Abstract
Background
The Fontan circulation is critically dependent on elevated venous pressures to sustain effective venous return. We hypothesize that chronically increased systemic venous pressures lead to adaptive changes in regional and peripheral vessels to maintain cardiac output, especially when upright.
Methods and Results
9 post Fontan patients (age 13-24 y) and 6 age and gender matched controls were compared, using techniques to measure circulatory responses (peripheral and compartmental blood flow, venous capacity and microvascular filtration). Parameters studied: Strain gauge plethysmography (SPG) measures of peripheral circulatory function, regional blood volume distribution by impedance plethysmography (IPG) and head-up tilt (HUT) testing. Important differences between Fontan and controls were seen in several vascular compartments: 1.Calf capacitance was lower (median 3.5 vs. 5.5ml/100ml tissue; p=0.005), and resting venous pressure (Pv) higher (13.0 vs.10.5 mmHg p=0.004). 2. Higher leg arterial resistance (32.1 vs. 22.2 p=0.03) 3. Microvascular filtration pressures and threshold for edema were elevated. 4. With HUT, splanchnic flow did not reduce in Fontan vs. Controls (Fractional change +4% vs. -32% p=0.004) and splanchnic arterial resistance did not increase as expected. (Fractional change +8% vs +79% p= 0.003).
Conclusions
Reduced venous compliance and increased filtration thresholds may act as adaptive mechanisms in maintaining venous return in Fontan circulation. Well compensated Fontan subjects demonstrate superior orthostatic tolerance resulting from decreased compartmental fluid shifts in response to HUT and higher vascular resistance. This results from increased venous stiffness and decreased splanchnic capacitance and may also be an adaptive mechanism to maintain venous return in these patients while standing.
Keywords: Fontan, venous capacitance, filtration threshold, splanchnic reservoir
Introduction
Univentricular heart is a common feature of many forms of complex cardiac disease, and the Fontan repair comprising total cavopulmonary connection (TCPC) offers a final common pathway to palliate these patients.1-5 This is a highly abnormal circulation, where the right ventricle (RV) is absent as an effective pump that allows pulsatile flow to be distributed to the pulmonary arteries.5,6 Systemic venous return to the heart is highly dependent on peripheral venous properties and pulmonary artery pressure.3 Abnormalities in circulatory regulation after TCPC have been sporadically reported. However, integrative features of peripheral vascular function after long-term adaptation to the surgery have not been well characterized.5-12
We hypothesize that chronically increased systemic venous pressure leads to structural and functional changes in arteries and veins which alter venous capacitance, venous resistance, microvascular filtration, regional venous tone, peripheral and regional arterial regulation and autonomic function corresponding to compartmental redistributions in blood volume. For example, venous compliance and function changes may produce increased microvascular filtration resulting in edema and extravascular fluid collection often found in “failed-Fontan” patients.13-15 Although this is most easily evident in dependent extremities, we hypothesize that vascular properties are also abnormal within the splanchnic vasculature with more important circulatory consequences because the splanchnic compartment is the single largest venous reservoir. This may be of special importance in the development of protein losing enteropathy, a frequent and dire sequel to Fontan palliation.16 It is possible that subjects who are functioning well many years after the Fontan repair develop protective adaptations in the vascular system in order to maintain normal function in activities of daily living, especially in the upright state.
In order to investigate these hypotheses, we compared well compensated patients with Fontan physiology with age and gender matched normal control subjects using non-invasive techniques. Peripheral venous capacity and microvascular filtration properties were studied using venous occlusion strain-guage plethysmography (SPG).17 Impedance plethysmography (IPG) was used to measure regional circulatory responses at rest and during neurovascular stress including head up tilt (HUT) and forced Valsalva maneuver, and to measure limb, thoracic and splanchnic blood flows.
Materials and Methods
Subjects and Experimental Outline
Nine subjects (4 female and 5 male) aged 13-24 years who had undergone Fontan surgery 8 to 19 years prior to testing and 6 age matched healthy controls (3 males and 3 females), volunteered for the study (Subject characteristics and hemodynamics are described in Table 1). The control subjects were healthy, normotensive and were free from any systemic disorders. There were no smokers in either group. There were no competitive athletes in either group. Post-Fontan patients who were medically unfit for testing lasting several hours, those with clinical evidence of cardiac failure, those on anti arrhythmia medications, patients with other serious systemic diseases – including renal or hepatic disorders and “failed Fontans”(patients with severe edema, anasarca and malabsorption suggesting gut edema) were excluded. Our Fontan patients therefore comprised those with a “good” outcome only. Six of the Fontan subjects were taking low dose aspirin, 3 of whom were additionally taking very low dose (2.5 to 5 mg per day) of enalapril, 1 was taking Coumadin, and 2 were taking no medications. A thorough clinical examination, electrocardiography, echocardiography and baseline laboratory testing were obtained in all patients. Echocardiography revealed good single ventricular function with no significant valvar dysfunction and electrocardiography (and periodic holter monitoring) ruled out any arrhythmias in all subjects. All laboratory values were within normal range. Written informed consent was obtained and all protocols were approved by the institutional committee for protection of human subjects (IRB). All the authors have had full access to the data and take responsibility for its integrity. All the authors have read and agree to the manuscript as written.
Table 1.
Fontan subject Characteristics and Surgical Data
| Pt. | Sex | Age (years) | Diagnosis (Systemic Ventricle) | Type of surgery | Years since surgery |
|---|---|---|---|---|---|
| 1 | M | 18 | HLHS (RV) | Lateral tunnel | 14 |
| 2 | M | 13 | TA, PS (LV) | ECF | 8 |
| 3 | M | 16 | TA, PA (LV) | Lateral tunnel | 12 |
| 4 | F | 16 | HLHS (RV) | Lateral tunnel | 13 |
| 5 | M | 13 | Ebstein, PA (LV) | ECF | 8 |
| 6 | M | 24 | HLHS (RV) | Lateral tunnel | 18 |
| 7 | F | 13 | DILV, PS (LV) | ECF | 8 |
| 8 | F | 19 | TA, PS (LV) | Lateral tunnel | 16 |
| 9 | F | 17 | TA, PS (LV) | Lateral tunnel | 13 |
Abbreviations: HLHS (hypoplastic left heart syndrome), TA (tricuspid atresia, PA (pulmonary atresia), DILV (double inlet left ventricle), PS (pulmonary stenosis), ECF (Extracardiac Fontan), M (male), F (female).
Study Protocol
All experiments were completed on a single visit, with the subject arriving in the morning, 2 hours or more after a light breakfast. Electrocardiogram (ECG) was monitored continuously and recorded to assess heart rate and rhythm changes. Blood pressure was monitored using a finger plethysmograph (Finometer, TNO Biomedical Instrumentation, Amsterdam, The Netherlands) placed on the right index finger and recalibrated every 5 minutes against oscillometric sphygmomanometer pressure. Leg blood pressure was measured intermittently by oscillometry on the calf contralateral to the strain gauge and used to calculate the calf mean arterial pressure (MAP). A respiratory impedance plethysmograph (model 200 noninvasive monitoring system, Respitrace) monitored respiration. Respiratory, ECG and Finometer pressure data were interfaced to a personal computer using an analog-digital converter (DataQ Ind; Milwaukee, WI) and custom designed software was used to store data on a continuous basis. These data were multiplexed with a strain gauge, and were effectively synchronized. The study was started after an initial 30 minute acclimatization period.
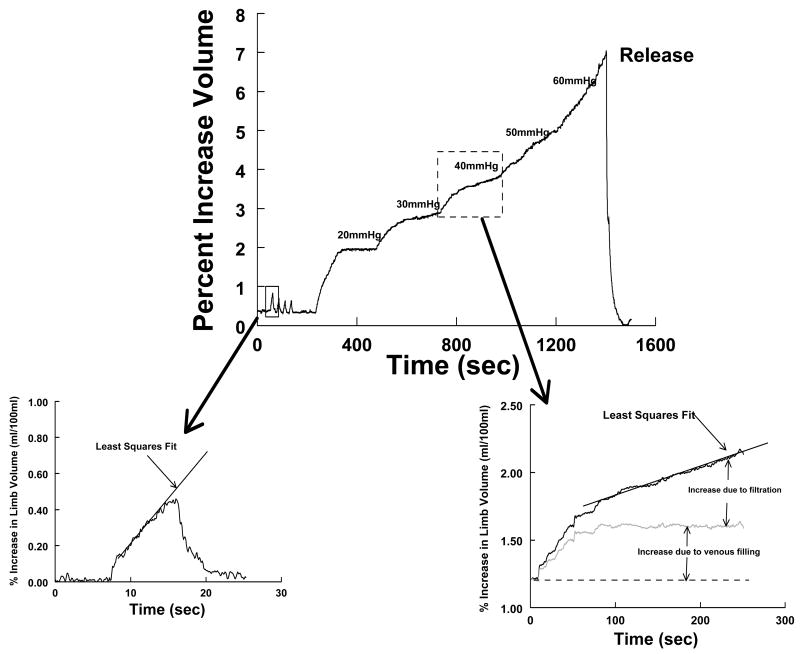
Strain-gauge measurements include peripheral blood flow by venous occlusion plethysmography, resting venous pressure, peripheral arterial resistance, and venous capacitance. Our facility and others have extensively reported on these methods before.18-23 The methods used are summarized in Figure 1.
Figure 1.
Measurements made during venous occlusion plethysmography (arterial flow and venous filling). Top: Typical experiment in which blood flow is measured in triplicate by venous occlusion followed by incremental occlusions using 10 mm steps to determine compliance and microvascular filtration relationships. Bottom left: Derivation of limb flow by fitting a slope to the initial portion of the occlusion curve. Bottom right: Venous filling and capillary filtration. Means by which volume changes during pressure “steps” is partitioned into contributions from venous filling and microvascular filtration.
Venous occlusion strain-gauge plethysmography to measure peripheral blood flow.17,21
(SPG) was used to measure forearm and calf blood flow (Figure 1 upper panel and left lower panel); compliance (volume-pressure relationship, Figures 2 and 3) and microvascular filtration (flow-pressure relationship, Figures 2 and 4) in the supine steady state in all subjects. Supine measurements were made after a 30 minute equilibration period using occlusion cuffs placed approximately 10 cm above the knee and elbow. Strain gauges attached to a Whitney-type strain-gauge plethysmograph (Hokanson) were placed around the maximum diameter of the forearm and calf. Blood flows were estimated while subjects were in the supine position using rapid cuff inflation to a pressure below diastolic pressure (e.g., 40 mmHg) but above the venous pressure to prevent venous egress. Systolic and diastolic blood pressures of the arm and leg were determined by oscillometry. Arterial inflow in milliliters/100 ml tissue/minute was estimated as the rate of change of the rapid increase in the limb cross-sectional area (Figure 1 upper panel, and left lower panel).
Figure 2.
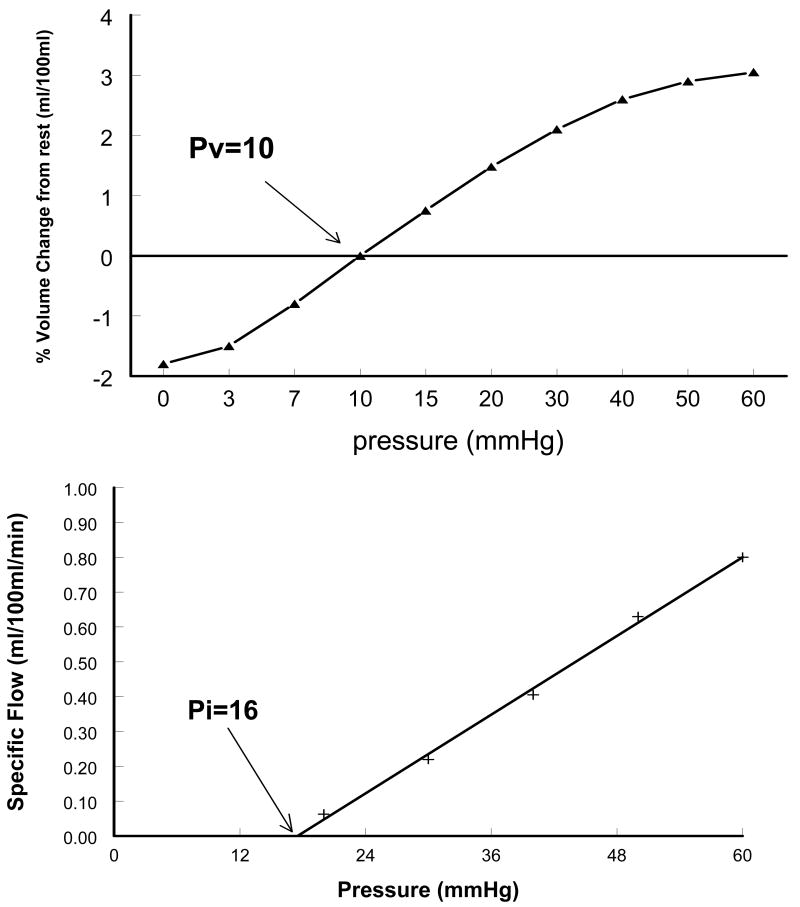
Compliance Relationship and Microvascular filtration relation derived from the Fontan sub-group. Upper graph: X-axis intercept of the “volume- pressure” curve (Pv) is the resting venous pressure. Lower graph: X-axis intercept of the “flow-pressure” line (Pi) is the pressure at which microvascular filtration begins.
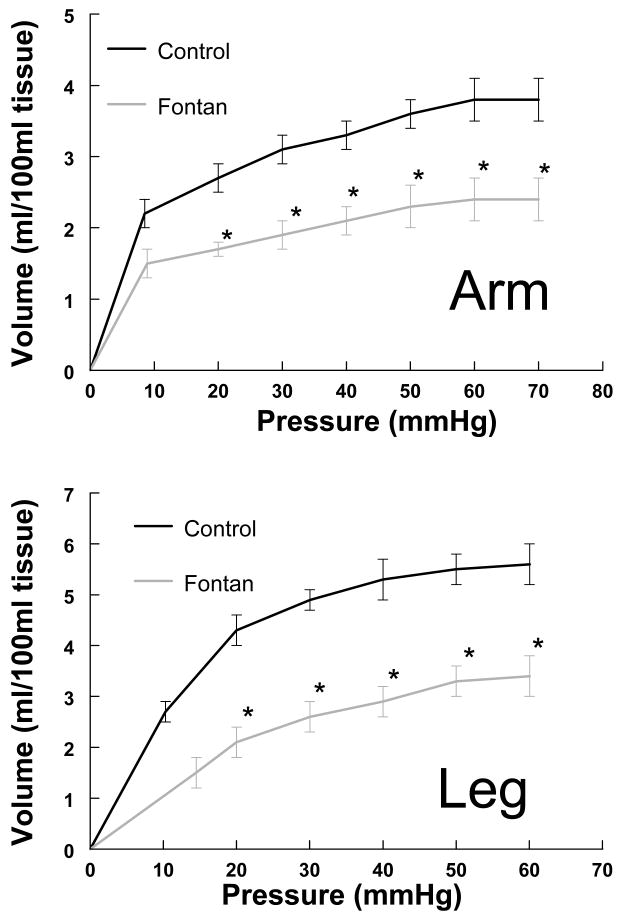
Figure 3.
Volume Pressure curves in arm and leg in Fontan vs Control subjects. Note - for each pressure point, the change in volume was significant in both the arms and legs. Ptime shows comparison over time and P time*·group shows group and time interaction when variables were compared by repeated measures (ANCOVA). *(Ptime<0.001, Ptime*·group =0.044 in arm and Ptime =0.002 and Ptime*·group =0.050 in leg) indicating reduced limb venous capacitance.
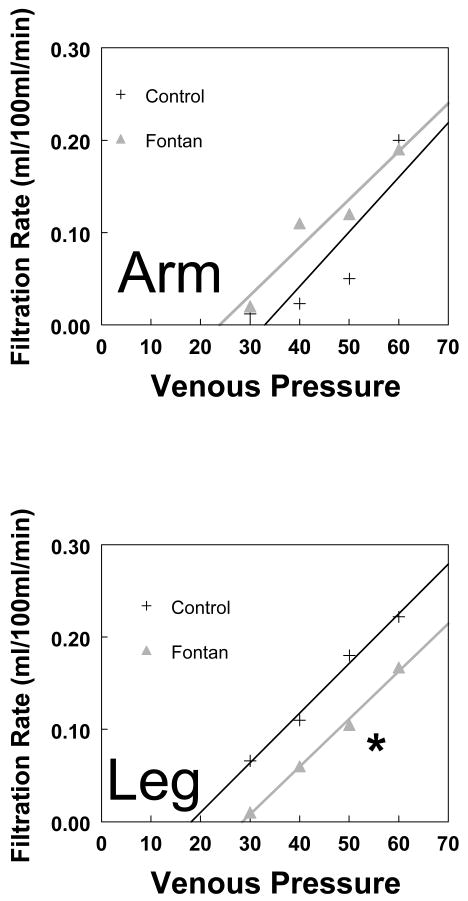
Figure 4.
The relation between filtration rate and venous pressure in the arm and leg in Fontan subjects vs controls. The filtration threshold in the Fontan group was similar to controls in arms, but significantly higher in the legs. *(p<0.05).
Capacitance vessel (resting venous) pressure (Pv) was assessed in the steady state. Once strain-gauge dimension returned to baseline after blood flow measurement, the occlusion cuff pressure was gradually increased until an increase in limb volume occurred- at which point, Pv was measured.
Peripheral resistance was calculated in the forearm and calf using the formula: (MAP-Pv)/blood flow, where MAP is mean arterial pressure.
Venous capacitance was measured using our previously documented techniques.18-22 Since over 75% of the blood volume at any given point resides in the veins, they are the principal capacitance vessels. Venous capacitance is defined as the relative amount of blood volume that can be held in the peripheral veins at a given point in time. In brief (Figure 1), while subjects were in the supine position, the limb was gently raised above the heart level until no further decrease in volume was obtained. This was used to empty the limb and establish zero filling. After recovery with the limb once again flat, we used 10 mmHg increases in pressure, starting at the first multiple of 10 larger than Pv, to a maximum of 60 mmHg. Thus, if Pv were 5 mmHg, the first occlusion pressure would be 10 mmHg, while if Pv were 14 mmHg, the first occlusion pressure would be 20mmHg. This produced progressive limb enlargement. Pressure was maintained for 4 min until a steady state was achieved as specified by Gamble.21 At lower congestion pressures, the limb size reaches a plateau representing venous filling alone. At higher pressures, a plateau is not reached. Instead, there are two components superimposed: a linear component representing microvascular filtration, which can be extracted from the total curve by least-squares methods, and a residual curve that reaches a plateau, which represents filling of capacitance vessels (Figure 1, right lower panel). Once the volume response is partitioned, capacitance is calculated from the sum of residual portions, to which is added the estimate of supine venous volume obtained from raising the limb.
Curvilinear graphs of volume versus occlusion pressure were calculated and represent the volume-pressure or capacitance relation (Figure 2). Linear graphs of filtration rate as a function of occlusion pressure were also calculated. The slope of the slow increase in volume of the limb once rapid venous filling is complete provides an estimate of filtered flow. The linear relation between filtered flow and pressure at each pressure increment defines the microvascular filtration coefficient.
The pressure intercept of the filtration curve is denoted the Isovolumetric pressure (Pi) which is defined as the minimum occlusion pressure required to produce a net increase in limb interstitial fluid.18,23 Above Pi, intravascular fluid begins to extravasate due to microvascular filtration. Below Pi, there is no net accumulation of filtrate.
Impedance plethysmography to measure segmental blood flows
Impedance plethysmography (IPG) can detect internal volume shifts, estimate cardiac output, measure regional fluid flows and quantify relative body fluid volumes.24,25 Relations between impedance and fluid compartmentalization have been well established.24 A tetrapolar IPG was used to measure fluid volumes and blood flows in the thorax (neck to Xyphoid process), splanchnic (Xyphoid process to Iliac crest), pelvic (Iliac crest to knee), and lower leg (knee to ankle) segments during test sequences. Measurements of baseline resistance, R0, and pulsatile resistance changes, ΔR, were made. Disposable spot EKG electrodes were attached to the foot of the dominant leg at segmental limits on the same side of the body, and on the dominant arm at the back of the hand. The IPG introduced a high frequency (50 kHz), low amperage (0.1 mA RMS) constant current signal between the foot and hand electrodes which is not felt by the subject. Simultaneous R values were measured in each segment continuously. Records of R0 were used to determine the fluid volume of each segment during each test sequence. The pulsatile ΔR changes were used to obtain the total (ml/min) and relative (ml/100 ml of body tissue/min) blood flow responses of each body segment to each test condition. These methods have been previously validated.24-26 The measurements allow us to trace fluid shifts from thoracic to peripheral compartments during orthostatic stress.
Upright circulatory measurements
After supine measurements were obtained and the subjects were allowed to return to an equilibrium state, they were subjected to an upright tilt to 70 degrees for 10 minutes, or until symptoms necessitated a return to supine position. Limb blood flows were measured each minute by SPG, and IPG fluid shifts, BP, HR, and respirations were recorded continuously. SPG was performed to measure limb arterial blood flow and capacitance volume-pressure relation, and the patient returned to supine. In general Pv changes during tilt but reaches a steady state. Pi was also measured. Arterial and venous properties during tilt were compared with supine data to define the relative contribution of active limb venoconstriction to the compliance relation, and to determine changes in arterial and venous flow resistance, micro vascular filtration, compartmental fluid shifts, and changes in cardiac output and blood volume with orthostatic stress.
Quantitative Valsalva maneuver
The quantitative valsalva maneuver was used to characterize sympathetic vasoconstrictive and parasympathetic cardiac responsiveness. The maneuver was performed in the supine position by blowing with an open glottis into a mouthpiece connected to the mercury column of a sphygmomanometer with an air leak. A 40-50 mmHg pressure was maintained for 15 seconds. BP recovery in phase II and cardiopressor response in phase IV were used as indices of vasoconstrictor and contractile integrity. Baroreceptor mediated tachycardia in phase II and bradycardia in phase IV determine if cardiovagal reflexes are intact. Prior to the maneuver, blood pressure was averaged over 20 beats, and mean resting heart rate calculated. The BP response was quantified during straining and during the pressure overshoot. Since arterial tonometry was used to measure BP, decreases of greater than 20 mmHg during early phase II combined with absent phase IV or late phase II was considered abnormal and indicative of blunted vasoconstrictive response.27
Statistics
Data were digitalized and stored in a computer and were analyzed off-line with custom software. We used non-parametric independent samples test (Wilcoxon–Mann–Whitney) for comparing hemodynamic data between Fontan and control subjects (Table 2), segmental arterial resistances (Figure 5) and segmental blood flows (Figure 6). Repeated measures analysis of variance (ANOVA) was used for repeated measurements over time (change in volume with increase in pressure in Figure 3). Adjustment for multiple comparisons was done using Bonferroni correction. Filtration curves between two groups were compared by linear regression in Figure 4. Results were calculated by using SPSS (Statistical Package for the Social Sciences) software version 14.0 and graphed using GraphPad prism (California) software version 4. Results are reported as median and range; p<0.05 was considered significant. We measured normalized blood flow-blood flow divided by segmental volume, in units of ml/minute/100 ml of tissue, and normalized arterial resistance-arterial resistance divided by segmental volume, in units of mmHg/ml/minute/100 ml of tissue.
Table 2.
Supine Hemodynamic Data
| Variable | Control (n=6) | Fontan (n=9) | p value |
|---|---|---|---|
| Median Age (range in years) | 16.2 (13-24) | 17.3 (13-24) | 0.4 |
| Height (cm) | 161.5(151-169) | 160.0(145-175) | 0.5 |
| Weight (Kg) | 58.9(47-64) | 562(49-98) | 0.3 |
| BSA (Sq.cm) | 1.6(1.43-1.67) | 1.6(1.5-1.71) | 0.5 |
| Heart Rate | 74.5(67-77) | 63(49-97) | 0.6 |
| Respiration | 20(17-25) | 20(14-24) | 0.8 |
| Systolic Blood pressure (mm Hg) | 118(96-128) | 124(95-146) | 0.8 |
| Diastolic Blood pressure(mmHg) | 61(52-70) | 70(50-83) | 0.1 |
| Mean Blood Pressure (mm Hg.) | 80(66-89) | 88(68-104) | 0.4 |
| End Tidal CO2 | 38(35-45) | 32(26-34) | 0.001* |
| Hemoglobin (gm/dl) | 13.2 (12.1-14.2) | 13.4 (12.6-14) | 0.6 |
| Arm Pv, mm Hg | 8.6(6-11) | 8.9(5-14) | 0.6 |
| Arm arterial resistance ml/100ml/min | 5.43(3.5-17.5) | 4.2(1.9-11.5) | 0.4 |
| Arm Capacity, ml/100ml | 3.7(3-4.6) | 2.5(1.3-2.9) | 0.006* |
| Arm flux intercept | 39.9(23.3-48.7) | 25.4(11.9-43.5) | 0.3 |
| Leg Pv, mm Hg | 10.5(8-12) | 13(12-18) | 0.004* |
| Leg arterial resistance ml/100ml/min | 22.2(13.0-28.2) | 32.0(13.2-55) | 0.03* |
| Leg Capacity, ml/100ml | 5.5(4.6-6.8) | 3.5(2.2-5) | 0.005* |
| Leg flux intercept | 15.1(11.7-30) | 27.4(20-38.5) | 0.05* |
Values are Median (range)
p < 0.05
CO2: Carbon Dioxide
Figure 5.
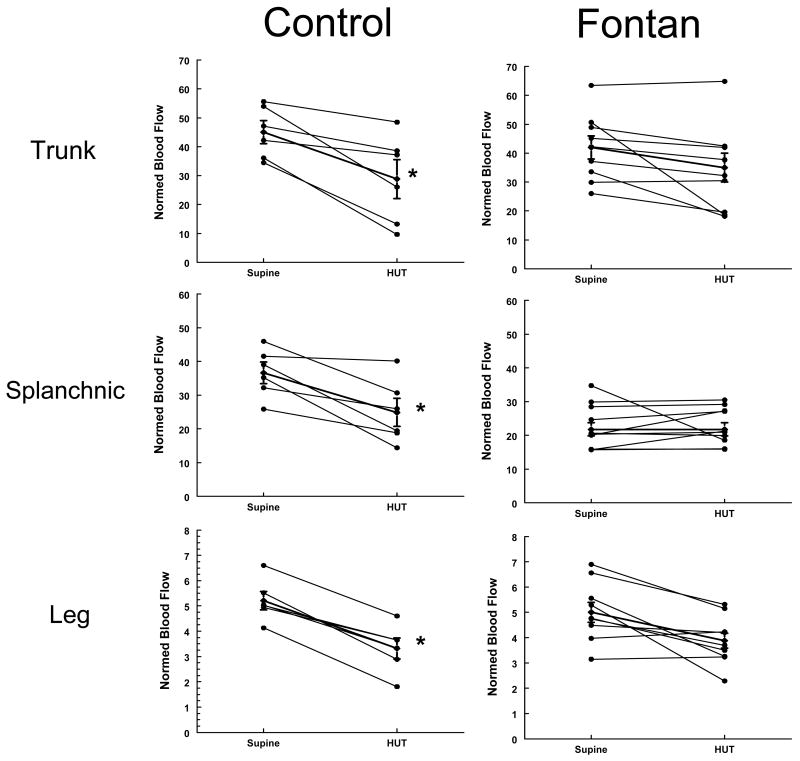
Fractional changes in segmental blood flows in the thoracic, splanchnic and leg with HUT in Control (left sided panels) and Fontan subjects (right sided panels). The Y-axis shows the normalized segmental blood flows (ml/100mltissue/min) and X-axis shows the change in value between supine and HUT in each individual subject. The thicker line in each panel depicts the median for each group. *The change in flow after HUT was significant in the control group (p<0.05 in each compartment), but not in the Fontan group.
Figure 6.
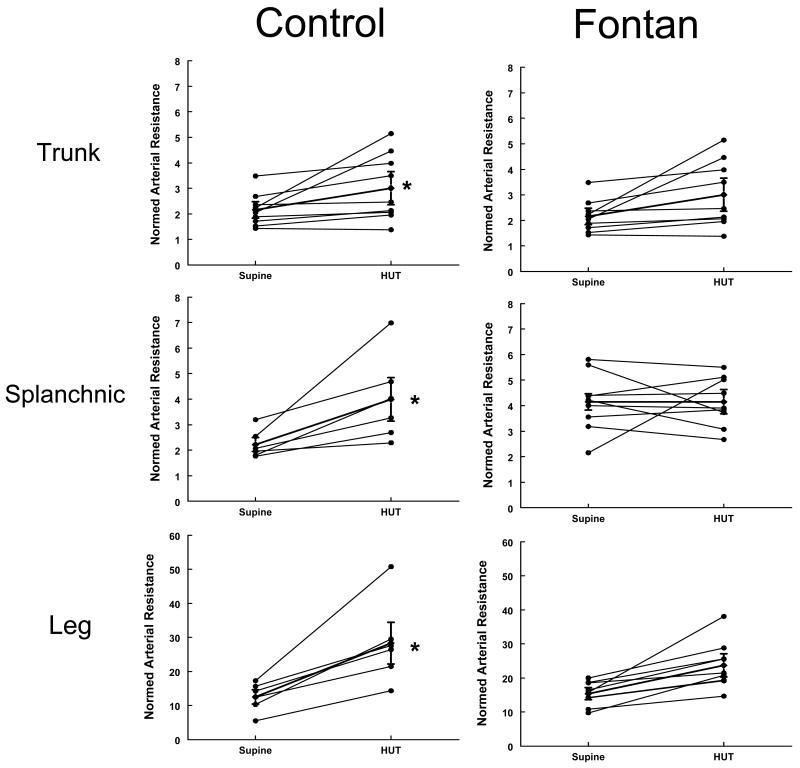
Fractional changes in resistances at baseline and after HUT in control (left panels) and Fontan subjects (right panels). The Y-axis shows the nomalized segmental arterial resistances (mmHg/ml/100mltissue/min) and X-axis shows the change in value between supine and HUT in each individual subject. The thicker line in each panel depicts the median for each group. *The change in resistances after HUT was significant in the control group (p<0.05 in each compartment), but not in the Fontan group. Note - the splanchnic segment showed no change at all.
Results
Results are quantified in Table 2 with median and range shown. The two groups were similar for age, height, weight, BSA, heart rate, respiratory rates and blood pressures.
Supine Hemodynamics
The Fontan subjects taking enalapril had blood pressures which were not statistically different from those of subjects not taking the medication. Fontan subjects had significantly lower ETCO2 throughout the testing period (p=0.001). Both groups had normal oxygen saturations, in the 97-100% range. There was no significant difference between peripheral blood flows in both arms and legs between the 2 groups by SPG measurements. The peripheral resistance was significantly elevated in the leg in Fontan subjects as compared to controls, but was comparable in the arms in the two groups.
Volume Pressure Relation (Capacitance) and peripheral venous properties by SPG
Figure 3 depicts arm and leg volume-pressure curves. Using Bonferroni correction, Ptime and P time *group in the arm curves were <0.001 and 0.044 and in the leg were 0.002 and 0.050 respectively (both curves being statistically significant over time and between groups). The curves show that as we increase the limb occlusive cuff pressure, the limb volume initially increases steeply, followed by a more gradual increase. The volume change in both arms and legs in the Fontan group was less than in the controls. The difference at each given pressure was statistically significant, suggesting reduced limb venous capacitance. The steeper slope of the capacitance relation in the control group suggests higher compliance as compared to Fontan subjects, thus implying “stiffer” capacitance vessels in the Fontan circulation. Forearm Pv was similar for both groups (median 8.9 vs 8.6 mm Hg) and did not reach statistical significance. Supine calf Pv was significantly (13 vs 10.5, p=0.004) increased in Fontan group, indicating increased supine ambient pressure in the leg veins. Figure 4 demonstrates the microvascular filtration relation (filtration rate vs Pv) in the arm and leg in Fontan vs control subjects. As Pv increased, the rate of filtration increased in both groups. There was no difference between the regression fits in the arms for Fontan and control subjects. Regression slopes were also not different in the legs, while the X intercept (Pi) was significantly greater in Fontan patients suggesting a higher filtration threshold in the leg. Thus, for a given venous pressure Pv, filtration rate is significantly smaller in the lower extremities in the Fontan group. Net microvascular filtration begins at significantly higher leg venous pressures (higher Pi) in the Fontan group compared to controls, implying a higher threshold for edema formation.
Head up Tilt (HUT) testing
Impedance and segmental volume changes (Figures 5 and 6). There were significant differences in the response to HUT in the 2 groups. In contrast to the normal increase in heart rate and diastolic pressure observed in control subjects, there was insignificant change in heart rate and blood pressures with HUT in the Fontan group. None of the subjects developed any symptoms of orthostatic intolerance (dizziness, nausea, heat, or headache) necessitating early return to supine position. In the control group, 4/6 subjects mentioned lightheadedness and some nausea, although the changes in hemodynamics did not dictate early return to supine position. At the baseline, compartmental arterial resistances (trunk, splanchnic and leg) were significantly higher in Fontan subjects compared to control subjects. Control subjects demonstrated the expected decrease in splanchnic (-32%) and trunk blood flow (-35%) and increase in splanchnic (+79%) and trunk arterial resistance (+86%) with orthostasis. In contrast, Fontan subjects showed unchanged splanchnic blood flow and arterial resistances (+4%and +8%) with orthostasis, while trunk flow decreased (-19%) and resistance increased (+39%) to a lesser extent compared to control subjects. As shown in figures 5 and 6, the segmental changes in both blood flows and arterial resistances in the Fontan group parallels the controls, however the changes were minimal and insignificant with HUT, especially in the splanchnic compartment. Figure 5 shows the directional changes in segmental blood flows in the thoracic, splanchnic and leg with HUT in individual control (left sided panels) and Fontan subjects (right sided panels) and Figure 6 shows the directional changes in resistances in individual control and Fontan subjects at baseline and after HUT.
Valsalva maneuver
In both the Fontan and control subjects, as expected, the Valsalva maneuver produced decreased trunk flow during early phase 2 (- 8% and - 15%) while splanchnic flow increased (1% vs 4%). The responses were again directionally similar but blunted in the Fontan group, although differences were not statistically significant in this small group of subjects.
Discussion
The “Fontan circulation” has long puzzled cardiologists and physiologists alike 2,3,6,11,28,29. Conceptually, at the level of the heart, systemic and pulmonary venous flows are separated and a single ventricle provides the pumping function. However, the sustainability of this situation over a lifetime after surgery has been a source of intense speculation and research.2-15, 16, 28, 29 The question why some patients remain symptom free in the face of such abnormal circulatory physiology whilst others become edematous, develop protein losing enteropathy and succumb early to cardiac failure (failed Fontan) remains unclear but is of paramount importance for patient health.2-14,16
This is the first study to systematically study alterations in arterial blood flow and in the peripheral venous capacitance and microvascular filtration properties in subjects who have successfully adapted to the Fontan repair. Circulatory adjustments and alteration of venous properties especially in the lower extremities have been postulated to result from chronic exposure to the Fontan circulation, with the venous pressure sustaining forward flow to the pulmonary circuit.3-5,8,10,11 Our study reports several key physiological findings in the peripheral circulation:
Vascular capacitance and compliance are decreased in post-Fontan patients to sustain the increased venous pressure needed to propel venous return from the legs. This results in increased venous pressure at any given level of venous volume thereby enhancing the gradient for venous return to the heart. This is of particular importance in a post Fontan patient while standing, in which increased distensibility at the higher venous pressures necessary for sustaining Fontan blood flow would produce markedly increased venous pooling in the lower extremities further compromising circulation. The level of lower limb venous pressure (Pv) impacts critically on microvascular filtration. The elevated Pv with lower capacitance is evidence of a stiffer lower extremity venous system in patients after creation of the Fontan circulation.3
Microvascular filtration: We have shown that the relationship of venous pressure to microvascular filtration is linear, with the Fontan subjects showing onset of filtration at significantly higher pressures than the controls. These data indicate a higher filtration threshold in Fontan subjects. This may be an adaptive mechanism developed in response to chronic exposure to the Fontan circulation, preventing the onset of edema. We speculate that failure of such adaptation in some patients may be responsible for edema and anasarca formation in the “failing Fontan” circulation.
Peripheral arterial resistance: Lower limb peripheral vascular resistance and splanchnic resistance were significantly elevated in Fontan subjects compared to control subjects, but the arm resistances were similar. This also suggests a chronic adaptive mechanism. The higher leg resistance post Fontan prevents leg pooling and enhances venous filling of the heart. Higher peripheral resistance increases elastic recoil of the capacitance vessels, thereby, increasing venous return to the heart and maintaining pre-load.3,4,5,28,30 A normal person suddenly given Fontan physiology would be expected to pool all the blood in the lower body when upright, thereby losing postural tone and perhaps causing death. However, chronic exposure to increased lower body venous pressure results in functional adaptations of the vasculature that sustain venous return and prevent fluid extravasation.30
Reduced ETCO2: The Fontan subjects had significantly lower end-tidal CO2 than the controls, despite equal respiratory rate and respiratory volumes. Gisolf et al in an elegant study of end tidal CO2 changes in human beings with postural change demonstrated that cardiac output changes made an important contribution to alveolar CO2.31 Senzaki et al tested well-functioning Fontan patients with beta- adrenergic stimulation and atrial pacing and demonstrated evidence of reduced cardiac output and B-adrenergic reserve, basically as a function of impaired pre-load and afterload properties of the circulation.32 Thus, chronic reduction in cardiac output may lead to alterations in gas exchange, reflected as reduced alveolar CO2 as was seen in our Fontan subjects. Further, relatively lower blood flow in the legs with higher arterial resistance and lower capacitance may cause relatively lower capillary circulation and alter gas exchange especially in the legs, reducing the amount of CO2 returning to the lungs.
Better orthostatic tolerance: Our studies showed that asymptomatic, well preserved Fontan patients had a significantly blunted response to HUT. The tilt does not translate blood as much out of the thoracic compartment. This is caused by reduced venous compliance (i.e. increased stiffness) as well as increased arterial resistance in Fontan subjects compared to control subjects. These combine to reduce venous pooling in general, in particular to reduce splanchnic pooling, which enhance venous return to the heart. Similar decreases in Valsalva responses are noted consistent with increased splanchnic venous stiffness and increased vasoconstriction.
Limitations of the study
Only asymptomatic Fontan patients were studied in the present study. This is because the study protocol was a demanding one and since all the tests were performed on a single day, each subject had to be able to tolerate the lengthy testing. Therefore we created a selection bias and our findings can only be applied to well-adapted post-Fontan patients. Nevertheless, the results of investigating the peripheral vasculature of these preselected subjects may provide clues as to successful adaptations of asymptomatic Fontan patients. Further studies using larger patient numbers and including symptomatic patients are needed. Treatment methods in edematous patients such as external compression to functionally decrease leg capacitance and increase leg peripheral resistance might be considered as acute therapy for such patients, if our results are confirmed in symptomatic patients as well. Our results indicate overall stiffer segmental vascular compartments, suggesting a contracted blood volume. However, blood volume estimates were not performed as a part of the study protocol in our subjects and controls. The data do suggest that the volume-pressure-capacitance relationship is altered in Fontan subjects, independent of the overall blood volume. The instruments used in our laboratory cannot be used in an inpatient setting and further modifications would be necessary to study acutely sick or early post-operative patients. These complex methodologies do not lend themselves to routine outpatient testing and are at present utilized only for research purposes. Additionally, 3/9 of our patients were on very low doses of enalapril (which did not alter their diastolic blood pressures), however other effects of angiotensin converting enzyme inhibitors (on the bradykinin system) were not measured as a part of the protocol and is a potential limitation in this study.
In summary, our data indicate that there are significant adjustments to the peripheral venous circulation noted in subjects who were evaluated several years after the Fontan procedure, which help to prevent development of edema, maintain venous return and to sustain cardiac output. Further, there is increased vascular resistance in other vascular compartments like the splanchnic and thoracic compartments, with less fluid shifts in response to orthostasis and valsalva maneuvers, leading to better orthostatic tolerance. One may infer a contracted blood volume, with poor tolerance to blood loss (or sudden vasodilatory stress like in sepsis), leading to sudden and severe hemodynamic deterioration, even in relatively asymptomatic Fontan subjects.
Acknowledgments
Funding Sources: Children's Hospital Foundation of Maria Fareri Children's Hospital and Westchester Medical Center and R01HL074873, R01HL087803, R21HL091948 from the National Heart Lung and Blood Institute of the National Institutes of Health.
Footnotes
Conflict of Interest: None to report for any of the authors.
This study involves 9 well compensated patients several years after the Fontan repair, who were compared with 6 age matched controls. Important differences between the Fontan and control subjects were seen in several vascular compartments. Calf capacitance was lower and resting venous pressure higher in the Fontan group, suggesting stiffer peripheral venous compartment especially in the lower extremities. The reduced venous compliance and increased filtration thresholds possibly act as adaptive mechanisms in maintaining venous return in Fontan circulation. These well compensated Fontan subjects also demonstrated superior orthostatic tolerance resulting from decreased compartmental fluid shifts in response to HUT and higher vascular resistance. This results from increased venous stiffness and decreased splanchnic capacitance and may also be an adaptive mechanism to maintain venous return in these patients while standing. One may infer a contracted blood volume, with poor tolerance to blood loss (or sudden vasodilatory stress like in sepsis), leading to sudden and severe hemodynamic deterioration, even in relatively asymptomatic Fontan subjects.
We believe that this study contributes to the understanding of the post-operative single-ventricle physiology- especially about compartmental fluid shifts as well as changes in the peripheral vascular properties in well compensated patients exposed to the Fontan circulation over a long period of time. This study would form the baseline for further studies both in the acute post operative period as well as in decompensated patients to study if these protective mechanisms were somehow deranged causing the circulatory decompensation in failing Fontan patients.
Contributor Information
Usha S. Krishnan, Pediatrics, NY Medical College.
Indu Taneja, Pediatrics, Medicine and Pharmacology, NY Medical College.
Michael Gewitz, NY Medical College.
Richard Young, NY Medical College.
Julian Stewart, Medicine and Physiology, NY Medical College.
References
- 1.Fontan F, Baudet E. Surgical repair of Tricuspid atresia. Thorax. 1971;26:240–8. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowgill D. The Fontan Procedure: A historical review. Ann Thorac Surg. 1991;51:1026–30. doi: 10.1016/0003-4975(91)91044-v. [DOI] [PubMed] [Google Scholar]
- 3.Kelley JR, Mack GW, Fahey JT. Diminished venous vascular capacitance in patients with univentricular hearts after the Fontan operation. Am J Cardiol. 1995;76:158–63. doi: 10.1016/s0002-9149(99)80049-6. [DOI] [PubMed] [Google Scholar]
- 4.Guyton AC, Abernathy H, Langston JB, Kaufmann BN, Fairchild HM. Relative importance of venous and arterial resistances in controlling venous return and cardiac output. Am J Physiol. 1959;196:1008–14. doi: 10.1152/ajplegacy.1959.196.5.1008. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RH, Becker AE, Tynan M, Macartney FJ, Rigby ML, Wilkinson JL. The univentricular atrioventricular connection: getting to the root of a thorny problem. Am J Cardiol. 1984;54:822–828. doi: 10.1016/s0002-9149(84)80214-3. [DOI] [PubMed] [Google Scholar]
- 6.Goff DA, Blume ED, Gauvreau K, Mayer JE, Lock JE, Jenkins KJ. Clinical outcome of fenestrated Fontan patients after closure: the first 10 years. Circulation. 2000;102:2094–2099. doi: 10.1161/01.cir.102.17.2094. [DOI] [PubMed] [Google Scholar]
- 7.Border WL, Syed AU, Michelfelder EC, Khoury P, Uzark KC, Manning PB, Pearl JM. Impaired systemic ventricular relaxation affects postoperative short-term outcome in Fontan patients. J Thorac Cardiovasc Surg. 2003;126:1760–4. doi: 10.1016/j.jtcvs.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Kaulitz R, Bergman P, Luhmer I, Paul T, Hausdorf G. Instantaneous pressure–flow velocity relations of systemic venous return in patients with univentricular circulation. Heart. 1999;82:294–299. doi: 10.1136/hrt.82.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohuchi H, Ohashi H, Takasugi H, Yamada O, Yagihara T, Echigo S. Restrictive ventilatory impairment and arterial oxygenation characterize rest and exercise ventilation in patients after fontan operation. Pediatr Cardiol. 2004;25:513–21. doi: 10.1007/s00246-003-0652-7. [DOI] [PubMed] [Google Scholar]
- 10.Szabo G, Buhmann V, Graf A, Melnitschuk S, Bahrle S, Vahl CF, Hagl S. Ventricular energetics after the fontan operation: Contractility-afterload mismatch. J Thorac Cardiovasc Surg. 2003;125:1061–1069. doi: 10.1067/mtc.2003.405. [DOI] [PubMed] [Google Scholar]
- 11.Houlind K, Stenbøg EV, Sørensen KE, Emmertsen K, Hansen OK, Rybro L, Hjortda VE. Pulmonary and caval flow dynamics after total cavopulmonary connection. Heart. 1999;8:67–72. doi: 10.1136/hrt.81.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohuchi H, Takasugi H, Ohashi H, Yamada O, Watanabe K, Yagihara T, Echigo S. Abnormalities of neurohormonal and cardiac autonomic nervous activities relate poorly to functional status in fontan patients. Circulation. 2004;110:2601–2608. doi: 10.1161/01.CIR.0000145545.83564.51. [DOI] [PubMed] [Google Scholar]
- 13.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after fontan-type operation. Circulation. 2003;107:3204–3208. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 14.Mace L, Dervanian P, Bourriez A, Mazmanian GM, Lambert V, Losay J, Neveux JY. Changes in venous return parameters associated with univentricular fontan circulations. Am J Physiol Heart Circ Physiol. 2000;279:2335–43. doi: 10.1152/ajpheart.2000.279.5.H2335. [DOI] [PubMed] [Google Scholar]
- 15.Buchhorn R, Bartmus D, Buhre W, Bursch J. Pathogenetic mechanisms of venous congestion after the fontan procedure. Cardiol Young. 2001;11:161–168. doi: 10.1017/s1047951101000051. [DOI] [PubMed] [Google Scholar]
- 16.Rychik J, Gui-Yang S. Relation of mesenteric vascular resistance after Fontan operation and protein losing enteropathy. Am J Cardiol. 2002;90:672–674. doi: 10.1016/s0002-9149(02)02584-5. [DOI] [PubMed] [Google Scholar]
- 17.Whitney RJ. The measurement of changes in human limb-volume by means of a mercury-in rubber strain gauge. J Physiol. 1949;109(12) Proc, 5. [PubMed] [Google Scholar]
- 18.Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2003;285:2749–56. doi: 10.1152/ajpheart.00429.2003. [DOI] [PubMed] [Google Scholar]
- 19.Stewart JM, Medow MA, Bassett B, Montgomery LD. Effects of thoracic blood volume on valsalva maneuver. Am J Physiol Heart Circ Physiol. 2004;287:798–804. doi: 10.1152/ajpheart.01174.2003. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JM, Medow MS, Montgomery LD, McLeod K. Decreased skeletal muscle pump activity in patients with postural tachycardia syndrome and low peripheral blood flow. Am J Physiol Heart Circ Physiol. 2004;286:1216–22. doi: 10.1152/ajpheart.00738.2003. [DOI] [PubMed] [Google Scholar]
- 21.Gamble J, Gartside IB, Christ F. A reassessment of mercury in silastic strain gauge plethysmography for microvascular permeability assessment in man. J Physiol. 1993;464:407–22. doi: 10.1113/jphysiol.1993.sp019642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart JM. Pooling in chronic orthostatic intolerance: Arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105:2274–2281. doi: 10.1161/01.cir.0000016348.55378.c4. [DOI] [PubMed] [Google Scholar]
- 23.Stewart JM. Microvascular filtration is increased in postural tachycardia syndrome. Circulation. 2003;107:2816–2822. doi: 10.1161/01.CIR.0000070951.93566.FC. [DOI] [PubMed] [Google Scholar]
- 24.Ebert TJ, Smith JJ, Barney JA, Merill DC, Smith GK. The use of thoracic impedence for determining thoracic blood volume changes in man. Aviat Space Environ Med. 1986;57:49–53. [PubMed] [Google Scholar]
- 25.Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2004;287:1319–27. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obeid AN, Barnett NJ, Dougherty G, Ward G. A critical review of laser Doppler flowmetry. J Med Eng Technol. 1990;14:178–181. doi: 10.3109/03091909009009955. [DOI] [PubMed] [Google Scholar]
- 27.Stewart JM, Medow MA, Montgomery LD, Glover JL, Millonas MM. Splanchnic hyperemia and hypervolemia during the valsalva maneuver in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2005;288:752–758. doi: 10.1152/ajpheart.00194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khairy P, Poirier N, Mercier LA. Univentricular heart. Circulation. 2007;115:800–812. doi: 10.1161/CIRCULATIONAHA.105.592378. [DOI] [PubMed] [Google Scholar]
- 29.Hsia TY, Khambadkone S, Deanfield JE, Taylor JF, Migliavacca F, De Leval MR. Subdiaphragmatic venous hemodynamics in the Fontan circulation. J Thorac Cardiovasc Surg. 2001;121:436–47. doi: 10.1067/mtc.2001.112527. [DOI] [PubMed] [Google Scholar]
- 30.Magder S. Vascular mechanics of venous drainage in dog hindlimbs. Am J Physiol Heart Circ Physiol. 1990;259:1789–95. doi: 10.1152/ajpheart.1990.259.6.H1789. [DOI] [PubMed] [Google Scholar]
- 31.Gisolf J, Wilders R, Immink RV, Van Lieshout JJ, Karemaker JM. Tidal volume, cardiac output and functional residual capacity determine end-tidal CO2 transient during standing up in humans. J Physiol. 2004;554:579–590. doi: 10.1113/jphysiol.2003.056895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senzaki H, Masutani S, Ishido H, Taketazu M, Kobayashi T, Sasaki N, Asano H, Katogi T, Kyo S, Yokote Y. Cardiac Rest and Reserve Function in Patients with Fontan Circulation. J Am Coll Cardiol. 2006;47:2528–35. doi: 10.1016/j.jacc.2006.03.022. [DOI] [PubMed] [Google Scholar]