Abstract
Rationale
Recent studies have raised the possibility that antagonists of H3 histamine receptors possess cognitive-enhancing and antipsychotic properties. However, little work has assessed these compounds in classic animal models of schizophrenia.
Objectives
The purpose of this study was to determine if a prototypical H3 antagonist, thioperamide, could alter behavioral deficits caused by the NMDA receptor antagonist, MK-801, in adult male rats. MK-801 was chosen for study since it produces a state of NMDA receptor hypofunction in rats that may be analogous to the one hypothesized to occur in schizophrenia.
Methods
The interaction between thioperamide and MK-801 was measured in three behavioral tests: locomotor activity, prepulse inhibition (PPI), and delayed spatial alternation. In each test, rats received a subcutaneous injection of saline or thioperamide (3.0 & 10 mg/kg) followed 20 minutes later by a subcutaneous injection of saline or MK-801 (0.05, 0.10, & 0.30 mg/kg).
Results
Locomotor activity was significantly elevated by MK-801 in a dose-dependent manner. Thioperamide pretreatment alone did not alter locomotor activity, however its impact on MK-801 was dose-dependent. Each thioperamide dose enhanced the effects of two lower doses of MK801 but reduced the effect of a higher MK-801 dose. Clear deficits in PPI and delayed spatial alternation were produced by MK-801 treatment, but neither impairment was significantly modified by thioperamide pretreatment.
Conclusions
H3 receptors modulate responses to NMDA antagonists in behaviorally-specific ways and dependent upon the level of NMDA receptor blockade.
Keywords: Antipsychotic, cognition, locomotor activity, prepulse inhibition, spatial working memory, NMDA receptor
Introduction
The H3-type histamine receptor is one of four G-coupled receptors that serve as a target for the neurotransmitter, histamine (Hancock 2006). The H3 receptor is found on presynaptic terminals in the brain and acts as an autoreceptor to control histamine release (Schwartz et al., 2003). This receptor appears to be constitutively active in the absence of ligand (Schwartz et al., 2003), suggesting that antagonists act as inverse agonists at this site. The imidazole drugs, thioperamide and ciproxifan, are considered the prototypical antagonists at this receptor (Schwartz et al., 2003; Fox et al., 2003; Fox et al., 2005). H3 receptor mRNA expression is fairly high throughout the telencephalon and diencephalon, but actual receptor binding is greatest in the deep layers of cortex, amygdala, and pallidum (Pillot et al., 2002). The physiological function of the H3 receptor is still being determined. Receptor antagonists increase histamine turnover in cortical synpatosomes, an effect mimicked by the atypical antipsychotic drugs, clozapine and risperidone (Morriset et al., 1999). Moreover, H3 receptor antagonists have been shown to stimulate acetylcholine release in slices of entorhinal cortex (Clapham & Kilpatrick 1992) and in the cortex of freely moving rats (Blandina et al., 1996). A more recent study by Fox et al. (2005) demonstrated that a newer H3 receptor antagonist, ABT-239, elevates acetylcholine release in the prefrontal cortex and hippocampus, and dopamine release in the prefrontal cortex.
Many behavioral studies of H3 antagonists have focused on their potential as cognitive enhancers, in addition to assessing their possible antipsychotic and anti-obesity properties (Schwartz et al., 2003; Hancock 2006). Several studies have suggested that H3 antagonists, including thioperamide and ciproxifan, may improve attention, inhibitory avoidance, and social and spatial memory (Flood et al., 1998; Ligneau et al., 1998; Fox et al. 2003; 2005). Moreover, thioperamide has been shown to reduce the memory-impairing effects of the muscarinic antagonist, scopolamine, in a T-maze task (Orsetti et al., 2001) and similar effects of the NMDA receptor antagonist, MK-801, on inhibitory avoidance behavior (Bernearts et al., 2004). Additional preclinical testing has suggested the utility of H3 antagonists as treatments for schizophrenia. For example, H3 antagonists have been reported to reverse the hyperlocomotion induced by amphetamine and methamphetamine (Clapham & Kilpatrick 1994; Morisset et al., 2002; Fox et al., 2005). Deficits in prepulse inhibition (PPI) that are observed in DBA/2 mice can also be ameliorated by H3 receptor antagonists (Browman et al., 2004; Fox et al., 2005)
One of the better-established animal models of schizophrenia is the NMDA hypofunction model. This model is based on observations that NMDA antagonists, such as ketamine and phencyclidine, can cause a profile of behavioral changes that are similar to the positive, negative, and cognitive symptoms of schizophrenia (see Farber et al., 1998 for review; Newcomer et al., 1999). When studied in animals, NMDA antagonists cause a constellation of behavioral changes including hyperactivity, deficits in PPI, and memory impairment (Jacobs et al., 2000; Bakshi & Geyer, 1998; Bardgett et al., 2008), that are, in some cases, sensitive to remediation by antipsychotic drugs (see Bardgett 2004 for review). The similarities between the behavioral phenotype produced by NMDA antagonists in humans and animals, and the sensitivity of the phenotype in animals to antipsychotic drugs strongly suggest that the NMDA hypofunction model serves as a valid preclinical assay for drugs with antipsychotic and cognitive enhancing activity.
The purpose of this study was to determine if a prototypical H3 antagonist, thioperamide, could alleviate the behavioral deficits observed in rats after treatment with the NMDA antagonist, MK-801. Several studies (Bernaerts et al., 2004; Huang et al., 2004; Faucard et al., 2006; Ligneau et al., 2007) have assessed the effects of H3 antagonists on behavioral changes produced by NMDA antagonists, yet additional characterization of the interaction between H3 and NMDA receptors at a behavioral level is needed. In the present study, doses of thioperamide (3 & 10 mg/kg) were chosen based upon their ability to maximally increase histamine release in the brain (Ligneau et al., 1998), improve learned avoidance behavior in rat pups (Fox et al., 2003), decrease amphetamine-induced activity in mice (Clapham & Kilpatrick, 1994), and improve spatial memory (Komater et al., 2005). The doses of MK-801 chosen for study were based on their effects in previous locomotor and memory studies performed in our lab (Jacobs et al., 2000; Bardgett et al., 2008), although a higher dose of MK-801 was chosen for a second locomotor experiment since previous work (Faucard et al., 2006; Ligneau et al., 2007) investigated the effects of H3 antagonists on activity produced by this dose. Locomotor activity, prepulse inhibition, and delayed spatial alternation were chosen for study since the effects of NMDA antagonists on these behaviors are well-known (Bakshi & Geyer, 1998; Jacobs et al., 2000; Bardgett et al., 2008) and because the latter two behaviors are clearly altered in schizophrenia (see Bardgett 2004 for review).
Method
Subjects
Forty-nine adult male Long-Evans rats (200–225 grams) were purchased from Harlan Bioproducts (Indianapolis, IN). The rats were group-housed three per cage with free access to food and water except where noted. Lighting in the animal colony was maintained on a 12-hour light/dark schedule with lights on at 07:00. All experimental procedures were performed according to the Current Guide for the Care and Use of Laboratory Animals (USPHS) under a protocol approved by the Northern Kentucky University Institutional Animal Use and Care Committee.
Drugs and treatment procedure
Thioperamide was kindly provided by the National Institute of Mental Health’s Chemical Synthesis and Drug Supply program, and MK-801 was purchased from Sigma (St. Louis, MO). Doses of 3.0 and 10.0 mg/kg of thioperamide and 0.05 and 0.10 mg/kg of MK-801 were used in each experiment, except for the second locomotor experiment when a dose of 0.30 mg/kg of MK-801 was used. In each experiment, thioperamide or saline was injected 40 minutes prior to testing, and MK-801 or saline was injected 20 minutes prior to testing, except in the locomotor testing when activity was recorded for two hours immediately after the second injection. All injections were administered subcutaneously. In each experiment, each rat received all nine possible pretreatment/treatment combinations. All testing occurred with at least a 48-hour interval between treatments. A different set of rats was used for the first (n = 12) and second locomotor (n = 12), sensorimotor gating (n = 12), and delayed alternation tests (n = 13).
Locomotor testing
Clear polypropylene cages that measured 25.9 cm wide × 47.6 cm long × 20.9 cm high were used to test locomotor activity. These cages were filled with wood chips to cover the bottom and were placed in Hamilton-Kinder (Poway, CA) Smart-Frame photocell-based, activity-monitoring racks. Rats were placed in the test cages for two hours a day for three consecutive days prior to any drug testing. The overhead lights were off during testing. On drug test days, the rats were placed in the testing cages immediately after injection of MK-801 or saline. The number of consecutive photobeam breaks was recorded every ten minutes for two hours, and this data was used to compare the effects of each pretreatment-treatment condition.
A second locomotor activity experiment was conducted after the one described above in order to ascertain the effects of thioperamide on an even higher dose of MK-801 (0.3 mg/kg). A separate group of 12 rats was used for this study. In this experiment, rats received pretreatment with saline, 3.0 mg/kg of thioperamide, or 10.0 mg/kg of thioperamide followed twenty minutes later by treatment with 0.3 mg/kg of MK-801 or saline. All other procedures were identical to the first experiment.
Sensorimotor gating
A Hamilton-Kinder Startle Monitor (Hamilton-Kinder, Poway, CA) system was used to test sensorimotor gating after drug treatment. Prior to any drug testing, animals were first exposed to a baseline session of predominantly startle trials and two additional sessions of predominantly PPI trials. This first session consisted a five-minute acclimation period in which the rat was exposed to continuous 70 dB background noise. The rat was then exposed to, in random order, seventeen startle trials (40 ms 120 dB tone), five prepulse inhibition (PPI) trials (20 ms tones that were 12 dB above background followed 80 ms later by a startle tone), and three no startle trials. The average intertrial interval was 15 seconds. In the next two sessions, held on the next two consecutive days, rats were acclimated as in the first baseline session and then exposed to four consecutive startle trials. Following these trials, rats were given two blocks of testing that included five random exposures to each of the following trial types within each testing block: startle, no startle, 5 dB PPI, 10 dB PPI, and 15 dB PPI. The last testing block was followed by four startle trials. In each trial, response to startle stimuli was measured in newtons for 250 ms after the presentation of the last stimulus and averaged across that time. Data from the first and last four startle trials were not included in the data analysis. On drug testing days, animals were tested using this procedure 20 minutes after drug treatment.
For data analyses, startle was defined as the response on the startle trials minus the response on the no startle trials. The percentage of prepulse inhibition was calculated as follows: 100 × (1 - (response on the trials at a specific PPI level (e.g. 5 dB PPI) - response on the no startle trials/response on the startle trials - the response on the no startle trials)).
Discrete-trials rewarded delayed spatial alternation
The T-maze used in these experiments was constructed of wire-mesh and wood, and painted black. It contained walls that measured 10 cm in height and 9 cm in width. The stem was 40 cm long, each arm was 45 cm long, and the entire maze had a wire mesh bottom. One week prior to habituation and testing, all rats were placed on a food-restricted diet and reduced to ~90% of their free-feeding weight. During habituation, all rats were placed on the T-maze until they ate two pieces of food or 90 seconds had elapsed. This was repeated three times a day with a five-minute intertrial interval for four days. During testing, rats received six trials a day with a five-minute intertrial interval. Each test trial consisted of two runs, a forced run and a free run. On the forced run, rats were forced to obtain a piece of food from one goal arm of the T-maze. Goal arm entries were defined as placing four paws in the arm. Ten or 40 seconds after the end of the forced run, the free run was conducted. At the beginning of the free run, the rats were returned to the start arm and allowed to choose either goal arm. If the rats returned to the same arm that they were forced into on the previous run, they received no food reward. If the rats chose the opposite arm, they received a food reward. Rats were given three 10-second and three 40-second delay trials during each day of testing, with three forced runs to the right arm and three to the left arm. The sequence of delays and forced run food locations (left or right) were randomized each day, with the stipulation that the same delay or the same forced arm location could not be used for three trials in a row. All animals were tested for at least one week before drug testing began. The number of correct choices at each delay under each pretreatment-treatment condition was used in the data analysis.
Data analysis
For the locomotor data, an overall three-way analysis of variance (ANOVA) was conducted that compared the effects of time (as a repeated measure), pretreatment (saline, 3.0, and 10.0 mg/kg of thioperamide), and treatment (saline, 0.05, and 0.1 mg/kg of MK-801) on the number of photobeam breaks made every ten minutes. In the sensorimotor and delayed alternation experiments, each dependent measure, as defined above, was analyzed using a two-way ANOVA with pretreatment and treatment serving as independent variables. Significant differences were accepted if p < .05 (two-tailed).
Results
Effects of thioperamide on hyperactivity induced by MK-801
The first locomotor activity experiment tested the interaction between pretreatment with two doses of thioperamide (3.0 & 10.0 mg/kg) and treatment with two doses of MK-801 (0.05 & 0.10 mg/kg). A three-way ANOVA revealed significant pretreatment × treatment (F(4, 99) = 3.1, p < .02), pretreatment × time (F(22, 1089) = 5.2, p < .0001), and treatment × time (F(22, 1089) = 17.2, p < .0001) interactions, as well as significant main effects for each independent variable (Pretreatment effect: F(2, 99) = 10.2, p < .0001; Treatment effect: F(2, 99) = 131.3, p < .0001; Time effect: F(11, 1089) = 10.9, p < .001) on levels of activity every ten minutes. Treatment with 0.05 and 0.1 mg/kg of MK-801 caused a dose-dependent increase in locomotor activity over a two-hour period (Figure 1B & C). The 0.05 mg/kg dose elevated locomotor activity more than two-fold over the amount of activity observed after saline injection, whereas the 0.1 mg/kg dose produced a four-fold increase in comparison to saline treatment.
Figure 1.
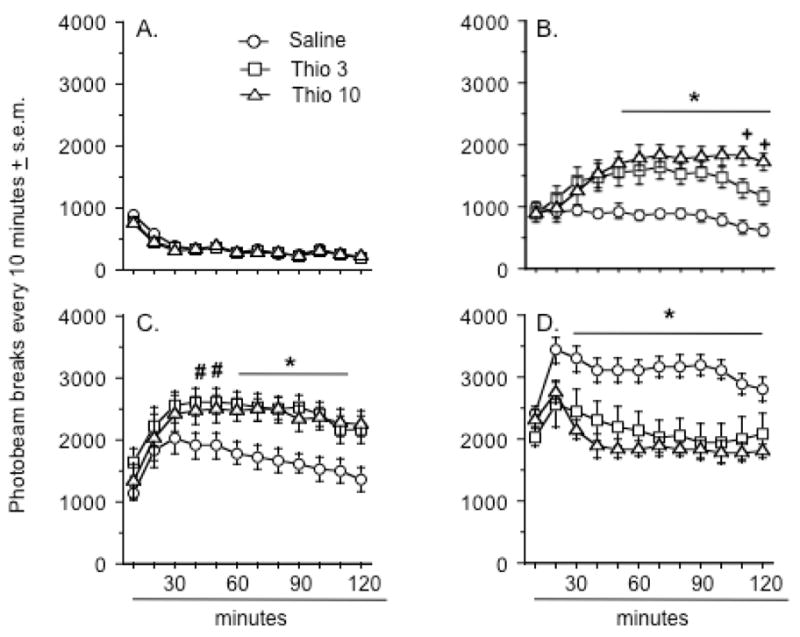
Effects of thioperamide pretreatment on locomotor activity after A.) saline treatment, B.) MK-801 0.05 mg/kg, C.) MK-801 0.1 mg/kg., and D.) MK-801 0.3 mg/kg. An asterisk (*) indicates a significant difference between rats pretreated with either dose of thioperamide and those pretreated with saline. In B., the plus sign (+) indicates a significant difference between all three pretreatment groups. In C., the number sign (#) indicates a significant difference only between the groups pretreated with saline and 10 mg/kg of thioperamide. Data represent mean number of photobeam breaks every 10 minutes ± s.e.m. There were 12 animals in each group although a separate cohort of 12 rats was used to generate the data depicted in D.
Thioperamide pretreatment in the absence of MK-801 treatment did not significantly alter locomotor activity (Figure 1A), although there was a significant decrease in activity over time in all rats treated with saline (Time effect: F(11, 363) = 40.5, p < .0001).
Each thioperamide dose significantly enhanced the effect of the low MK-801 dose on activity (Pretreatment × time interaction: F(22, 363) = 6.7, p < .0001) (Figure 1B). The enhancing effect of thioperamide emerged at approximately 40 minutes after MK-801 injection (Figure 1B). At approximately 110 and 120 minutes after MK-801 injection, the activity levels of the rats pretreated with the 3.0 mg/kg dose of thioperamide remained significantly higher than the levels seen in the saline pretreated rats, but were significantly lower that the levels observed in the rats pretreated with 10 mg/kg of thioperamide.
In the case of the 0.1 mg/kg dose of MK-801, pretreatment with thioperamide elevated the effects of this dose on activity (Pretreatment effect: F(2, 33) = 4.5, p < .02) (Figure 1C). The higher dose of thioperamide significantly enhanced the MK-801 effect beginning approximately 40 minutes post-treatment, while the lower thioperamide dose began to significantly enhance the MK-801 effect at 60 minutes post-treatment. There were no differences between the thioperamide groups at the end of testing with the high dose of MK-801.
Because other studies (Faucard et al., 2006; Ligneau et al., 2007) have tested the interaction between H3 antagonists and even higher doses of MK-801, a second experiment was conducted to determine if thioperamide affected locomotor responses to a 0.3 mg/kg dose of MK-801. Using a different cohort of 12 rats, it was determined that each dose of thioperamide reduced the activity produced by a high dose of MK-801 (Pretreatment effect: F(2, 33) = 8.6, p < .001)(Figure 1D). The suppressive effect of thioperamide was statistically significant beginning at 30 minutes post-MK-801 treatment and lasted for the remainder of the testing period. Informal observations of rats pretreated with thioperamide and MK-801 noted that these rats demonstrated a constant, ataxic-like or uncoordinated forward “swimming” behavior, marked by constant movement on the rat’s stomach, flailing of the limbs, and occasional rolling of the body.
Effects of thioperamide on the enhanced startle and PPI deficits produced by MK-801
The same doses of MK-801 and thioperamide were used to assess the effects of each drug on auditory startle and PPI. There were no significant drug effects on activity during the “No Startle” trials (Figure 2A). MK-801 significantly increased startle responses regardless of dose or thioperamide pretreatment (Treatment effect: F(2, 66) = 101.6, p < .0001)(Figure 2B). PPI appeared to be increased as a function of prepulse intensity (Figure 3). MK-801 significantly decreased PPI at each prepulse intensity (Treatment effect: F(2, 66) = 93.2, 160.3, & 151.9, p < .0001 for 5, 10, & 15 dB prepulse intensities, respectively)(Figure 3). Thioperamide pretreatment alone did not significantly alter PPI or modify the disruptive effect of MK-801 on PPI at any of the prepulse intensities studied. There was a trend towards pretreatment x treatment interaction at the 10 dB prepulse intensity (Interaction term: F(4, 66) = 2.0, p = .11)(Figure 3, middle graph), but none of the other analyses approached significance.
Figure 2.
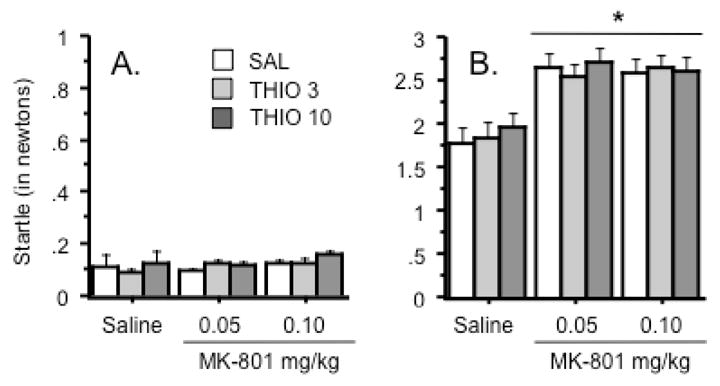
Effects of thioperamide and MK-801 on A.) baseline (“No startle”) activity and B.) auditory startle in the sensorimotor testing. Both doses of MK-801 enhanced auditory startle regardless of thioperamide pretreatment, as indicated by an asterisk. Data represent average force (expressed in newtons) ± s.e.m during no startle and startle trials. n = 12 animals per group.
Figure 3.
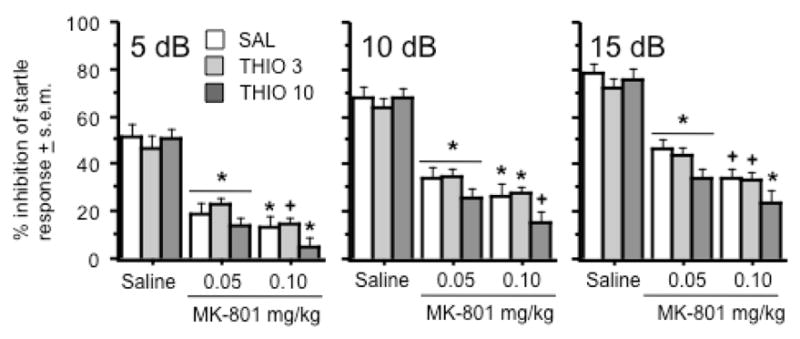
Effects of thioperamide and MK-801 on prepulse inhibition after presentation of a 5dB (left graph), 10dB (middle graph), and 15 dB prepulse (right graph). Symbols indicate significant differences within each of the three thioperamide pretreatment groups – an asterisk (*) indicates a significant difference from saline treatment and a cross (+) indicates a significant difference from saline and MK-801 (0.05 mg/kg) treatments. Data represent average % of prepulse inhibition (see text for further definition) ± s.e.m during prepulse inhibition trials. n = 12 animals per group.
Effect of thioperamide on the delayed spatial alternation impairment produced by MK-801
Rats were tested in the delayed spatial alternation task as previously described (Bardgett et al., 2006a & 2006b; 2008), using a 10 and 40 second delay between free and forced choice runs. As shown in Figure 4, MK-801 was found to decrease the number of correct choices at each delay in a dose-dependent manner (Treatment effect: F(2, 72) = 42.1 & 44.8, p < .0001 for the 10 and 40 second delays, respectively). There were no significant effects of thioperamide alone on task performance or overall interactions of thioperamide with MK-801 treatment (Figure 4).
Figure 4.
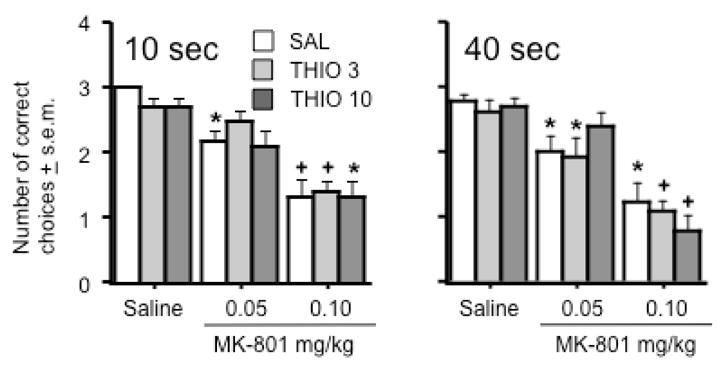
Effects of thioperamide and MK-801 on rewarded delayed spatial alternation at a 10 second (left graph) or 40 second delay (right graph) between forced and free choice trials. Symbols indicate significant differences within each of the three thioperamide pretreatment groups - an asterisk (*) indicates a significant difference from saline treatment and a cross (+) indicate a significant difference from saline and MK-801 (0.05 mg/kg) treatments. Data represent average number of correct choices ± s.e.m. n = 13 animals per group.
Discussion
Many studies over the past two decades have assessed the effects of H3 antagonists on several dimensions of animal behavior. The overall consensus of this work is that H3 antagonists possess pro-cognitive and even antipsychotic properties. If so, an important “next step” in the field is to test prototypical compounds in animal models of memory impairment and schizophrenia. The present study was undertaken to determine if a prototypical H3 antagonist, thioperamide, could modulate behavioral responses to MK-801, a drug that induces memory deficits and psychotomimetic behavioral changes in rats. The results demonstrate that thioperamide does not alter the memory impairment and PPI deficit produced by MK-801, but modulates the hyperactivity induced by MK-801 in a manner dependent on the MK-801 dose. Taken together, the data array generated in this study indicates that H3 and NMDA-type glutamate receptors may interact in behaviorally specific ways as a function of the level of NMDA antagonism.
Previous work has suggested a modulatory role of H3 receptors in rodent locomotor activity. Most observations of locomotor activity after treatment with thioperamide have noted little if any change in locomotor activity (Clapham & Kilpatrick 1994; Perez-Garcia et al., 1999) and the results of the present study are consistent with these observations. Studies assessing the interactive effects of thioperamide and psychostimulants on locomotion have suggested that thioperamide reduces stimulant-induced hyperactivity. Clapham and Kilpatrick (1994) found that a 10 mg/kg dose of thioperamide effectively reduced locomotor responses to amphetamine, apomorphine, and cocaine in mice. Similar results have been reported in mice using other H3 antagonists such as ciproxifan (Morriset et al., 2002) or a newer, non-imidazole compound ABT-239 (Fox et al., 2005). On the other hand, thioperamide can potentiate cocaine-induced hyperactivity (Brabant et al., 2009), indicative of a complex interaction between H3 receptors and psychostimulants. As for interactions with NMDA antagonists, Faucard et al. (2006) reported that ciproxifan could retard the hyperlocomotion observed in mice after treatment with a 0.3 mg/kg dose of MK-801. Likewise, a novel H3 antagonist, BF2.649, was found to reduce the locomotor response of mice to a 0.2 mg/kg dose of MK-801 (Ligneau et al., 2007).
The present results attest to the complex relationship between H3 receptors and psychostimulants. In our initial experiment, thioperamide increased the effects of two doses of MK-801 (0.05 & 0.1 mg/kg) on locomotor activity. These doses of MK-801 were chosen mainly for comparative reasons with our prepulse inhibition and delayed alternation experiments, wherein we avoided using higher doses of MK-801 that produce significant ataxia (Wozniak et al., 1990; Brosnan-Watters et al., 1996). The result from this initial experiment was in contrast to previous findings that had shown a suppressive effect of H3 antagonists on hyperactivity produced by MK-801 (Faucard et al., 2006; Ligneau et al., 2007). Yet, these latter papers had investigated doses of MK-801 that were higher than those used in the present study. In order to address the discrepancy between our results and previous findings, we conducted a second locomotor experiment that examined the effects of thioperamide on a 0.3 mg/kg dose of MK-801. The results from this study were consistent with previous work, since thioperamide was found to decrease the hyperactivity associated with higher dose of MK-801. Informal observations of animals treated with thioperamide and the high dose of MK-801 indicated that their activity mainly consisted of forward “swimming” behaviors and body rolling and not actual ambulation accomplished upright on all four paws.
The most parsimonious explanation of the results is that thioperamide simply shifts the dose-response curve for MK-801 to the left. Animals treated with a low dose of MK-801 and thioperamide show activity levels similar to those treated with slightly higher doses of MK-801. Animals treated with a high dose of MK-801 and thioperamide experience more of the ataxia produced by even higher doses of MK-801 and fail to break as many photobeams in the locomotor activity monitors. We would conclude that these experiments indicate that antagonism of H3 receptors may enhance the effects of NMDA receptor antagonism on motor behavior.
However, several caveats must be considered before this conclusion can be accepted. Our findings may be an idiosyncratic effect of thioperamide, and other H3 antagonists may interact differently with MK-801 and other drugs. As precedent for this idea, Brabant et al. (2009) reported that thioperamide enhances cocaine-induced locomotor activity in mice but a non-imidazole H3 antagonist, A-331440, does not. Similarly, Zhang et al. (2005) found that catalepsy induced by risperidone and haloperidol in rats was not altered by the non-imidazole H3 antagonists, ABT-239 and A-431404, yet was augmented by thioperamide and ciproxifan. However, unpublished work in our lab has determined that ciproxifan alters the motor effects of MK-801 in the same manner as thioperamide, suggesting that imidazole-type H3 antagonists have similar effects on MK-801-related behavior.
Another factor that should be considered regarding the interaction between H3 antagonists and MK-801 is the species used. Rats were used in the present study instead of mice since most of our previous work (e.g., Bardgett et al., 1997; Jacobs et al., 2000) involved the former species. It is possible that the interaction between central glutamatergic and histaminergic pathways, or the H3 receptor sequence itself (Hancock 2006), is sufficiently different between species that locomotor responses to MK-801 are differentially sensitive to H3 receptor blockade in mice versus rats. While H3 receptor antagonists have been found to reduce locomotor responses to MK-801 and other psychostimulants in mice (Clapham & Kilpatrick, 1994; Faucard et al., 2006; Ligneau et al., 2007), it should be noted that others (Brabant et al., 2009) have found that thioperamide can enhance locomotor responses to psychostimulant, such as cocaine, in mice. Overall, the results from the mouse studies are not dramatically different from the bi-directional pattern of responses to MK-801 that were observed after thioperamide pretreatment in rats in the present study.
While species and drug differences may partially account for complex interaction between thioperamide and MK-801, consideration of two procedural issues may also be informative. The earlier work by Faucard et al. (2006) and Ligneau et al. (2007) used a one-hour interval between H3 antagonist pretreatment and MK-801 treatment. These studies also habituated animals to the activity cages for one hour prior to drug testing, whereas rats in this study were habituated to the activity cages for two hours a day for three consecutive days prior to drug testing. These differences may have altered baseline activity in a manner that influenced drug responses and drug interactions.
If, as suggested, thioperamide works to augment the actions of MK-801 on neurons involved in motor activity, then this interaction must be accounted for at a cellular level. Histamine reduces glutamate release via presynaptic H3 receptors, while H3 antagonists block this effect (Brown & Haas, 1999; Garduño-Torres et al., 2007). These findings would suggest that H3 antagonists effect a permissive environment for the neuronal excitability produced by NMDA antagonists (Takahata & Moghaddam 2003), and would potentiate behavioral responses to MK-801. An alternative interpretation of the interaction between thioperamide and MK-801 is that these drugs work in concert to over-inhibit neurons. Neurotoxicity studies have shown that thioperamide and another H3 antagonist, clobenpropit, block NMDA-induced cell death in cultured cortical neurons via increased GABA release (Dai et al., 2006; 2007). The combination of H3 antagonist-induced GABA release and NMDA receptor blockade by MK-801 may work to inhibit neurons critical in maintaining normative rates of locomotor activity. Why this interaction affects only locomotor behavior as opposed to all of the behavioral alterations caused by MK-801 is not clear. Obviously, much work is needed to characterize the exact relationship between H3 and NMDA receptors, and how this relationship impacts specific behavioral processes.
Previous research has implicated a role of H3 receptors in sensorimotor gating. While most studies have indicated that H3 antagonists do not alter auditory startle, they have suggested that such drugs alone enhance PPI in mice (Browman et al., 2004, Fox et al., 2005; Ligneau et al., 2007). Browman et al. (2004) found that a high dose of thioperamide (30 mg/kg) but not a moderate dose (10 mg/kg) improves PPI in DBA/2 mice, a mouse strain that demonstrates deficient PPI. Ligneau et al. (2007) reported that BF2.649 could reverse the debilitating effects of the dopamine agonist, apomorphine, on PPI in mice. Our results in rats suggest that the PPI deficits resulting from NMDA antagonism are impervious to thioperamide pretreatment. One potential explanation of this outcome is that, unlike the NMDA receptors and brain regions that regulate locomotor activity, the receptors and brain regions that regulate PPI are not modulated by H3 receptors. Another explanation is that the dose of thioperamide was insufficient. This explanation is questionable since the same dose of thioperamide was effective in the locomotor assay. Moreover, the chosen dose of thioperamide is significantly higher than the reported IC50 for binding to H3 receptors (Le et al., 2008) – suggesting that effects obtained with substantially higher doses might involve binding to non-H3 receptors. Nonetheless, since it has been shown that the effective dose of other H3 antagonists can vary depending on the specific behavioral assay (Fox et al., 2005; Ligneau et al., 2007), the issue of dose deserves attention in the interpretation of the PPI and spatial memory results.
The ability of H3 antagonists to elevate cortical and hippocampal acetylcholine release (Blandina et al., 1996; Clapham & Kilpatrick 1992; Fox et al., 2005) would seem to strongly imply that these compounds possess cognitive enhancing properties. To this end, behavioral work has shown that, when administered alone, H3 antagonists improve attention, inhibitory avoidance, and social and spatial memory (Flood et al., 1998; Ligneau et al., 1998; Fox et al. 2003; 2005). More importantly, inhibitory avoidance deficits produced by MK-801 can be alleviated by systemic thioperamide treatment (Bernearts et al., 2004), while spatial memory impairment caused by MK-801 can be remediated by intra-hippocampal infusion of clobenpropit (Huang et al., 2004). The present study found that thioperamide neither improved delayed spatial alternation memory when administered alone or reversed the memory impairment observed after MK-801 treatment. These results, taken in the context of earlier reports, suggest that the mnemonic effects of H3 antagonists are highly dependent on the specific antagonist used, the route of administration, or the specific memory task. The T-maze delayed spatial alternation task is test of spatial working memory that depends the integrity of the hippocampus (Bardgett et al., 2008) and the prefrontal cortex (Mogensen et al., 2008). It is possible that each brain region mediates different aspects of the task (e.g., processing of spatial information versus short-term memory burden). Given that modulation of histamine receptors in the hippocampus can alter the spatial memory deficits produced by intrahippocampal MK-801 infusion (Huang et al., 2004), it may be of interest to determine if H3 antagonists would be more efficacious in cognitive assays (e.g., Morris Swim Maze) that rely solely on hippocampal function.
It has been suggested that H3 antagonists possess antipsychotic properties along with the ability to improve cognition. Our results do not discount this latter possibility but call into question the likelihood that H3 antagonists may act as antipsychotic drugs. However, it is important to qualify this charge: our results suggest that H3 antagonists, or specifically thioperamide, may exacerbate psychosis driven by physiological states of NMDA receptor hypofunction. It remains possible that such compounds may be efficacious in combating the psychosis linked to dysfunction in other neurotransmitter systems, e.g., elevated dopaminergic tone. As some have stated previously (Bardgett 2004; Roth et al. 2004), it is important to recognize that different pathophysiological pathways may lead to forms of psychosis or dyscognition that have similar presentations. The development of new treatments for these disorders should be tailored to target these specific pathways. In this sense, H3 antagonists may still represent a viable strategy for remediating some, but not all, forms of pathophysiology that lead to mental disorders.
Acknowledgments
This research was supported by National Center for Research Resources Grant P20 RR16481 and National Institute of Mental Health Grant R15 MH076788. We would like to acknowledge the NIMH Chemical Synthesis and Drug Supply Program providing the thioperamide used in this study. The experiments reported here comply with Current Guide for the Care and Use of Laboratory Animals (USPHS) and were performed under a protocol approved by the Northern Kentucky University Institutional Animal Use and Care Committee.
References
- Bardgett ME. Behavioral models of atypical antipsychotic drug action. In: Csernansky JG, Laurello J, editors. Atypical antipsychotics: From bench to bedside. Marcel Dekker; New York: 2004. pp. 61–93. [Google Scholar]
- Bardgett ME, Griffith MS, Foltz RF, Hopkins JA, Massie CM, O’Connell SM. The effects of clozapine on delayed spatial alternation in rats with hippocampal damage. Neurobiol Learn Mem. 2006;85:86–94. doi: 10.1016/j.nlm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Hon JG, Lee NM, O’Connell SM, Baum KM. Effects of risperidone on locomotor activity and spatial memory in rats with hippocampal damage. Neuropharmacology. 2006;51:1156–1162. doi: 10.1016/j.neuropharm.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Jacobs PS, Jackson JL, Csernansky JG. Kainic acid lesions enhance locomotor responses to novelty, saline, amphetamine, and MK-801. Behav Brain Res. 1997;87:47–55. doi: 10.1016/s0166-4328(96)00132-5. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Points M, Ramsey-Faulkner C, Topmiller J, Roflow J, McDaniel T, Lamontagne T, Griffith MS. The effects of clonidine on discrete-trial delayed spatial alternation in two rat models of memory loss. Neuropsychopharmacology. 2008;33:1980–1991. doi: 10.1038/sj.npp.1301580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. J Neurosci. 1998;18:8394–8401. doi: 10.1523/JNEUROSCI.18-20-08394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaerts P, Lamberty Y, Tirelli E. Histamine H3 antagonist thioperamide dose-dependently enhances memory consolidation and reverses amnesia induced by dizocilpine or scopolamine in a one-trial inhibitory avoidance task in mice. Behav Brain Res. 2004;154:211–219. doi: 10.1016/j.bbr.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Blandina P, Giorgetti M, Bartolini L, Cecchi M, Timmerman H, Leurs R, Pepeu G, Giovannini MG. Inhibition of cortical acetylcholine release and cognitive performance by histamine H3 receptor activation in rats. Br J Pharmacol. 1996;119:1656–1664. doi: 10.1111/j.1476-5381.1996.tb16086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant C, Alleva L, Grisar T, Quertemont E, Lakaye B, Ohtsu H, Lin JS, Jatlow P, Picciotto MR, Tirelli E. Effects of the H(3) receptor inverse agonist thioperamide on cocaine-induced locomotion in mice: role of the histaminergic system and potential pharmacokinetic interactions. Psychopharmacology. 2009;202:673–687. doi: 10.1007/s00213-008-1345-y. [DOI] [PubMed] [Google Scholar]
- Brosnan-Watters G, Wozniak DF, Nardi A, Olney JW. Acute behavioral effects of MK-801 in the mouse. Pharmacol Biochem Behav. 1996;53:701–711. doi: 10.1016/0091-3057(95)02073-x. [DOI] [PubMed] [Google Scholar]
- Browman KE, Komater VA, Curzon P, Rueter LE, Hancock AA, Decker MW, Fox GB. Enhancement of prepulse inhibition of startle in mice by the H3 receptor antagonists thioperamide and ciproxifan. Behav Brain Res. 2004;153:69–76. doi: 10.1016/j.bbr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Brown RE, Haas HL. On the mechanism of histaminergic inhibition of glutamate release in the rat dentate gyrus. J Physiol. 1999;515:777–786. doi: 10.1111/j.1469-7793.1999.777ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham J, Kilpatrick GJ. Histamine H3 receptors modulate the release of [3H]-acetylcholine from slices of rat entorhinal cortex: Evidence for the possible existence of H3 receptor subtypes. Br J Pharmacol. 1992;107:919–923. doi: 10.1111/j.1476-5381.1992.tb13386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham J, Kilpatrick GJ. Thioperamide, the selective histamine H3 receptor antagonist, attenuates stimulant-induced locomotor activity in the mouse. Eur J Pharmacol. 1994;259:107–114. doi: 10.1016/0014-2999(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Dai H, Fu Q, Shen Y, Hu W, Zhang Z, Timmerman H, Leurs R, Chen Z. The histamine H3 receptor antagonist clobenpropit enhances GABA release to protect against NMDA-induced excitotoxicity through the cAMP/protein kinase A pathway in cultured cortical neurons. Eur J Pharmacol. 2007;563:117–123. doi: 10.1016/j.ejphar.2007.01.069. [DOI] [PubMed] [Google Scholar]
- Dai H, Zhang Z, Zhu Y, Shen Y, Hu W, Huang Y, Luo J, Timmerman H, Leurs R, Chen Z. Histamine protects against NMDA-induced necrosis in cultured cortical neurons through H receptor/cyclic AMP/protein kinase A and H receptor/GABA release pathways. J Neurochem. 2006;96:1390–1400. doi: 10.1111/j.1471-4159.2005.03633.x. [DOI] [PubMed] [Google Scholar]
- Farber NB, Newcomer JW, Olney JW. The glutamate synapse in neuropsychiatric disorders. Focus on schizophrenia and Alzheimer’s disease. Prog Brain Res. 1998;116:421–37. doi: 10.1016/s0079-6123(08)60453-7. [DOI] [PubMed] [Google Scholar]
- Faucard R, Armand V, Héron A, Cochois V, Schwartz JC, Arrang JM. N-methyl-D-aspartate receptor antagonists enhance histamine neuron activity in rodent brain. J Neurochem. 2006;98:1487–1496. doi: 10.1111/j.1471-4159.2006.04002.x. [DOI] [PubMed] [Google Scholar]
- Fox GB, Pan JB, Radek RJ, Lewis AM, Bitner RS, Esbenshade TA, Faghih R, Bennani YL, Williams M, Yao BB, Decker MW, Hancock AA. Two novel and selective nonimidazole H3 receptor antagonists A-304121 and A-317920: II. In vivo behavioral and neurophysiological characterization. J Pharmacol Exp Ther. 2003;305:897–908. doi: 10.1124/jpet.102.047241. [DOI] [PubMed] [Google Scholar]
- Flood JF, Uezu K, Morley JE. Effect of histamine H2 and H3 receptor modulation in the septum on post-training memory processing. Psychopharmacology. 1998;140:279–84. doi: 10.1007/s002130050768. [DOI] [PubMed] [Google Scholar]
- Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, Browman KE, Buckley MJ, Ballard ME, Komater VA, Miner H, Zhang M, Faghih R, Rueter LE, Bitner RS, Drescher KU, Wetter J, Marsh K, Lemaire M, Porsolt RD, Bennani YL, Sullivan JP, Cowart MD, Decker MW, Hancock AA. Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther. 2005;313:176–190. doi: 10.1124/jpet.104.078402. [DOI] [PubMed] [Google Scholar]
- Garduño-Torres B, Treviño M, Gutiérrez R, Arias-Montaño JA. Pre-synaptic histamine H3 receptors regulate glutamate, but not GABA release in rat thalamus. Neuropharmacology. 2007;52:527–535. doi: 10.1016/j.neuropharm.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Hancock AA. The challenge of drug discovery of a GPCR target: Analysis of preclinical pharmacology of histamine H3 antagonists/inverse agonists. Biochem Pharmacol. 2006;71:1103–1113. doi: 10.1016/j.bcp.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Huang YW, Hu WW, Chen Z, Zhang LS, Shen HQ, Timmerman H, Leurs R, Yanai K. Effect of the histamine H3-antagonist clobenpropit on spatial memory deficits induced by MK-801 as evaluated by radial maze in Sprague-Dawley rats. Behav Brain Res. 2004;151:287–293. doi: 10.1016/j.bbr.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Komater VA, Buckley MJ, Browman KE, Pan JB, Hancock AA, Decker MW, Fox GB. Effects of histamine H3 receptor antagonists in two models of spatial learning. Behav Brain Res. 2005;159:295–300. doi: 10.1016/j.bbr.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Le S, Gruner JA, Mathiasen JR, Marino MJ, Schaffhauser H. Correlation between ex vivo receptor occupancy and wake-promoting activity of selective H3 receptor antagonists. J Pharmacol Exp Ther. 2008;325:902–909. doi: 10.1124/jpet.107.135343. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Landais L, Perrin D, Piriou J, Uguen M, Denis E, Robert P, Parmentier R, Anaclet C, Lin JS, Burban A, Arrang JM, Schwartz JC. Brain histamine and schizophrenia: potential therapeutic applications of H3-receptor inverse agonists studied with BF2.649. Biochem Pharmacol. 2007;73:1215–1224. doi: 10.1016/j.bcp.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, Stark H, Elz S, Schunack W, Schwartz J. Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist. J Pharmacol Exp Ther. 1998;287:658–666. [PubMed] [Google Scholar]
- Jacobs PS, Taylor BM, Bardgett ME. Maturation of locomotor and Fos responses to NMDA antagonists, PCP and MK-801. Dev Brain Res. 2000;122:91–95. doi: 10.1016/s0165-3806(00)00059-6. [DOI] [PubMed] [Google Scholar]
- Mogensen J, Boyd MH, Nielsen MD, Kristensen RS, Malá H. Erythropoietin improves spatial delayed alternation in a T-maze in rats subjected to ablation of the prefrontal cortex. Brain Res Bull. 2008;77:1–7. doi: 10.1016/j.brainresbull.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Morisset S, Pilon C, Tardivel-Lacombe J, Weinstein D, Rostene W, Betancur C, Sokoloff P, Schwartz JC, Arrang JM. Acute and chronic effects of methamphetamine on tele-methylhistamine levels in mouse brain: Selective involvement of the D(2) and not D(3) receptor. J Pharmacol Exp Ther. 2002;300:621–628. doi: 10.1124/jpet.300.2.621. [DOI] [PubMed] [Google Scholar]
- Morisset S, Sahm UG, Traiffort E, Tardivel-Lacombe J, Arrang JM, Schwartz JC. Atypical neuroleptics enhance histamine turnover in brain via 5-Hydroxytryptamine2A receptor blockade. J Pharmacol Exp Ther. 1999;288:590–596. [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia C, Morales L, Cano MV, Sancho I, Alguacil LF. Effects of histamine H3 receptor ligands in experimental models of anxiety and depression. Psychopharmacology. 1999;142:215–220. doi: 10.1007/s002130050882. [DOI] [PubMed] [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, Arrang JM. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Orsetti M, Ghi P, Di Carlo G. Histamine H(3)-receptor antagonism improves memory retention and reverses the cognitive deficit induced by scopolamine in a two-trial place recognition task. Behav Brain Res. 2001;124:235–242. doi: 10.1016/s0166-4328(01)00216-9. [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: Selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004b;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, Schunack W, Ganellin CR, Arrang JM. Therapeutic implications of constitutive activity of receptors: the example of the histamine H3 receptor. J Neural Transm. 2003;(Suppl):1–16. doi: 10.1007/978-3-7091-6020-6_1. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Olney JW, Kettinger L, 3rd, Price M, Miller JP. Behavioral effects of MK-801 in the rat. Psychopharmacology. 1990;101:47–56. doi: 10.1007/BF02253717. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ballard ME, Pan L, Roberts S, Faghih R, Cowart M, Esbenshade TA, Fox GB, Decker MW, Hancock AA, Rueter LE. Lack of cataleptogenic potentiation with non-imidazole H3 receptor antagonists reveals potential drug-drug interactions between imidazole-based H3 receptor antagonists and antipsychotic drugs. Brain Res. 2005;1045:142–149. doi: 10.1016/j.brainres.2005.03.018. [DOI] [PubMed] [Google Scholar]


