cues were designed to be salient reported those cues to be noticeable (M across 4 promoting cues = 4.85, SD = 1.77); a result paralleled by participants responding to inhibiting cues (M across 4 inhibiting cues = 4.44, SD = 1.75). In the promoting cues condition, 36 out of 39 participants (92%) rated at least one of the 4 promoting cues as 7 or higher on the 9-point scale assessing noticeabilty; and, in the inhibiting cues condition, 39 out of 42 participants (93%) rated at least one of the 4 inhibiting cues as 7 or higher. Indeed, the minimum rating given to the most noticeable of the 4 promoting cues was 4 out of 9 (a result provided by only 2 participants), and, to the most noticeable of the 4 inhibiting cues, 5 out of 9 (a result provided by 1 participant).
Participants in the promoting cues conditions also reported significantly more anger toward the confederate (M = 3.49, SD = 1.78) than did those in the inhibiting cues conditions (M = 2.43, SD = 1.68), t(79) = 2.75, p < .01. However, there were no main effects or interactions involving arousal on either the noticeability or anger measure, nor did treating the anger measure as a covariate influence the pattern of results for the primary dependent measure reported below.
Aggression
We made use of Anderson and Dill’s (2000) procedure for analyzing the reaction-time game data, as these researchers had employed a design similar to our own. In particular, participants’ responses on trials after which they had just lost the reaction time game to the confederate constituted our primary measure of aggression (i.e., 4 of the 8 trials). Following Carnagey and Anderson (2005), an aggressive energy score was calculated for each of these trials by multiplying a participant’s chosen level of intensity of noise by the square root of the noise duration and forming a composite score by averaging across the four trials. This approach has been shown to produce a valid measure of aggressive behavior (Baron & Bell, 1975; Bartholow, Anderson, Carnagey, & Benjamin, 2005). Participants’ aggressive energy scores on the practice trials following a loss, in essence a baseline measure of noise-blast behavior, served as a covariate. In addition, participants’ responses on the post-task questionnaire indicated some significant arousal-based differences in their ratings of the task. In order to control for these differences, we formed a composite measure of how stressful, tiring, difficult, and fun (reverse coded) they found the task (Cronbach’s alpha = .75), which served as a second covariate.
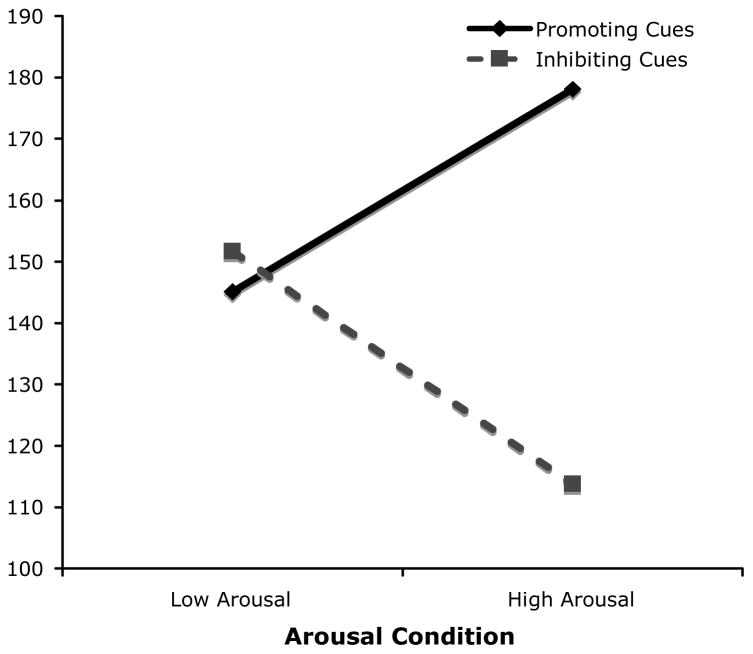
An Arousal × Aggression Cue analysis of covariance (ANCOVA; with practice trial performance and post-task ratings as covariates) revealed an expected main effect of cue condition, F(1, 74) = 4.38, p = .04, ηp2 = .05, with those in the aggression-promoting conditions behaving more aggressively than those in the aggression-inhibiting conditions, and, of greater interest, an interaction between arousal and cue condition, F(1, 74) = 7.26, p = .009, ηp2 = .09. 2 When cues promoted aggression, elevated arousal was associated with increased aggression, F(1, 74) = 3.91, p = .052, a result that was consistent with both past research and the predictions of our model. By contrast, when cues inhibited aggression, increased arousal served to decrease aggression, a finding unique to the experiment reported here, F(1, 74) = 7.76, p = .019. 3 In particular, participants experiencing high arousal in the aggression-promoting condition (n = 15) engaged in higher levels of aggression (M = 205.72, SD = 86.02; adjusted M = 178.12) than did those experiencing low arousal (n = 23; M =152.70, SD= 74.27; adjusted M = 145.11). By contrast, participants experiencing high arousal in the aggression-inhibiting condition (n = 17) engaged in lower levels of aggression (M = 112.75, SD = 61.99; adjusted M = 113.76) than did those experiencing low arousal (n = 25; M = 128.82, SD = 72.92; adjusted M = 151.67; see Figure 1).
Figure 1.
Mean level of aggression as a function of arousal and cue condition (note: means are adjusted for relevant covariates).
Mood
An Arousal × Cue ANOVA conducted on the negative mood measure (the ten negative affect items plus the four anger-hostility items) revealed only a main effect of arousal, F(1, 77) = 8.21, p < .01. Participants in the high arousal condition reported more negative mood (M = 2.61, SD = 1.30) than did those in the low arousal condition (M = 1.89, SD .94). However, the introduction of mood as a covariate into our analysis of the aggression data did not alter the pattern of results reported above, suggesting that the primary results reported here were not mediated by changes in mood.
Discussion
The present set of results offers support for the attentional myopia model, confirming its prediction that aroused individuals would behave more in line with highly salient situational cues than would non-aroused individuals. In particular, this study suggests that elevated arousal can either increase or decrease aggression, depending on whether salient situational cues promote or inhibit aggression, respectively.
The finding that high exercise-induced arousal can sometimes act as a potent inhibitory force may initially seem to conflict with “real-world” observations, in which it is common for arousal to be associated with aggression. For example, after a chase, a police officer may use excessive physical violence to subdue a suspect, or during a heated sports contest a fight may break out between athletic teams. In such situations, however, aggression-promoting cues are likely to be both more common and more salient than aggression-inhibiting cues. In fact, because provocations to anger are themselves arousing, many real-life situations naturally involve both high arousal and strong cues provoking aggression. A baseball player, for instance, may become angry with a pitcher after being hit by a pitch, an incident that would both elevate arousal and serve as a highly salient aggression-promoting cue for the aroused athlete. In situations like these, inhibiting cues, such as a moral commitment to good sportsmanship or a vague threat of punishment for fighting, are likely to be less salient and therefore to fall under the radar of the aroused individual.
Instances of aggression are rarely observed, however, in other situations in which people are aroused. When performing yoga, for example, people are typically physiologically aroused but unlikely to become aggressive. In fact, according to the attentional myopia model, participants may feel especially peaceful during a yoga class because they are aroused but surrounded by aggression-inhibiting cues. Similarly, classic research on anger-management treatments has found that rehearsal of inhibiting strategies can be as effective as combining such strategies with relaxation (Novaco, 1976). Such a finding suggests that even when physiologically aroused, anger-prone individuals can inhibit their anger if they can attend to potent restraining cues. In sum, the lesson for those seeking to control aggressive behavior, whether it be their own or that of another, is that the age-old advice of taking time to “cool off” during an interpersonal conflict may not always be necessary to deter an act of aggression (indeed, such advice may be counterproductive, especially with men, who may use the time to seethe [Zillmann, 1993]). Instead, the introduction of salient inhibitory cues into a heated conflict situation may be sufficient to prevent aroused individuals from behaving aggressively. At the same time, individuals who wish to control their own aggressive impulses may be well-served to rehearse, and thereby render automatic, strong cues to inhibit aggression cues that they can draw upon the next time they find themselves in highly arousing circumstances (see Gollwitzer, 1999).
Limitations
It is important to note that the findings reported in these experiments confirmed the predictions of the attentional myopia model for male participants only. The selection of males as research participants seemed appropriate in light of the fact that a divergence in aggressive tendencies is among the most consistently documented psychological sex differences in both laboratory and real world environments (Maccoby & Jacklin, 1974). Nonetheless, future research will help determine whether females behave according to the predictions of the model in similar situations and/or those involving other forms of aggression (e.g., verbal or “relational” aggression [Dodge & Schwartz, 1997]), as they have in other behavioral domains (Mann & Ward, 2004; Ward & Mann, 2000).
Although participants in this study reported noticing cues that had been designed to be salient, we made no attempt to assess participants’ perception of the level of influence of those cues on their relevant behavior. Given individuals’ notorious failure to accurately self-report similar phenomena (Nisbett & Wilson, 1977), we suspect that such a measure would be less than revealing. As we have stated elsewhere, “Expecting participants to notice cues designed to be salient is one thing; expecting them to accurately intuit the degree of influence those cues exerted on their behavior is something else entirely” (Parent, Ward, & Mann, 2007, p. 164). However, in theory such a result, were it to be found, could provide additional mediational-type evidence for the hypothesized role played by relevant cues on aggressive behavior—cues that, our results suggest, operate though cognitive-attentional channels, rather than through an affective or mood-based route. At the very least, future research might ask participants to indicate how noticeable they found cues deliberately designed to not be salient (which, according to our model, should be less noticeable under conditions of high arousal than under low arousal) as a means of confirming the attentional narrowing postulated to underlie the effects documented here.
Finally, it is important to acknowledge the somewhat artificial nature of the study reported here, which relied on arousal induced through exercise and did not involve physical contact between individuals. Future research will determine whether other forms of arousal and/or aggression are linked in the same manner as reported here. Nonetheless, although the only aggression performed by our participants was accomplished through the administration of noxious noise, and strong arousal was induced through the use of a stairclimber, we believe that the results speak to instances of real-world violence by aggressors experiencing heightened arousal.
Implications
Additional experiments might also shed light on the specific physiological and even neurological mechanisms underlying attentional myopia effects, an approach that holds great promise in light of recent neuroscientific theorizing with regard to attention (Posner, 2004). At this point, what is clear is that appropriate cues can serve not only to provoke physiologically aroused individuals into acting aggressively; they can also result in a reduction in aggression among aroused individuals—a finding with potentially significant implications for those committed to reducing aggression in our society.
The results reported here also contribute to a growing body of literature suggesting that whether one observes excessively inhibited or disinhibited behavior performed by a particular individual depends on the cognitive-attentional state of that individual and the relative balance of appropriate internal and external cues, such as cues motivating eating versus dieting (Mann & Ward, 2004; Ward & Mann, 2000); safe versus unsafe sexual behavior (MacDonald et al., 2000); or what Metcalfe and Mischel (1999) have termed “hot” versus “cold” pressures (see Parent et al., 2007). At the same time, classic research documenting “misattribution of arousal” effects (e.g., Schachter & Singer, 1962) may be properly reconceptualized as the effects of narrowed attention in the presence of salient emotionally-relevant cues (see Mann & Ward, 2007). Finally, although research dating back 100 years has argued that arousal can enhance dominant responses (Yerkes & Dodson, 1908), the findings detailed in this article suggest that what counts as a “dominant response” can entail more than habitual or well-practiced behaviors (Hull, 1943; Spence, 1956; Zajonc, 1965). If appropriate cues are sufficiently salient, then arousal can be associated with a relatively unusual or novel response, namely, enhanced inhibition of aggression in the face of a provocateur.
Acknowledgments
This research was supported by grant MH63795 from the National Institutes of Mental Health (T.M. & A.W.); a Swarthmore College Faculty Research grant and sabbatical funds from the Solomon Asch Center for Study of Ethnopolitical Conflict (A.W.); a University of California Council on Research Grant (T.M.); and a Joel Dean Summer Research Grant from Swarthmore College (J.P.E.). We acknowledge the efforts of our anger-inducing confederates Hector Garcia, John Sistona, Ashot Tadevosian, and Jeffrey Wright, and our talented experimenters Theresa Nguyen, Madelynn Diaz, and Rachelle Flores. We would also like to thank Lyle Brenner and Jennifer Smith, who, as always, provided invaluable statistical assistance; Wendy Mendes and Robert Levenson, who provided advice on analyzing physiological data; and Jean Twenge, Brad Bushman, and Frank Durgin, who furnished us with key materials and equipment.
Footnotes
Five participants were excluded from one of the SA analyses because the heArt rate or blood pressure equipment failed to record.
This result remained statistically significant without the addition of the second (task-ratings) covariate (note that one participant failed to complete measures assessing this covariate, resulting in the loss of one degree of freedom in the relevant analyses). In addition, regression analyses that included predictor variables representing interactions between each of the two covariates and each independent variable failed to yield significant results (suggesting the homogeneity of regression assumption for covariance had been met), and inclusion of the relevant interaction terms failed to alter the significance levels of any of the analyses of covariance results reported here (see Yzerbyt, Muller, & Judd, 2004).
This result remained significant with the addition of data from the four omitted “outlier” participants.
Contributor Information
Andrew Ward, Swarthmore College.
Traci Mann, UCLA.
Erika H. Westling, UCLA
J. David Creswell, UCLA.
Jeffrey P. Ebert, Swarthmore College
Matthew Wallaert, Swarthmore College.
References
- Adam JJ, Teeken JC, Ypelaar PJC, Verstappen FTJ, Paas FGW. Exercise-induced arousal and information processing. International Journal of Sport Psychology. 1997;28:217–226. [Google Scholar]
- Anderson CA, Anderson KB, Deuser WE. Examining an affective aggression framework: Weapon and temperature effects. Personality and Social Psychology Bulletin. 1996;22:366–376. [Google Scholar]
- Anderson CA, Bushman BJ. Human aggression. Annual Review of Psychology. 2002;53:27–51. doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Dill KE. Video games and aggressive thoughts, feelings, and behavior in the laboratory and in life. Journal of Personality and Social Psychology. 2000;78:772–790. doi: 10.1037//0022-3514.78.4.772. [DOI] [PubMed] [Google Scholar]
- Ariely D, Loewenstein G. The heat of the moment: The effect of sexual arousal on sexual decision making. Journal of Behevioral Decision Making. 2006;19:87–98. [Google Scholar]
- Bandura A. Aggression: A social leaning analysis. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Barclay AM. Linking sexual arousal and aggressive motives: Contributions of “irrelevant” arousal. Journal of Personality. 1971;39:481–492. doi: 10.1111/j.1467-6494.1971.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Baron RA. Magnitude of victim’s pain cues and level of prior anger arousal as determinants of adult aggressive behavior. Journal of Personality and Social Psychology. 1971;17:236–243. doi: 10.1037/h0030595. [DOI] [PubMed] [Google Scholar]
- Baron RA. The aggression-inhibiting influence of heightened sexual arousal. Journal of Personality and Social Psychology. 1974;30:318–322. doi: 10.1037/h0036887. [DOI] [PubMed] [Google Scholar]
- Baron RA, Bell PA. Aggression and heat: Mediating effects of prior provocation and exposure to an aggressive model. Journal of Personality and Social Psychology. 1975;31:825–832. doi: 10.1037/h0076647. [DOI] [PubMed] [Google Scholar]
- Baron RS. Arousal, capacity, and intense indoctrination. Personality and Social Psychology Review. 2000;4:238–254. [Google Scholar]
- Bartholow BD, Anderson CA, Carnagey NL, Benjamin AJ. Interactive effects of life experience and situational cues on aggression: The weapons priming effect in hunters and non-hunters. Journal of Experimental Social Psychology. 2005;41:48–60. [Google Scholar]
- Berkowitz L. The contagion of violence: An S-R mediational analysis of some effects of observed aggression. In: Arnold WJ, Page MM, editors. Nebraska Symposium on Motivation. Vol. 18. Lincoln, NE: University of Nebraska Press; 1970. pp. 95–135. [Google Scholar]
- Berkowitz L. Aggression: Its causes, consequences, and control. Philadelphia: Temple University Press; 1993. [Google Scholar]
- Bodenhausen GV. Emotions, arousal, and stereotypic judgments: A heuristic model of affect and stereotyping. In: Mackie DM, Hamilton DL, editors. Affect, cognition, and stereotyping. New York: Academic Press; 1993. pp. 13–37. [Google Scholar]
- Bushman BJ, Baumeister RF, Stack AD. Catharsis, aggression, and persuasive influence: Self-fulfilling or self-defeating prophecies? Journal of Personality and Social Psychology. 1999;76:367–376. doi: 10.1037//0022-3514.76.3.367. [DOI] [PubMed] [Google Scholar]
- Carnagey NL, Anderson CA. The effects of reward and punishment in violent video games on aggressive affect, cognition, and behavior. Psychological Science. 2005;16:882–889. doi: 10.1111/j.1467-9280.2005.01632.x. [DOI] [PubMed] [Google Scholar]
- Chajut E, Algom D. Selective attention improves under stress: Implications for theories of social cognition. Journal of Personality and Social Psychology. 2003;85:231–248. doi: 10.1037/0022-3514.85.2.231. [DOI] [PubMed] [Google Scholar]
- Cohen D, Nisbett RE, Bowdle B, Schwarz N. Insult, aggression, and the Southern culture of honor: An “experimental ethnography. Journal of Personality and Social Psychology. 1996;70:945–960. doi: 10.1037//0022-3514.70.5.945. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Schwartz D. Social information-processing mechanisms in aggressive behavior. In: Stoff DM, Breling J, Maser JD, editors. Handbook of antisocial behavior. New York: Wiley; 1997. pp. 171–180. [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Eysenck MW. Attention and arousal: Cognition and performance. Berlin: Springer; 1982. [Google Scholar]
- Giancola PR, Corman MD. Alcohol and aggression: A test of the attention-allocation model. Psychological Science. 2007;18:649–655. doi: 10.1111/j.1467-9280.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Zeichner A. Construct validity of a competitive reaction-time aggression paradigm. Aggressive Behavior. 1995;21:199–204. [Google Scholar]
- Gladwell M. Blink. New York: Little, Brown & Company; [Google Scholar]
- Geen RG, O’Neal EC. Activation of cue-elicited aggression by general arousal. Journal of Personality and Social Psychology. 1969;11:289–292. doi: 10.1037/h0026885. [DOI] [PubMed] [Google Scholar]
- Gollwitzer PM. Implementation intentions: Strong effects of simple plans. American Psychologist. 1999;54:493–503. [Google Scholar]
- Granholm E, Asarnow RF, Marder SR. Display visual angle and attentional scanpaths on the Span of Apprehension task in schizophrenia. Journal of Abnormal Psychology. 1996;105:17–24. doi: 10.1037//0021-843x.105.1.17. [DOI] [PubMed] [Google Scholar]
- Hanoch Y, Vitouch O. When less is more: Information, emotional arousal and the ecological reframing of the Yerkes-Dodson law. Theory and Psychology. 2004;14:427–452. [Google Scholar]
- Hebb DO. Drives and the C.N.S. (Conceptual Nervous System) Psychological Review. 1955;62:243–254. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- Hull CL. Principles of behavior. New York: Appleton-Century-Crofts; 1943. [Google Scholar]
- Humphreys MS, Revelle W. Personality, motivation, and performance: A theory of the relationship between individual differences and information processing. Psychological Review. 1984;91:153–184. [PubMed] [Google Scholar]
- Jaffee Y, Malamuth N, Feingold J, Feshbach S. Sexual arousal and behavioral aggression. Journal of Personality and Social Psychology. 1974;30:759–764. doi: 10.1037/h0037526. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and Effort. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Klauer KC, Teige-Mocigemba ST. Controllability and resource dependence in automatic evaluation. Journal of Experimental Social Psychology. 2007;43:648–655. [Google Scholar]
- Leonard KE. The impact of explicit aggressive and implicit nonaggressive cues on aggression in intoxicated and sober males. Personality and Social Psychology Bulletin. 1989;15:390–400. [Google Scholar]
- Lindsay JJ, Anderson CA. From antecedents to violent actions: A general affective aggression model. Personality and Social Psychology Bulletin. 2000;26:533–547. [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct disorders: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Maccoby EE, Jacklin CN. Psychology of Sex Differences. Stanford, CA: Stanford University Press; 1974. [Google Scholar]
- MacDonald TK, Fong GT, Zanna MP, Martineau AM. Alcohol myopia and condom use: Can alcohol intoxication be associated with more prudent behavior? Journal of Personality & Social Psychology. 2000;78:605–619. doi: 10.1037//0022-3514.78.4.605. [DOI] [PubMed] [Google Scholar]
- Mandler G. Mind and emotion. New York: Wiley; 1975. [Google Scholar]
- Mann T, Ward A. To eat or not to eat: Implications of the attentional myopia model for restrained eaters. Journal of Abnormal Psychology. 2004;113:90–98. doi: 10.1037/0021-843X.113.1.90. [DOI] [PubMed] [Google Scholar]
- Mann T, Ward A. Attention, self-control, and health behaviors. Current Directions in Psychological Science. 2007;16:280–283. [Google Scholar]
- McNaughton N, Gray JA. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. Journal of Affective Disorders. 2000;61:161–176. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Wilson TD. Telling more than we can know: Verbal reports on mental processes. Psychological Review. 1977;84:231–259. [Google Scholar]
- Novaco RW. Treatment of chronic anger through cognitive and relaxation controls. Journal of Consulting and Clinical Psychology. 1976;44:681. doi: 10.1037//0022-006x.44.4.681. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: A meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Parent S, Ward A, Mann T. Health information processed under limited attention: Is it better to be “hot” or “cool”? Health Psychology. 2007;26:159–164. doi: 10.1037/0278-6133.26.2.159. [DOI] [PubMed] [Google Scholar]
- Paulhus DL, Lim DTK. Arousal and evaluative extremity in social judgments: A dynamic complexity model. European Journal of Social Psychology. 1994;24:89–99. [Google Scholar]
- Posner MI, editor. Cognitive neuroscience of attention. New York: Guilford Press; 2004. [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biological Psychology. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Rule BG, Nesdale AR. Emotional arousal and aggressive behavior. Psychological Bulletin. 1976;83:851–863. [PubMed] [Google Scholar]
- Rusting CL, Nolen-Hoseksema S. Regulating responses to anger: Effects of rumination and distraction on angry mood. Journal of Personality and Social Psychology. 74:790–803. doi: 10.1037//0022-3514.74.3.790. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer JE. Cognitive, social and physiological determinants of emotional state. Psychological Review. 1962;69:379–99. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Smit AS, Eling PATM, Hopman MT, Coenen AML. Mental and physical effort affect vigilance differently. International Journal of Psychophysiology. 2005;57:211–217. doi: 10.1016/j.ijpsycho.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Spence KW. Behavior theory and conditioning. New Haven: Yale University Press; 1956. [Google Scholar]
- Steele CM, Josephs RA. Alcohol myopia: Its prized and dangerous effects. American Psychologist. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- Taylor SP. Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. Journal of Personality. 1967;35:297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Thayer RE. The biopsychology of mood and arousal. New York: Oxford University Press; 1989. [Google Scholar]
- Tomporowski PD, Ellis NR. Effects of exercise on cognitive processes: A review. Psychological Bulletin. 1986;99:338–346. [Google Scholar]
- Ward A, Mann T. Don’t mind if I do: Disinhibited eating under cognitive load. Journal of Personality and Social Psychology. 2000;78:753–763. doi: 10.1037//0022-3514.78.4.753. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative and Neurological Psychology. 1908;18:459–482. [Google Scholar]
- Yzerbyt VY, Muller D, Judd CM. Adjusting researchers’ approach to adjustment: On the use of covariates when testing interactions. Journal of Experimental Social Psychology. 2004;40:424–431. [Google Scholar]
- Zajonc RB. Social Facilitation. Science. 1965;149:269–274. doi: 10.1126/science.149.3681.269. [DOI] [PubMed] [Google Scholar]
- Zillmann D. Cognitive-excitation interdependencies in aggressive behavior. Aggressive Behavior. 1988;14:51–64. [Google Scholar]
- Zillmann D. Mental control of angry aggression. In: Wegner DM, Pennebaker JW, editors. Handbook of mental control. Englewood Cliffs, NJ: Prentice Hall; 1993. [Google Scholar]
- Zillmann D, Bryant J. Effects of residual excitation on the emotional response to provocation and delayed aggressive behavior. Journal of Personality and Social Psychology. 1974;30:782–791. doi: 10.1037/h0037541. [DOI] [PubMed] [Google Scholar]
- Zillmann D, Bryant J, Cantor JR, Day KD. Irrelevance of mitigating circumstances in retaliatory behavior at high levels of excitation. Journal of Research in Personality. 1975;9:282–293. [Google Scholar]
- Zillmann D, Johnson RC, Day KD. Attribution of apparent arousal and proficiency of recovery from sympathetic activation affecting excitation transfer to aggressive behavior. Journal of Experimental Social Psychology. 1974;10:503–515. [Google Scholar]