Abstract
Asian women have lower rates of hip and forearm fractures despite lower areal BMD (aBMD) by DXA compared with white women and other racial groups. We hypothesized that the lower fracture rates may be explained by more favorable measurements of volumetric BMD (vBMD) and microarchitectural properties, despite lower areal BMD. To address this hypothesis, we used high-resolution pQCT (HRpQCT), a new method that can provide this information noninvasively. We studied 63 premenopausal Chinese-American (n = 31) and white (n = 32) women with DXA and HRpQCT. aBMD by DXA did not differ between groups for the lumbar spine (1.017 ± 0.108 versus 1.028 ± 0.152 g/cm2; p = 0.7), total hip (0.910 ± 0.093 versus 0.932 ± 0.134 g/cm2; p = 0.5), femoral neck (0.788 ± 0.083 versus 0.809 ± 0.129 g/cm2; p = 0.4), or one-third radius (0.691 ± 0.052 versus 0.708 ± 0.047 g/cm2; p = 0.2). HRpQCT at the radius indicated greater trabecular (168 ± 41 versus 137 ± 33 mg HA/cm3; p = <0.01) and cortical (963 ± 46 versus 915 ± 42 mg HA/cm3; p < 0.0001) density; trabecular bone to tissue volume (0.140 ± 0.034 versus 0.114 ± 0.028; p = <0.01); trabecular (0.075 ± 0.013 versus 0.062 ± 0.009 mm; p < 0.0001) and cortical thickness (0.98 ± 0.16 versus 0.80 ± 0.14 mm; p < 0.0001); and lower total bone area (197 ± 34 versus 232 ± 33 mm2; p = <0.001) in the Chinese versus white women and no difference in trabecular number, spacing, or inhomogeneity before adjustment for covariates. Similar results were observed at the weight-bearing tibia. At the radius, adjustment for covariates did not change the direction or significance of differences except for bone, which became similar between the groups. However, at the tibia, adjustment for covariates attenuated differences in cortical BMD and bone area and accentuated differences in trabecular microarchitecture such that Chinese women additionally had higher trabecular number and lower trabecular spacing, as well as inhomogeneity after adjustment. Using the high-resolution technology, the results provide a mechanistic explanation for why Chinese women have fewer hip and forearm fractures than white women.
Key words: race, Asian, volumetric BMD, microarchitecture, white
INTRODUCTION
Asian women have lower areal BMD (aBMD) as measured by DXA compared with white women and other racial groups.(1–5) Despite lower areal BMD, numerous studies indicate paradoxically low rates of hip fracture among Asian populations, a finding that has remained poorly understood.(6–8) A recent study also indicated a lower incidence of wrist and arm fractures in Asian women compared with whites and Hispanic women.(1)
DXA measures aBMD. aBMD is defined as BMC divided by bone area (expressed as g/cm2). As a 2D index, it is influenced by bone size. Moreover, it does not reflect true BMD, which is a 3D index (g/cm3). DXA underestimates true BMD in those with small bone size, whereas it overestimates true BMD in those with large bones.(9–11) A number of studies have indicated that when aBMD is adjusted for racial differences in body weight or when bone size is taken into account by calculating bone mineral apparent density from DXA measurements (which provides a value expressed in g/cm3 and gives an estimate of volumetric density(9,10)), BMD racial differences are attenuated.(6,12,13) This observation raised the possibility that a technology that can directly measure volumetric BMD (vBMD) and also give additional information about microarchitecture, noninvasively, might help to explain the apparent paradox in Chinese women whose fracture rates are lower despite having lower BMD by DXA.
Measurement of BMD by QCT offers the advantage of direct assessment of vBMD, which is not confounded by bone size. Although data regarding racial differences in vBMD are limited,(14–17) one recent study using central QCT (cQCT) has shown greater trabecular vBMD and greater cortical thickness at the proximal femur in Asian and black men.(15) As noted by the authors, it was unclear whether the significantly higher trabecular BMD observed in this study could have been caused, at least in part, by microarchitectural features because cQCT cannot resolve the image to measure these properties.(15)
HRpQCT is a new technology that allows measurement not only of trabecular and cortical vBMD separately but also permits assessment of trabecular and cortical microarchitecture noninvasively. Recent data suggest that alterations in bone microarchitecture as assessed by HRpQCT are associated with fracture and give key information that cannot be obtained by conventional DXA.(18) Differences in vBMD and microarchitectural elements by HRpQCT between Asian and white women could have important implications for addressing the paradox of lower aBMD and lower fracture rates among Asian women. The purpose of this study is to address the hypothesis that Asian women have higher vBMD and microarchitectural advantages in comparison with white women. To address this hypothesis, we used HRpQCT in a study of Chinese and white women.
MATERIALS AND METHODS
Subjects
Sixty-three premenopausal women (32 white and 31 Chinese American) were studied. We chose to study Chinese women, because according to one study, Chinese Americans have the lowest rates of hip fracture among any Asian-American subgroups.(19) Participants were recruited by newspaper and internet advertisements, flyers, and directly at primary care physician offices. Inclusion criteria were self-reported full Chinese or white descent (all four grandparents), regular menses, and age between 29 and 40 yr old. This age range was selected to study women who have reached peak bone mass and in whom a perimenopausal transition or menopause had not influenced bone and mineral metabolism. Women were screened by history and biochemical evaluation for conditions or medications known to affect bone metabolism. Exclusion criteria included amenorrhea ≥6 mo; current pregnancy or lactation; current hyperthyroidism, kidney dysfunction (glomerular filtration rate [GFR] ≤ 60 ml/min); liver dysfunction; intestinal malabsorption from any cause; history of malignancy other than nonmelanomatous skin cancer; metabolic bone disease; human immunodeficiency virus; organ transplantation; and current or past use of anti-epileptic medications, glucocorticoids >3 mo, tacrolimus, cyclosporine or methotrexate, bisphosphonates, teriparatide, raloxifene, calcitonin, or aromatase inhibitors. All patients gave written, informed consent. Patients were compensated for study participation and travel expenses. This study was approved by the Institutional Review Board of Columbia University Medical Center.
Clinical evaluation
Information regarding medical and surgical history, medications, tobacco and alcohol use, reproductive history, oral contraceptive (OCP) use, and exposure to sunlight was collected. Daily dietary calcium and vitamin D intake was assessed with a standardized food frequency questionnaire.(20) Physical activity was evaluated with the Modified Baecke Questionnaire, which allows for calculation of Baecke work, sport, and leisure physical activity indices.(21) Alcohol intake was assessed as number of drinks (1 drink = 12 oz beer, 5 oz wine, or 1 oz liquor) per week and converted to grams per week. Participants were considered alcohol users if they had at least one drink per month. Tobacco use was quantified as current, past, and never use. Pack-years were calculated for current and past smokers. Sun exposure was assessed by time spent outdoors per day (1 = <15 min; 2 = 15 min to 1 h; 3 = 1-2 h; 4 = ≥2 h). Weight and height were measured by balance beam and a wall-mounted, calibrated Harpenden stadiometer, respectively.
Biochemical evaluation
Serum calcium, creatinine, and alkaline phosphatase activity were measured with colorimetric or spectrophotometric methods. Intact PTH and thyroid stimulating hormone (TSH) were measured by chemiluminescence assay. 25-Hydroxyvitamin D was measured by liquid chromatography tandem mass spectrometry.
Bone densitometry and microarchitecture
aBMD was measured at the lumbar spine, L1–L4 (LS); total hip (TH); femoral neck (FN); and one-third radius (1/3 Rad) using a QDR 4500A (Hologic, Waltham, MA, USA). Participants were measured on the same densitometer, using the same software, scan speed, and technologist, certified by the International Society of Clinical Densitometry. In vivo precision, determined according to the standard method, at this facility is 1.28% at the lumbar spine, 1.36% at the hip, and 0.70% at the distal radius (one-third site).(22)
vBMD and microarchitecture were measured at the nondominant forearm and tibia using a HRpQCT instrument (XtremeCT; Scanco Medical, Bassersdorf, Switzerland) as previously described.(18,23,24) The following variables were assessed at the radius and tibia: total (D100), cortical (Dcort), and trabecular (Dtrab) vBMD; total cross-sectional area (CSAtot); cortical thickness (Ct.Th) and trabecular thickness (Tb.Th); bone volume to tissue volume (BV/TV, %); trabecular number (Tb.N), trabecular spacing (Tb.Sp), and trabecular inhomogeneity (Tb.1./N.SD). In vivo precision values at our center are 0.7–1.0% for density and 1.0–7.3% for trabecular architecture.
Statistics
Data are expressed as mean ± SD. Comparisons of clinical and biochemical variables between the Chinese-American and white groups were evaluated by independent two-sided t-test. Criterion values were adjusted for unequal variances where appropriate. Pearson correlation coefficients were computed for BMD and microarchitectural variables with each potential covariate. Covariates with p < 0.05 were included in multiple regression models. These included age, weight, tobacco use, age of menarche, Baecke sport index, PTH, and 25-hydroxyvitamin D. Although BMI, height, pregnancy number, time outdoors, and total calcium were associated with BMD and microarchitectural variables, they were not included in final models because they also correlated with other covariates showing stronger associations with the dependent variables.
BMD and microarchitecture for each site were first compared between the two racial groups without adjustment using two-sided t-tests and compared again after adjustment for the above covariates using generalized linear mixed models. Both unadjusted group means and means adjusted for covariates are reported to show the influence of covariates on estimated bone parameters. aBMD values adjusted only for body weight and age are also shown. For all analyses, a two-tailed p ≤ 0.05 was considered to indicate statistical significance. Statistical analysis was performed using SAS Version 9.1.3 (SAS Institute, Cary, NC, USA).
RESULTS
As shown in Table 1, Chinese and white women did not differ in age, BMI, calcium or vitamin D intake, or age at menarche. Chinese women were shorter, and there was a trend toward lower weight compared with white women. White women were more physically active in sports and during leisure compared with Chinese women. There was a trend toward less time outside exposed to sunlight in Chinese women. White women were more likely to drink alcohol (80% versus 45%; p = 0.005), although total intake in both groups was low. There was no difference in current tobacco use (3.1% versus 6.9%; p = 0.53). White women were more likely to use OCPs (43% versus 18%; p = 0.036) and had a longer duration of use. 25-Hydroxyvitamin D levels were lower and PTH levels were higher in Chinese compared with white women (Table 1).
Table 1.
Anthropometric, Lifestyle Factors, and Biochemical Data
| White (mean ± SD) | Chinese (mean ± SD) | p value (white vs. Chinese) | |
| Age (yr) | 34 ± 3.5 | 35 ± 3.6 | 0.51 |
| Height (in) | 64.8 ± 2.6 | 63.4 ± 1.9 | <0.02 |
| Weight (lb) | 135 ± 36 | 123 ± 11 | 0.08 |
| BMI (m/kg2) | 22.5 ± 5.0 | 21.6 ± 2.6 | 0.36 |
| Daily calcium intake (mg) | 1069 ± 528 | 873 ± 579 | 0.17 |
| Daily vitamin intake (IU) | 582 ± 442 | 490 ± 464 | 0.42 |
| Physical activity | |||
| Baecke work index | 2.4 ± 0.6 | 2.3 ± 0.5 | 0.38 |
| Baecke sport index | 1.6 ± 0.6 | 1.0 ± 0.6 | <0.01 |
| Baecke leisure index | 3.0 ± 0.5 | 2.6 ± 0.7 | <0.01 |
| Grams alcohol per week among users | 34 ± 33 | 21 ± 23 | 0.20 |
| Pack-years among past or current smokers | 5.3 ± 6.3 | 7.1 ± 7.7 | 0.65 |
| Age at menarche (yr) | 13.0 ± 1.4 | 12.6 ± 1.0 | 0.19 |
| OCP duration (yr) among past or current OCP users | 7.0 ± 6.4 | 2.8 ± 2.6 | <0.01 |
| Time outdoors score | 2.75 ± 0.95 | 2.35 ± 0.75 | 0.07 |
| Calcium (mg/dl) | 9.4 ± 0.3 | 9.5 ± 0.3 | 0.23 |
| Phosphorus (mg/dl) | 3.5 ± 0.6 | 3.4 ± 0.4 | 0.73 |
| Creatinine (mg/dl) | 0.78 ± 0.1 | 0.72 ± 0.1 | <0.05 |
| 25-Hydroxyvitamin D (ng/ml) | 38 ± 11 | 25 ± 9 | <0.001 |
| PTH (pg/ml) | 28 ± 12 | 36 ± 12 | <0.01 |
| Alkaline phosphatase (u/liter) | 49.1 ± 14.1 | 48.5 ± 12.4 | 0.87 |
aBMD by DXA at the LS, TH, FN, and 1/3 Rad did not significantly differ between the two groups (Table 2), although absolute values were lower at all sites in Chinese compared with white women. Mean T- and Z-scores, calculated using a white reference, were in the normal range. When aBMD was adjusted for covariates, BMD was higher at the LS, TH, and FN but not the 1/3 Rad in Chinese compared with white women. Covariates responsible for differences between unadjusted and adjusted group means differed by bone site: LS density (g/cm2) because of weight (p = 0.0007) and PTH (p = 0.003); FN density because of weight (p < 0.0001); and TH because of weight (p = 0.0004) and vitamin D (p = 0.023). aBMD adjusted for weight and age alone did not significantly differ between the white and Chinese groups (LS: 0.994 ± 0.110 versus 1.028 ± 0.110 g/cm2, p = 0.22; FN: 0.782 ± 0.095 versus 0.795 ± 0.095 g/cm2, p = 0.61; TH: 0.907 ± 0.105 versus 0.914 ± 0.105 g/cm2, p = 0.78; 1/3 Rad: 0.703 ± 0.052 versus 0.691 ± 0.052 g/cm2, p = 0.40).
Table 2.
aBMD by DXA
|
Unadjusted |
Adjusted* |
|||||
| White (mean ± SD) | Chinese (mean ± SD) | p value (white vs. Chinese) | White (least square mean ± SD) | Chinese (least square mean ± SD) | p value (white vs. Chinese) | |
| LS (g/cm2) | 1.028 ± 0.152 | 1.017 ± 0.108 | 0.73 | 0.975 ± 0.111 | 1.058 ± 0.120 | <0.03 |
| LS T-score | −0.2 ± 1.4 | −0.3 ± 1.0 | 0.72 | −0.7 ± 1.0 | 0.1 ± 1.1 | <0.03 |
| LS Z-score | −0.1 ± 1.4 | −0.2 ± 1.0 | 0.74 | −0.6 ± 1.0 | 0.2 ± 1.1 | <0.03 |
| FN (g/cm2) | 0.809 ± 0.129 | 0.788 ± 0.083 | 0.44 | 0.757 ± 0.104 | 0.831 ± 0.111 | <0.05 |
| FN T-score | −0.4 ± 1.2 | −0.6 ± 0.8 | 0.46 | −0.8 ± 0.9 | −0.2 ± 1.0 | <0.05 |
| FN Z-score | −0.2 ± 1.2 | −0.4 ± 0.8 | 0.49 | −0.7 ± 0.9 | 0.0 ± 1.0 | <0.05 |
| TH (g/cm2) | 0.932 ± 0.134 | 0.910 ± 0.093 | 0.47 | 0.866 ± 0.108 | 0.963 ± 0.116 | <0.01 |
| TH T-score | −0.1 ± 1.1 | −0.3 ± 0.8 | 0.48 | −0.6 ± 0.9 | 0.2 ± 0.9 | <0.01 |
| TH Z-score | 0.0 ± 1.1 | −0.2 ± 0.8 | 0.50 | −0.5 ± 0.9 | 0.3 ± 9 | <0.01 |
| One-third radius (g/cm2) | 0.708 ± 0.047 | 0.691 ± 0.052 | 0.18 | 0.695 ± 0.056 | 0.709 ± 0.059 | 0.44 |
| One-third radius T-score | 0.2 ± 0.8 | −0.1 ± 0.9 | 0.18 | 0.0 ± 0.9 | 0.2 ± 1.0 | 0.44 |
| One-third radius Z-score | 0.5 ± 0.8 | 0.2 ± 0.9 | 0.20 | 0.2 ± 0.9 | 0.5 ± 1.0 | 0.45 |
* Adjusted for age, weight, age of menarche, sport index, tobacco use, PTH, and 25-hydroxyvitamin D levels.
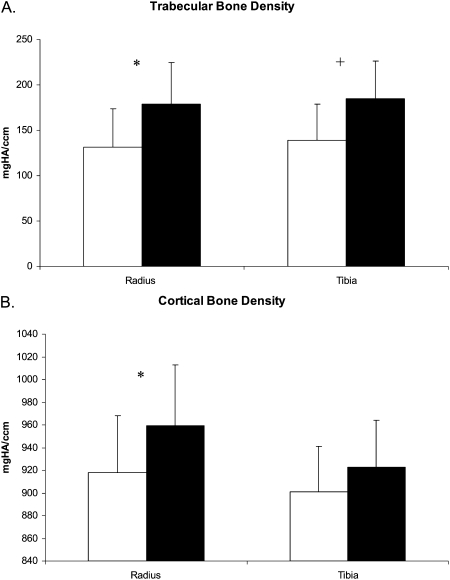
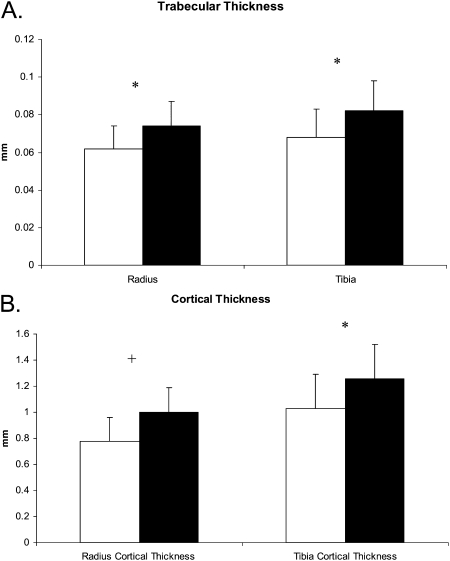
Before adjustment for covariates, there were significant racial differences as measured by HRpQCT (Table 3). Total CSA was 15% lower at the radius and 10% lower at the tibia in Chinese compared with white women. Trabecular BMD was 22% and 15% higher in Chinese compared with white women at the radius and tibia, respectively. Cortical BMD was 5% higher at the radius and 2% higher at the tibia in Chinese women compared with white women. Consistent with the trabecular BMD findings, BV/TV was also greater in Chinese compared with white women. Cortical thickness was 22.5% and 9% greater in Chinese compared with white women at the radius and radius, respectively. Trabecular thickness was also greater in Chinese women. There were no differences in trabecular number, spacing, or inhomogeneity at either the radius or tibia before adjustment of covariates.
Table 3.
vBMD and Microarchitecture
|
Unadjusted |
Adjusted* |
|||||
| White (mean ± SD) | Chinese (mean ± SD) | p value (white vs. Chinese) | White (least square mean ± SD) | Chinese (least square mean ± SD) | p value (white vs. Chinese) | |
| Radius | ||||||
| Densities | ||||||
| Average BMD (mg HA/cm3) | 321 ± 46 | 412 ± 70 | <0.0001 | 320 ± 71 | 418 ± 77 | <0.001 |
| Trabecular BMD (mg HA/cm3) | 137 ± 33 | 168 ± 41 | <0.01 | 131 ± 43 | 179 ± 46 | <0.01 |
| Cortical BMD (mg HA/cm3) | 915 ± 42 | 963 ± 46 | <0.0001 | 918 ± 50 | 959 ± 54 | <0.01 |
| Structure | ||||||
| Trabecular bone volume to tissue volume | 0.114 ± 0.028 | 0.140 ± 0.034 | <0.01 | 0.109 ± 0.035 | 0.149 ± 0.038 | <0.01 |
| Trabecular number (1/mm) | 1.84 ± 0.33 | 1.87 ± 0.32 | 0.73 | 1.76 ± 0.38 | 2.00 ± 0.41 | 0.06 |
| Trabecular thickness (mm) | 0.062 ± 0.009 | 0.075 ± 0.013 | <0.0001 | 0.062 ± 0.012 | 0.074 ± 0.013 | <0.01 |
| Trabecular spacing (mm) | 0.501 ± 0.125 | 0.476 ± 0.109 | 0.42 | 0.522 ± 0.141 | 0.440 ± 0.152 | 0.09 |
| Inhomogeneity of network (SD 1/Trab number) | 0.226 ± 0.123 | 0.212 ± 0.107 | 0.64 | 0.236 ± 0.142 | 0.195 ± 0.153 | 0.37 |
| Cortical thickness (mm) | 0.80 ± 0.14 | 0.98 ± 0.16 | <0.0001 | 0.78 ± 0.18 | 1.0 ± 0.19 | <0.001 |
| Total area (mm2) | 232 ± 33 | 197 ± 34 | <0.001 | 223 ± 38 | 207 ± 41 | 0.22 |
| Tibia | ||||||
| Densities | ||||||
| Average BMD (mg HA/cm3) | 275 ± 40 | 322 ± 59 | <0.001 | 268 ± 60 | 337 ± 61 | <0.001 |
| Trabecular BMD (mg HA/cm3) | 148 ± 35 | 170 ± 36 | 0.03 | 139 ± 40 | 185 ± 41 | <0.001 |
| Cortical BMD (mg HA/cm3) | 901 ± 34 | 921 ± 32 | 0.02 | 901 ± 40 | 923 ± 41 | 0.10 |
| Structure | ||||||
| Trabecular bone volume to tissue volume | 0.124 ± 0.029 | 0.142 ± 0.030 | 0.03 | 0.116 ± 0.033 | 0.154 ± 0.034 | <0.001 |
| Trabecular number (1/mm) | 1.81 ± 0.31 | 1.77 ± 0.25 | 0.59 | 1.70 ± 0.29 | 1.91 ± 0.30 | 0.03 |
| Trabecular thickness (mm) | 0.068 ± 0.012 | 0.080 ± 0.016 | <0.01 | 0.068 ± 0.015 | 0.082 ± 0.016 | <0.01 |
| Trabecular spacing (mm) | 0.500 ± 0.102 | 0.496 ± 0.078 | 0.84 | 0.535 ± 0.097 | 0.449 ± 0.099 | <0.01 |
| Inhomogeneity of network (SD 1/Trab number) | 0.226 ± 0.071 | 0.220 ± 0.041 | 0.71 | 0.249 ± 0.064 | 0.191 ± 0.065 | <0.01 |
| Cortical thickness (mm) | 1.1 ± 0.17 | 1.2 ± 0.24 | <0.01 | 1.03 ± 0.26 | 1.26 ± 0.26 | <0.01 |
| Total area (mm2) | 660 ± 82 | 596 ± 100 | 0.01 | 634 ± 98 | 615 ± 101 | 0.55 |
* Adjusted for age, weight, age of menarche, sport index, tobacco use, PTH, and 25-hydroxyvitamin D levels.
Adjustment for covariates did not change the direction or significance of differences of HRpQCT variables at the radius except for total area, which became similar between the groups (Table 3; Figs. 1 and 2). At the tibia, adjustment for covariates attenuated differences in cortical BMD and total area and increased differences in trabecular microarchitecture such that Chinese women had higher trabecular number and lower trabecular spacing, as well as inhomogeneity (Table 3). Consistent with these findings, there was a trend toward greater trabecular number and reduced spacing in Chinese compared with white women at the radius. Covariates responsible for differences between unadjusted and adjusted group means differed by bone site and HRpQCT measure: bone area because of weight (p = 0.0197 at the radius and p = 0.0028 at the tibia) and physical activity (p = 0.0049 at the radius and p = 0.06 at the tibia); and trabecular number, spacing, and inhomogeneity because of weight and vitamin D (all p < 0.01).
FIG. 1.
Racial differences in vBMD. Graphs depict mean (±SD) for (A) trabecular and (B) cortical BMD adjusted for age, weight, age of menarche, tobacco use, PTH, and 25-hydroxyvitamin D in white (white bars) and Chinese (black bars) women. *p < 0.01; +p < 0.001.
FIG. 2.
Racial differences in trabecular and cortical thickness. Graphs depict mean (±SD) for (A) trabecular and (B) cortical thickness adjusted for age, weight, age of menarche, tobacco use, PTH, and 25-hydroxyvitamin D in white (white bars) and Chinese (black bars) women. *p < 0.01; +p < 0.001.
DISCUSSION
Our results indicated that there are clear differences between Chinese and white women in trabecular and cortical BMD, as well as in skeletal microarchitecture, as measured by HRpQCT. These results were apparent before adjustment for any covariates such as weight. In contrast, the greater BMD by DXA at the spine and hip, but not at the radius, among Chinese women became apparent only after adjusted for weight and other covariates. These findings are consistent with the known effects of weight and/or bone size, as well as other biochemical and lifestyle factors on measurement of aBMD.(11,25) When we adjusted aBMD for weight and age alone, the racial groups did not significantly differ. This observation does not preclude the possibility that there would have been significant differences if our sample size was larger. Relevant to this point are the results showing that absolute values for BMD became higher at the LS, FN, and TH in the Chinese-American group compared with the white group. Although it has not yet been shown to predict fractures in prospective studies, the technology of HRpQCT could offer clear advantages over DXA in the measurement of BMD in Chinese women, because HRpQCT measurement of BMD is not influenced by bone size.
In unadjusted HRpQCT analyses, Chinese women had smaller bone size compared with white women, but higher trabecular and cortical BMD, and greater cortical and trabecular thickness. Adjustment for anthropometric (weight) and lifestyle factors (physical activity) attenuated differences in bone size at both the radius and tibia, suggesting that these factors may mediate, at least in part, differences in bone size. Adjustment for covariates (weight and vitamin D) increased racial differences in trabecular microarchitecture at the tibia greater than the radius such that Chinese women had greater trabecular number and lower trabecular spacing and inhomogeneity at the tibia after adjustment in addition to greater trabecular thickness. It is unclear why these microarchitectural differences were more apparent at the tibia rather than radius, but they may well be related to the greater effects of weight bearing at the tibia.
Recent data indicated that alterations in cortical and trabecular structure measured by HRpQCT are associated with fragility fractures.(18,23,24,26) In particular, one study indicated that osteopenic women with fractures have lower trabecular density and more trabecular inhomogeneity than women without fractures.(23) Another study showed that women with fragility fractures had lower total and trabecular volumetric BMD, BV/TV, cortical thickness, trabecular number, and trabecular thickness, whereas trabecular separation and inhomogeneity were greater. Therefore, differences in vBMD and microarchitecture that we observed in this study may confer advantages for Chinese women with regard to fracture risk.
Our results are consistent with the findings of Marshall et al.,(15) who also recently described greater trabecular density, smaller area, and greater cortical thickness at the proximal femur in Asian compared with white men. We extend their findings by providing evidence that not only are trabecular density and cortical thickness greater in Chinese compared with white women but that there are differences in trabecular microarchitecture. Additionally, we were able to adjust for the effects of calcitropic hormones (vitamin D and PTH), in addition to weight and lifestyle factors.
This study adds to our appreciation of the power of HRpQCT to discern differences in indices not measurable by DXA and thus helps to explain the problem at hand with regard to paradoxical observations on BMD measurements by DXA and fracture risk among white and Asian populations. Nevertheless, we do point out several limitations to this study. A number of other mechanisms to account for the discrepantly low hip fracture rates in Chinese women have been proposed and were not specifically examined in our study. Limited information from other studies points to shorter height,(27) lower incidence of falls,(28) and shorter hip axis length among Chinese women(29,30) as possible protective factors. High homocysteine levels have also recently been associated with altered collagen cross-linking, bone loss, and fracture risk.(31–34)
The cohort studied was a relatively small, convenience sample of women and thus the results could have been influenced by selection bias. The data are consistent, however, across other studies so that this is unlikely to be an issue. Additionally, we studied healthy premenopausal women, rather than postmenopausal, to avoid the potential confounding effect of racial differences in postmenopausal and age-related bone loss(35,36) and other factors (including medication use) that would have to be taken into account in the context of an aging population.(37–41) We do not know if the results would apply to postmenopausal women or those with medical conditions or taking medications that could influence BMD. Additional studies in postmenopausal women will be important to conduct.
Because race was self-reported, we can not exclude the possibility of racial diversity within the groups, although if present, this would have been expected to have diminished the ability to detect between group differences. Other limitations include those imposed by the methodology. Direct measurements of trabecular microstructure by HRpQCT are limited because the resolution of the XtremeCT is less than the width of individual trabeculae. Therefore, trabecular structure is assessed using a thickness-independent algorithm,(42,43) and results for trabecular microarchitecture could differ if obtained by direct analysis of this index by bone biopsies. These results show that Chinese women have higher trabecular and cortical BMD, as well as greater trabecular and cortical thickness at the radius and tibia than white women. Additionally, at the tibia, trabecular number is greater, whereas trabecular separation and inhomogeneity are lower. These differences may provide advantages with regard to fracture risk for Chinese women, thereby providing an explanation for their low rate of hip and forearm fractures.
ACKNOWLEDGMENTS
This work was supported by NIH Grants K23 AR053507 and UL1 RR024156, a National Osteoporosis Foundation grant, and the Mary and David Hoar Fellowship Program of the New York Community Trust and the New York Academy of Medicine. We thank Dr. Clyde Wu, whose vision and support were instrumental in the design and implementation of this study.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 2.Walker MD, Babbar R, Opotowsky AR, Rohira A, Nabizadeh F, Badia MD, Chung W, Chiang J, Mediratta A, McMahon D, Liu G, Bilezikian JP. A referent bone mineral density database for Chinese American women. Osteoporos Int. 2006;17:878–887. doi: 10.1007/s00198-005-0059-9. [DOI] [PubMed] [Google Scholar]
- 3.Woo J, Li M, Lau E. Population bone mineral density measurements for Chinese women and men in Hong Kong. Osteoporos Int. 2001;12:289–295. doi: 10.1007/s001980170118. [DOI] [PubMed] [Google Scholar]
- 4.Xiaoge D, Eryuan L, Xianping W, Zhiguang Z, Gan H, Zaijing J, Xiaoli P, Hongzhuan T, Hanwen W. Bone mineral density differences at the femoral neck and Ward's triangle: A comparison study on the reference data between Chinese and Caucasian women. Calcif Tissue Int. 2000;67:195–198. doi: 10.1007/s002230001139. [DOI] [PubMed] [Google Scholar]
- 5.Russell-Aulet M, Wang J, Thornton JC, Colt EW, Pierson RN., Jr Bone mineral density and mass in a cross-sectional study of white and Asian women. J Bone Miner Res. 1993;8:575–582. doi: 10.1002/jbmr.5650080508. [DOI] [PubMed] [Google Scholar]
- 6.Ross PD, He Y, Yates AJ, Coupland C, Ravn P, McClung M, Thompson D, Wasnich RD. Body size accounts for most differences in bone density between Asian and Caucasian women. The EPIC (Early Postmenopausal Interventional Cohort) Study Group. Calcif Tissue Int. 1996;59:339–343. doi: 10.1007/s002239900137. [DOI] [PubMed] [Google Scholar]
- 7.Ross PD, Norimatsu H, Davis JW, Yano K, Wasnich RD, Fujiwara S, Hosoda Y, Melton LJ., III A comparison of hip fracture incidence among native Japanese, Japanese Americans, and American Caucasians. Am J Epidemiol. 1991;133:801–809. doi: 10.1093/oxfordjournals.aje.a115959. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Lu A, Zhao X, Chen X, Cummings SR. Very low rates of hip fracture in Beijing, People's Republic of China the Beijing Osteoporosis Project. Am J Epidemiol. 1996;144:901–907. doi: 10.1093/oxfordjournals.aje.a009024. [DOI] [PubMed] [Google Scholar]
- 9.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 10.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 11.Cundy T, Cornish J, Evans MC, Gamble G, Stapleton J, Reid IR. Sources of interracial variation in bone mineral density. J Bone Miner Res. 1995;10:368–373. doi: 10.1002/jbmr.5650100306. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang MH, Greendale GA. Ethnic variation in bone density in premenopausal and early perimenopausal women: Effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3057–3067. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 13.Bhudhikanok GS, Wang MC, Eckert K, Matkin C, Marcus R, Bachrach LK. Differences in bone mineral in young Asian and Caucasian Americans may reflect differences in bone size. J Bone Miner Res. 1996;11:1545–1556. doi: 10.1002/jbmr.5650111023. [DOI] [PubMed] [Google Scholar]
- 14.Gilsanz V, Skaggs DL, Kovanlikaya A, Sayre J, Loro ML, Kaufman F, Korenman SG. Differential effect of race on the axial and appendicular skeletons of children. J Clin Endocrinol Metab. 1998;83:1420–1427. doi: 10.1210/jcem.83.5.4765. [DOI] [PubMed] [Google Scholar]
- 15.Marshall LM, Zmuda JM, Chan BK, Barrett-Connor E, Cauley JA, Ensrud KE, Lang TF, Orwoll ES. Race and Ethnic Variation in Proximal Femur Structure and BMD Among Older Men. J Bone Miner Res. 2008;23:121–130. doi: 10.1359/JBMR.070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bousson V, Bergot C, Meunier A, Barbot F, Parlier-Cuau C, Laval-Jeantet AM, Laredo JD. CT of the middiaphyseal femur: Cortical bone mineral density and relation to porosity. Radiology. 2000;217:179–187. doi: 10.1148/radiology.217.1.r00se11179. [DOI] [PubMed] [Google Scholar]
- 17.Nelson DA, Barondess DA, Hendrix SL, Beck TJ. Cross-sectional geometry, bone strength, and bone mass in the proximal femur in black and white postmenopausal women. J Bone Miner Res. 2000;15:1992–1997. doi: 10.1359/jbmr.2000.15.10.1992. [DOI] [PubMed] [Google Scholar]
- 18.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: The OFELY study. J Bone Miner Res. 2007;22:425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 19.Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J. Hip fracture incidence among elderly Asian-American populations. Am J Epidemiol. 1997;146:502–509. doi: 10.1093/oxfordjournals.aje.a009304. [DOI] [PubMed] [Google Scholar]
- 20.Hertzler A, Frary R. A dietary rapid assessment method (RAM) Top Clin Nutr. 1994;9:76–85. [Google Scholar]
- 21.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 22.Bonnick SL, Johnston CC, Jr, Kleerekoper M, Lindsay R, Miller P, Sherwood L, Siris E. Importance of precision in bone density measurements. J Clin Densitom. 2001;4:105–110. doi: 10.1385/jcd:4:2:105. [DOI] [PubMed] [Google Scholar]
- 23.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–6515. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 24.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23:392–399. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 25.Finkelstein JS, Sowers M, Greendale GA, Lee ML, Neer RM, Cauley JA, Ettinger B. Ethnic variation in bone turnover in pre- and early perimenopausal women: Effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3051–3056. doi: 10.1210/jcem.87.7.8480. [DOI] [PubMed] [Google Scholar]
- 26.Sornay-Rendu E, Cabrera-Bravo JL, Boutroy S, Munoz F, Delmas PD. Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res. 2009;24:737–743. doi: 10.1359/jbmr.081223. [DOI] [PubMed] [Google Scholar]
- 27.Lau EM, Suriwongpaisal P, Lee JK, Das De S, Festin MR, Saw SM, Khir A, Torralba T, Sham A, Sambrook P. Risk factors for hip fracture in Asian men and women: The Asian osteoporosis study. J Bone Miner Res. 2001;16:572–580. doi: 10.1359/jbmr.2001.16.3.572. [DOI] [PubMed] [Google Scholar]
- 28.Davis JW, Nevitt MC, Wasnich RD, Ross PD. A cross-cultural comparison of neuromuscular performance, functional status, and falls between Japanese and white women. J Gerontol A Biol Sci Med Sci. 1999;54:M288–M292. doi: 10.1093/gerona/54.6.m288. [DOI] [PubMed] [Google Scholar]
- 29.Cummings SR, Cauley JA, Palermo L, Ross PD, Wasnich RD, Black D, Faulkner KG. Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4:226–229. doi: 10.1007/BF01623243. [DOI] [PubMed] [Google Scholar]
- 30.Faulkner KG, Cummings SR, Black D, Palermo L, Gluer CC, Genant HK. Simple measurement of femoral geometry predicts hip fracture: The study of osteoporotic fractures. J Bone Miner Res. 1993;8:1211–1217. doi: 10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 31.Leboff MS, Narweker R, Lacroix A, Wu L, Jackson R, Lee J, Bauer DC, Cauley J, Kooperberg C, Lewis C, Thomas AM, Cummings S. Homocysteine levels and risk of hip fracture in postmenopausal women. J Clin Endocrinol Metab. 2009;94:1207–1213. doi: 10.1210/jc.2008-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrmann M, Peter Schmidt J, Umanskaya N, Wagner A, Taban-Shomal O, Widmann T, Colaianni G, Wildemann B, Herrmann W. The role of hyperhomocysteinemia as well as folate, vitamin B(6) and B(12) deficiencies in osteoporosis: A systematic review. Clin Chem Lab Med. 2007;45:1621–1632. doi: 10.1515/CCLM.2007.362. [DOI] [PubMed] [Google Scholar]
- 33.Blouin S, Thaler HW, Korninger C, Schmid R, Hofstaetter JG, Zoehrer R, Phipps R, Klaushofer K, Roschger P, Paschalis EP. Bone matrix quality and plasma homocysteine levels. Bone. 2009;44:959–964. doi: 10.1016/j.bone.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 34.Zhu K, Beilby J, Dick IM, Devine A, Soos M, Prince RL. The effects of homocysteine and MTHFR genotype on hip bone loss and fracture risk in elderly women. Osteoporos Int. 2008;20:1183–1191. doi: 10.1007/s00198-008-0804-y. [DOI] [PubMed] [Google Scholar]
- 35.Cauley JA, Lui LY, Stone KL, Hillier TA, Zmuda JM, Hochberg M, Beck TJ, Ensrud KE. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc. 2005;53:183–189. doi: 10.1111/j.1532-5415.2005.53101.x. [DOI] [PubMed] [Google Scholar]
- 36.Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in rate of decline in bone mass in older men: The Baltimore men's osteoporosis study. J Bone Miner Res. 2005;20:1228–1234. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 37.Brown AF, Perez-Stable EJ, Whitaker EE, Posner SF, Alexander M, Gathe J, Washington AE. Ethnic differences in hormone replacement prescribing patterns. J Gen Intern Med. 1999;14:663–669. doi: 10.1046/j.1525-1497.1999.10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babbar RK, Handa AB, Lo CM, Guttmacher SJ, Shindledecker R, Chung W, Fong C, Ho-Asjoe H, Chan-Ting R, Dixon LB. Bone health of immigrant Chinese women living in New York City. J Community Health. 2006;31:7–23. doi: 10.1007/s10900-005-8186-y. [DOI] [PubMed] [Google Scholar]
- 39.Mikuls TR, Saag KG, George V, Mudano AS, Banerjee S. Racial disparities in the receipt of osteoporosis related healthcare among community-dwelling older women with arthritis and previous fracture. J Rheumatol. 2005;32:870–875. [PubMed] [Google Scholar]
- 40.Wei GS, Jackson JL, Herbers JE., Jr Ethnic disparity in the treatment of women with established low bone mass. J Am Med Womens Assoc. 2003;58:173–177. [PubMed] [Google Scholar]
- 41.Thomas PA. Racial and ethnic differences in osteoporosis. J Am Acad Orthop Surg. 2007;15(Suppl 1):S26–S30. doi: 10.5435/00124635-200700001-00008. [DOI] [PubMed] [Google Scholar]
- 42.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24:35–39. doi: 10.1016/s8756-3282(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 43.Laib A, Ruegsegger P. Comparison of structure extraction methods for in vivo trabecular bone measurements. Comput Med Imaging Graph. 1999;23:69–74. doi: 10.1016/s0895-6111(98)00071-8. [DOI] [PubMed] [Google Scholar]