Abstract
QCT provides a measure of volumetric BMD (vBMD) and distinguishes trabecular from cortical bone. Few studies have determined the factors related to vBMD in men, especially among men of African heritage. This study evaluated the relationship of anthropometric, medical, and behavioral factors and vBMD in a population-based cohort of men of African ancestry (n = 1901) ≥40 yr of age who had undergone screening for prostate cancer for the first time. Trabecular and cortical vBMD were measured at the radius and tibia by pQCT. Multiple linear regression analysis identified age, height, body weight, cigarette smoking, history of diabetes, fracture, and prostate cancer as the independent correlates of vBMD. However, associations with several variables differed between cortical and trabecular vBMD and between the radius and tibia. Longitudinal studies are needed to gain a better understanding of the mechanisms underlying these differential associations that may show new insight into the etiology of trabecular and cortical bone loss in men.
Key words: osteoporosis, volumetric BMD, pQCT, men, African
INTRODUCTION
Osteoporosis is a global public health problem in men. Although the prevalence of osteoporosis(1) and osteoporotic fractures(2–5) is lower among men of African ancestry, the incidence of this disorder increases considerably with age(2–5) such that the number of affected men is expected to increase dramatically during the next half century.(6) In stark contrast to our understanding of bone health and osteoporotic risk in women, there is a paucity of information about skeletal health in men, particularly among men of African ancestry.
BMD measured by DXA has been widely used to diagnose osteoporosis and to identify risk factors for low BMD. However, DXA is a 2D imaging technique that quantifies areal BMD (aBMD), a measure that is confounded by differences in bone size between individuals. DXA also measures integral BMD and does not distinguish between trabecular and cortical bone. In contrast, QCT measures volumetric BMD (vBMD), which is not confounded by bone size, and yields a separate measure of trabecular and cortical BMD.
Factors related to DXA measures of aBMD in men are well established.(7–12) In contrast, few studies have characterized the factors related to trabecular and cortical vBMD in men. Moreover, there is limited information available on the correlates of BMD among men of African ancestry in general.(12–17) In this study, we examined the anthropometric, lifestyle, and medical factors related to trabecular and cortical vBMD in a large population-based study of men of African ancestry. An aim of our study was to identify the correlates of vBMD and to determine whether the factors associated with cortical and trabecular vBMD differ.
MATERIALS AND METHODS
Study population
Between 1997 and 2003, 3170 men were recruited for a population-based prostate cancer screening study on the Island of Tobago, Trinidad and Tobago.(18) Briefly, this observational cohort study examined prostate cancer prevalence and incidence in otherwise healthy men ≥40 yr of age. To be eligible, men had to be ambulatory, non-institutionalized, and not terminally ill. Recruitment for the study was accomplished by flyers, public service announcements, posters, informing health care workers at local hospital and health centers, and word of mouth. Approximately 60% of all age-eligible men on the island participated. The recruited cohort was 97% African, 2% East Indian, <1% white, and <1% “other” as defined by participant report of paternal and maternal grandparents' ethnicity.
Between 2004 and 2007, men in the cohort were invited to return for a repeat examination and to complete a pQCT scan. A total of 2031 men in the prostate cohort (70% of survivors) returned at the time of this analysis. At the follow-up visit, 451 new participants ≥40 yr of age were recruited using similar methods. Of these men, 2153 underwent pQCT scans at the radius and tibia. We excluded men with incomplete data or who were not of African ancestry from this analysis. This analysis is limited to the 1901 men of African descent with complete pQCT scans and available information from questionnaire interview at the time of analysis.
pQCT
pQCT was performed at the nondominant forearm and left tibia using the Stratec XCT-2000 scanner (Stratec Medizintechnik, Pforzheim, Germany). Technicians followed a standardized protocol for patient positioning and scanning. A scout view was obtained before the pQCT scan to define an anatomical reference line for the relative location of the subsequent scans at the radius and tibia. Tibia length was measured from the medial malleolus to the medial condyle of the tibia, and forearm length was measured from the olecranon to the ulna styloid process. Scans were taken at 4% and 33% of the total length of forearm and tibia, respectively. The scans at the 4% radius and tibia sites represent predominantly trabecular bone, whereas the scans at the 33% sites represent predominantly cortical bone. A single axial slice of 2.5 mm thickness with a voxel size of 0.5 mm and a speed of 20 mm/s was taken at all locations. Image processing was performed by a single investigator using the Stratec software package (Version 5.5E). All ultradistal radius and tibia scans were analyzed using identical parameters for contour finding and separation of trabecular and cortical bone (contour mode 2, T = 169 mg/cm3; peel mode 1, area = 45%) to determine the vBMD (mg/cm3) of the trabecular-rich bone compartment. All proximal radius and tibia shaft scans were analyzed using identical parameters for contour finding and separation of total and cortical bone (contour mode 2, T = 169 mg/cm3;cortmode 1, T = 710 mg/cm3) to determine the vBMD (mg/cm3) of the cortical-rich bone compartment. CVs were determined for pQCT scans by replicating measurements on 15 subjects (CV ≤ 2.1% for all measures). Daily phantom scans were analyzed to ensure long-term scanner stability.
Anthropometric and body composition measurements
Body weight was measured in kilograms with participants wearing light clothing and without shoes using a calibrated balance beam scale. Height was measured in centimeters without participants wearing shoes using a wall-mounted height board. Two height measurements were taken, and the average was used in analysis. Waist circumference was measured at the umbilicus with an inelastic tape measure. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Handgrip strength was measured in kilograms for both the left and right hands as a surrogate for upper body and overall strength using a dynamometer (Preston Grip Dynamometer; JA Preston).
Body composition (bone mineral–free lean tissue mass and fat mass) was measured using DXA on a Hologic QDR-4500W scanner (Hologic, Bedford, MA, USA). For all participants, the same scanner was used, and DXA scans were completed using the array beam mode. Standardized positioning and utilization of QDR software was based on the manufacturer's recommended protocol. Scans were analyzed with QDR software version 8.26a. To ensure consistency, the DXA technician scanned a spine phantom daily and completed a weekly quality control whole body air scan, before completing any participant scans.
Other measurements
Trained interviewers and nurses administered questionnaires to participants. We focused on potential correlates of BMD based on the body of literature for men and women. We collected information pertaining to demographic characteristics, medical history, fracture history, physical activity, and lifestyle variables. Ethnicity was self-reported, and participants provided detailed information on the ethnic origin of their parents and grandparents. Respondents were assigned to an ethnic group if they reported that all four grandparents as belonging to that group. Afro-Caribbean men who reported having less than four African grandparents were later categorized as mixed African ethnicity.
Participants were asked whether they had been diagnosed by a health care provider with selected conditions including cardiovascular disease, diabetes, hypertension, and prostate cancer. We also obtained information on personal and parental fracture history. Disability was assessed with questions about the degree of difficulty (no difficulty; some difficulty; much difficulty; or unable to perform activity) in six activities of daily living that involved the back (bending down to pick up light-weight objects, lifting a 10-lb object from the floor, reaching for objects just above the head, putting on socks or stockings, getting in and out of an automobile, and standing for 2 h). Men who answered having any difficulty or who were unable to perform any of the six activities because of back pain were considered to have “difficulty performing daily activity due to back pain.”
Men were also asked to report their use of selected medications. We documented if men underwent androgen deprivation treatment (ADT) by hormonal or surgical castration for prostate cancer. Participants also rated their overall health status compared with men their own age (excellent/good/fair/poor/very poor).
Smoking history was categorized as never, past, and current. Men were asked whether they had smoked at least 100 cigarettes in their lifetime. Men who smoked <100 cigarettes were considered to have never smoked. Weekly alcohol drinking in the past 12 mo was categorized as none, occasional drinking, and 1–3, 4–7, 8–14, 15–21, 22–27, and >28 drinks/wk. Physical activity was assessed by the frequency and duration of walking in the past 7 days for exercise, to work, the store, or church. We also used hours of television watching per week as a surrogate of physical inactivity.
Men also reported their daily consumption of coffee, tea, and soda (not decaffeinated). We assumed that one cup of coffee, tea, and soda contained 95, 55, and 45 mg of caffeine, respectively. Dietary calcium intake was assessed by frequency of selected food items including fish, bone chewing, green leafy vegetables, beans, milk, cheese, and cheese dishes that contain high dietary calcium and are frequently consumed in the local diet. Supplemental intake of calcium and vitamin D was also assessed.
Statistical analysis
ANOVA was used to compare the unadjusted skeletal site-specific vBMD across 5-yr age groups. We also evaluated the age-adjusted (data not shown) and age- and weight-adjusted association of each predictor with cortical and trabecular vBMD at the radius and tibia using linear regression analysis. The relationships between potential correlates and vBMD were expressed as one unit increase for categorical variables or ∼1 SD increase for continuous variables, along with 95% CIs. The formula used to calculate the percent difference in vBMD per unit change of independent variable was as follows: [(β coefficient × unit)/mean vBMD] × 100. The corresponding CIs were calculated as follows: {[(β coefficient ± 1.96 × SE) × unit]/mean vBMD} × 100. To identify the independent correlates of vBMD, multiple linear regression analysis was performed separately using a stepwise procedure for trabecular and cortical vBMD at the radius and tibia. Age was forced into each multiple linear regression model. Variables with p < 0.10 in the age- and weight-adjusted univariate linear regression model were entered into the multiple variable model. We also assessed the multicollinearity of predictor variables using the variance inflation factor (VIF). If there was evidence of collinearity, the model was re-evaluated. If variables were from the same domain (e.g., “ever smoked” and “currently smoke”), we selected the variable with a stronger association with vBMD. In addition, multiple linear regression analyses of vBMD at each skeletal site were performed for three separate models that included anthropometric or body composition variables: (1) modeling BMI; (2) modeling body weight and height separately; and (3) modeling total body fat and lean mass instead of BMI or body weight. Results were similar and thus only the results for weight and height are shown. All statistical analyses were performed using the Statistical Analysis System (SAS, version 9.1; SAS Institute, Cary, NC, USA).
RESULTS
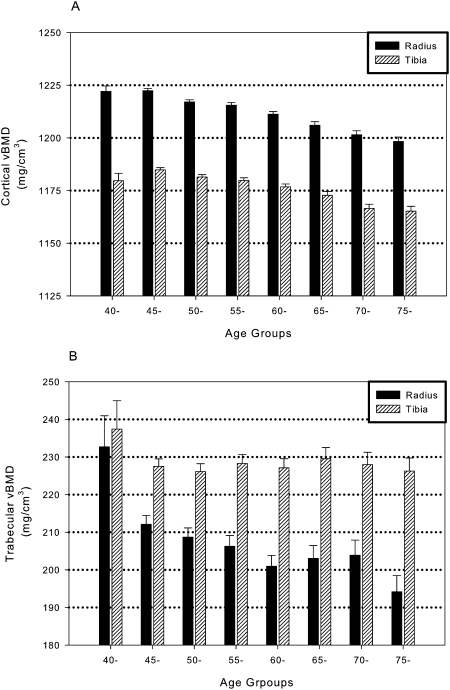
The mean age of the men was 59.1 ± 10.4 yr (range, 40–92 yr). The mean values and SD for trabecular and cortical vBMD were 206 ± 49 and 1213 ± 23 mg/cm3 at the radius and 228 ± 41 and 1177 ± 24 mg/cm3 at the tibia, respectively. Figure 1 showed the unadjusted age-related patterns for trabecular and cortical vBMD at each skeletal site. The oldest age group (75+) had 17% and 5% lower trabecular vBMD than the youngest age group (40–44 yr) at the radius (p < 0.0001) and tibia (p = 0.10), respectively. Men 45–49 yr of age had 9% (p = 0.01) and 4% lower (p = 0.01) trabecular vBMD than those 40–44 yr of age at the radius and tibia, respectively, but trabecular vBMD did not differ substantially between the older age groups. Cortical vBMD was 2% (p < 0.0001) and 1% (p = 0.0003) lower at the radius and tibia, respectively, among the oldest compared with youngest men.
FIG. 1.
Mean cortical (A) and trabecular (B) vBMD at the radius and tibia by 5-yr age group among men of African ancestry.
Age-adjusted regression results
Each SD (4.3 kg/m2) increase in BMI was associated with 3.0% and 4.6% higher trabecular vBMD and 0.37% and 0.36% lower cortical vBMD at the radius and tibia, respectively (Table 1). In general, the body weight and body composition–related variables were positively correlated with trabecular vBMD, whereas these variables were negatively associated with cortical vBMD. In addition, every 6.7-cm increase in height was associated with 4% lower trabecular vBMD at both the tibia and radius but 0.1% higher cortical vBMD at the radius.
Table 1.
Correlates of Trabecular and Cortical vBMD in Men of African Ancestry
| Variable | Mean (±SD) or frequency (prevalence) | Unit |
Age- and weight-adjusted percent difference in vBMD per unit (95% CI) |
|||
|
Radius |
Tibia |
|||||
| Trabecular vBMD | Cortical vBMD | Trabecular vBMD | Cortical vBMD | |||
| Age (yr)* | 59.1 ± 10.4 | 10 | −2.62 (−3.65, −1.58) | −0.62 (−0.70, −0.54) | −0.09 (−0.88, 0.69) | −0.52 (−0.61, −0.43) |
| Body weight (kg)† | 83.9 ± 14.5 | 10 | 1.11 (0.35, 1.87) | −0.26 (−0.31, −0.20) | 2.36 (1.79, 2.94) | −0.32 (−0.37, −0.25) |
| Standing height (cm) | 175.0 ± 6.7 | 6.7 | −4.07 (−5.25, −2.89) | 0.11 (0.02, 0.20) | −4.18 (−5.05, −3.30) | −0.08 (−0.17, 0.02) |
| BMI (kg/m2)† | 27.4 ± 4.3 | 4.3 | 3.00 (1.93, 4.07) | −0.37 (−0.45, −0.28) | 4.63 (3.83, 5.42) | −0.36 (−0.45, −0.27) |
| Waist circumference (cm) | 93.1 ± 10.7 | 10.7 | 0.76 (−1.46, 2.97) | 0.03 (−0.13, 0.20) | 2.70 (1.06, 4.35) | 0.15 (−0.03, 0.33)‡ |
| Grip strength (kg) | 42.7 ± 10.1 | 10.1 | 0.21 (−1.19, 1.60) | −0.09 (−0.19, 0.01)‡ | −1.24 (−2.29, −0.20) | −0.12 (−0.23, −0.00) |
| Daily dietary calcium intake (mg) | 465.4 ± 219.3 | 0.60 (−0.48, 1.68) | 0.02 (−0.07, 0.10) | 0.67 (−0.13, 1.48)‡ | −0.00 (−0.09, 0.08) | |
| Daily caffeine intake in top quartile | 364 (20.0) | 0.47 (−2.29, 3.23) | −0.20 (−0.41, 0.00)‡ | 0.99 (−1.07, 3.05) | −0.10 (−0.32, 0.12) | |
| Total body fat mass (kg)† | 17.6 ± 6.9 | 6.9 | 1.53 (0.45, 2.62) | −0.30 (−0.38, −0.22) | 3.29 (2.48, 4.11) | −0.33 (−0.42, −0.24) |
| Total body lean mass (kg)† | 64.2 ± 8.7 | 8.7 | 1.50 (0.33, 2.67) | −0.88 (−1.09, −0.67) | 6.88 (4.75, 9.01) | −1.16 (−1.39, −0.93) |
| Percent fat mass (%)† | 21.0 ± 5.9 | 5.9 | 5.15 (1.24, 9.06) | −0.36 (−0.49, −0.24) | 4.69 (2.48, 5.90) | −0.36 (−0.50, −0.23) |
| Mixed African ancestry | 170 (8.9) | −1.20 (−4.95, 2.55) | −0.37 (−0.65, −0.08) | 2.09 (−0.70, 4.89) | −0.31 (−0.61, −0.01) | |
| Diabetes | 311 (16.4) | 3.45 (0.48, 6.42) | 0.40 (0.18, 0.62) | 5.22 (3.01, 7.43) | 0.37 (0.13, 0.62) | |
| Hypertension | 620 (32.8) | 0.31 (−2.09, 2.71) | 0.04 (−0.14, 0.22) | 1.17 (−0.63, 2.96) | 0.10 (−0.09, 0.29) | |
| CVD | 67 (3.5) | 2.08 (−3.74, 7.9) | 0.20 (−0.24, 0.64) | 3.17 (−1.30, 7.63) | 0.07 (−0.42, 0.55) | |
| Prostate cancer | 291 (15.3) | −0.25 (−3.43, 2.92) | −0.39 (−0.63, −0.15) | 0.03 (−2.36, 2.43) | −0.33 (−0.59, −0.07) | |
| Androgen deprivation therapy | 61 (3.3) | 2.18 (−4.05, 8.41) | −0.84 (−1.33, −0.35) | 0.64 (−4.04, 5.32) | −0.88 (−1.42, −0.35) | |
| Excellent/good overall health | 1718 (91.3) | 1.74 (−2.14, 5.62) | −0.04 (−0.33, 0.25) | 0.27 (−2.65, 3.20) | −0.29 (−0.61, 0.02)‡ | |
| Personal history of fracture | 367 (19.3) | −3.24 (−5.97, −0.51) | −0.18 (−0.39, 0.02)‡ | −1.52 (−3.55, 0.51) | −0.26 (−0.48, −0.04) | |
| Maternal history of fracture | 80 (4.9) | −4.64 (−9.98, 0.69)‡ | 0.03 (−0.37, 0.43) | −1.50 (−5.45, 2.46) | −0.04 (−0.47, 0.38) | |
| Paternal history of fracture | 68 (4.3) | −0.80 (−6.62, 5.02) | −0.34 (−0.78, 0.10) | 0.50 (−3.77, 4.78) | −0.38 (−0.84, 0.09) | |
| Paternal or maternal fracture history | 141 (9.3) | −2.84 (−6.98, 1.30) | −0.16 (−0.47, 0.15) | −0.54 (−3.59, 2.51) | −0.26 (−0.59, 0.08) | |
| Difficulty performing daily activities caused by back pain | 176 (9.8) | −2.89 (−6.77, 0.98) | −0.20 (−0.49, 0.10) | −1.20 (−4.17, 1.76) | −0.11 (−0.43, 0.22) | |
| Ever smoked | 619 (32.7) | −5.16 (−7.44, −2.88) | −0.19 (−0.36, −0.02) | −3.89 (−5.59, −2.19) | −0.18 (−0.36, 0.01)‡ | |
| Smoking status | ||||||
| Past smoker vs. nonsmoker | 420 (22.2) | −3.74 (−6.36, −1.12) | −0.16 (−0.35, 0.04) | −3.06 (−5.02, −1.09) | −0.12 (−0.36, 0.06) | |
| Current smoker vs. nonsmoker | 199 (10.5) | −8.17 (−11.77, −4.58) | −0.27 (−0.54, −0.00) | −5.61 (−8.26, −2.96) | −0.23 (−0.52, 0.06) | |
| Alcohol intake ≥1 drink/wk | 358 (18.9) | −1.96 (−4.71, 0.79) | 0.13 (−0.08, 0.33) | −1.47 (−3.51, 0.57) | 0.12 (−0.10, 0.34) | |
| TV watching ≥14 h/wk | 722 (38.2) | 0.01 (−2.21, 2.22) | 0.12 (−0.04, 0.29) | −0.58 (−2.24, 1.07) | 0.19 (0.01, 0.37) | |
| Walk ≥3.5 h/wk | 545 (29.1) | −0.72 (−3.11, 1.68) | 0.06 (−0.12, 0.24) | 0.29 (−1.49, 2.08) | −0.00 (−0.20, 0.19) | |
| Walk ≥5–7 d/wk | 1192 (63.1) | 0.18 (−2.06, 2.42) | −0.11 (−0.28, 0.06) | 1.13 (−0.55, 2.79) | −0.03 (−0.21, 0.15) | |
| Bone chewing >5 d/wk | 278 (14.8) | 3.32 (0.27, 6.37) | 0.01 (−0.22, 0.23) | 2.38 (0.10, 4.65) | 0.01 (−0.24, 0.25) | |
| ≥1 glass of milk/d during teens | 1588 (84.2) | 0.57 (−2.38, 3.52) | −0.02 (−0.24, 0.20) | 0.73 (−1.48, 2.94) | 0.07 (−0.17, 0.31) | |
| ≥1 glass of milk/d at age 18–50 yr | 1260 (67.0) | 1.28 (−1.05, 3.61) | 0.02 (−0.15, 0.19) | 1.24 (−0.50, 2.97) | 0.03 (−0.16, 0.21) | |
| Calcium supplement ≥3 times/wk | 323 (17.5) | −1.45 (−4.31, 1.41) | 0.04 (−0.17, 0.26) | −1.27 (−3.40, 0.86) | −0.05 (−0.28, 0.18) | |
| Vitamin D supplement ≥3 times/wk | 221 (12.1) | 0.66 (−2.68, 3.99) | −0.03 (−0.28, 0.22) | 1.31 (−1.19, 3.80) | −0.24 (−0.51, 0.03) | |
| Use of nonthiadize diuretics | 26 (1.4) | −1.65 (−11.11, 7.80) | −0.03 (−0.78, 0.72) | 0.06 (−6.96, 7.08) | −0.45 (−1.25, 0.35) | |
| Use of thiadize diuretics | 195 (10.5) | 1.20 (−2.40, 4.80) | 0.01 (−0.26, 0.28) | 0.61 (−2.11, 3.32) | 0.07 (−0.22, 0.36) | |
| Use of glucocorticoids | 12 (0.64) | −2.60 (−16.14, 10.9) | −0.26 (−1.32, 0.79) | −3.00 (−13.99, 7.99) | −0.12 (−1.29, 1.06) | |
Bold indicates p < 0.05.
* Unadjusted
† Age adjusted only.
‡ Variables with p value between 0.05 and 0.10.
The prevalence of self-reported diabetes was 16.4% and diabetes was positively associated with vBMD at all four skeletal sites. For example, vBMD was ∼4% and 5% higher at the radius and tibia for trabecular vBMD but was only ∼0.4% higher at the radius and tibia for cortical vBMD among men with compared with without diabetes. Mixed African ancestry, prostate cancer history, and ADT (orchiectomy or currently taking Leuprorelin) were each associated with 0.3% to 0.9% lower cortical vBMD, but these variables were not associated with trabecular vBMD. Men who had experienced a previous fracture had lower vBMD compared with those without a history of fracture, but the association was only significant for trabecular vBMD at the radius and cortical vBMD at the tibia. Use of thiazide or nonthiazide diuretics was not associated with trabecular or cortical vBMD. Glucocorticoid use was low in this population and was not significantly associated with vBMD at either skeletal site.
Men who smoked >100 cigarettes in their lifetime had a 4–5% lower trabecular vBMD and ∼0.2% lower cortical vBMD than nonsmokers. Moreover, the association between smoking and vBMD was greater for current smokers than past smokers compared with nonsmokers. Alcohol drinking and physical activity were not significantly correlated with vBMD. Fifteen percent of the men chewed animal or fish bones at least 5 d/wk. Bone chewing was associated with 2–3% higher trabecular vBMD at both skeletal sites but was not associated with cortical vBMD. No other dietary-related factor was associated with vBMD including supplemental use of calcium and vitamin D, milk consumption, and caffeine intake.
Results from the multiple linear regression model
Age (−), body weight (+), standing height (−), diabetes (+), personal fracture history (−), cigarette smoking (−), and bone chewing (+) were independently associated with trabecular vBMD at the radius and explained 6% of the variation in vBMD at this skeletal site (Table 2). These variables, except fracture history, collectively explained 10% of the variation in trabecular vBMD at the tibia. Multiple linear regression models explained 16% and 13% of the variation in cortical vBMD at the radius and tibia, respectively. At the radius, age (−), body weight (−), standing height (+), grip strength (−), mixed African ethnicity (−), diabetes (+), prostate cancer (−), androgen deprivation therapy (−), and smoking (−) were significant correlates of cortical vBMD. However, for cortical vBMD at the tibia, only age (−), body weight (−), diabetes (+) and ADT (−) remained in the final model. Multiple linear regression models that included BMI or body fat and lean mass instead of body weight yielded similar results (data not shown). For instance, BMI was positively associated with trabecular vBMD, but negatively associated with cortical vBMD. Results were also similar after excluding men who had undergone ADT (data not shown).
Table 2.
Correlates of vBMD in Multivariable Models*
|
Variables |
Unit |
Percent difference in vBMD per unit (95% CI) |
|||
|
Radius |
Tibia |
||||
|
Trabecular vBMD† |
Cortical vBMD‡ |
Trabecular vBMD§ |
Cortical vBMD¶ |
||
| N | 1868 | 1805 | 1828 | 1801 | |
| Age (yr) | 10 | −3.30 (−4.42, −2.17) | −0.70 (−0.81, −0.60) | −0.44 (−1.27, 0.39)** | −0.63 (−0.72, −0.54) |
| Weight (kg) | 10 | 1.83 (1.00, 2.65) | −0.28 (−0.34, −0.22) | 4.52 (3.63, 5.40) | −0.31 (−0.38, −0.25) |
| Height (cm) | 6.7 | −3.87 (−5.06, −2.68) | 0.15 (0.05, 0.24) | −3.96 (−4.83, −3.07) | — |
| Grip strength (kg) | 10.1 | — | −0.13 (−0.24, −0.02) | — | |
| Mixed African ancestry | — | −0.37 (−0.66, −0.09) | — | — | |
| Diabetes | 3.40 (0.46, 6.35) | 0.39 (0.16, 0.61) | 5.18 (3.02, 7.35) | 0.41 (0.17, 0.65) | |
| Prostate cancer | — | −0.26 (−0.52, −0.01) | — | — | |
| ADT | — | −0.68 (−1.19, −0.18) | — | −0.86 (−1.40, −0.33) | |
| Personal fracture history | −3.01 (−5.71, −0.30) | — | — | — | |
| Smoking | |||||
| Past smoker vs. nonsmoker | −2.95 (−5.57, −0.33) | −0.20 (−0.40, −0.002) | −2.48 (−4.40, −0.55) | — | |
| Current smoker vs. nonsmoker | −7.30 (−10.89, −3.72) | −0.28 (−0.55, −0.004) | −4.84 (−7.43, −2.24) | — | |
| Chew bones >5 d/wk | 3.25 (0.25, 6.25) | — | 2.32 (0.11, 4.53) | — | |
| R2 | 0.06 | 0.16 | 0.10 | 0.13 | |
* Corresponding variables entered in multivariable model for each vBMD had p < 0.1; only those with p < 0.05 remained in the model.
† Age, weight, height, diabetes, fracture history, smoking (past and current smoker vs. nonsmoker), and bone chewing were enter into the model.
‡ Age, weight height, grip strength, mixed African ancestry, diabetes, fracture history, prostate cancer, ADT, top quartile caffeine intake, and smoking (past and current smoker vs. nonsmoker) were entered into the model.
§ Age, weight, height, grip strength, diabetes, daily calcium intake, smoking (past and current smoker vs. nonsmoker), and bone chewing were entered into the model.
¶ Age, weight, grip strength, mixed African ancestry, diabetes, prostate cancer, ADT, fracture history, ever smoked (dichotomous), TV watching, and good overall health were entered into the model.
** p > 0.5 (age was forced into the multiple linear regression model).
DISCUSSION
Osteoporosis is a growing public health problem and will increasingly affect populations of African ancestry throughout the world as the number of elderly increases.(6) To better understand the factors that influence bone mass in men of African ancestry, we comprehensively assessed anthropometric, lifestyle, and medical variables and examined their relationship with vBMD at the radius and tibia in a large population sample of middle-aged and elderly men. Several features of our study were unique, including its large sample size, careful measurement of variables, a focus on both cortical and trabecular volumetric BMD, and the wealth of information available about the study cohort. Our results validate associations described previously among white men, such as the major importance of body weight, but also illuminate previously unrecognized relationships such as an inverse correlation between weight, BMI, or adiposity with cortical vBMD. We also documented a novel association of mixed African ancestry and a strong association of ADT with cortical vBMD.
BMD decreases with advancing age in men of all race and ethnic backgrounds, and this trend most likely contributes to the increase in fracture rates with aging. In this study of African ancestry men, we observed different age-related patterns for cortical and trabecular vBMD. Similar results have been observed in other recent studies, but these reports have included only white men.(19,20) We found that cortical vBMD seemed to decrease cross-sectionally more slowly with advancing age than trabecular vBMD and the age pattern seemed to be more linear for cortical vBMD. The largest decline in trabecular vBMD occurred among men 40–44 and 45–49 yr of age and then seemed to decline more slowly at the radius or remain stable at the tibia. The apparent age declines were greater for trabecular than cortical vBMD and were greater at the radius than tibia. Although our study did not include men ≤40 yr of age, our results among men of African ancestry are consistent with the recent observations of an early reduction of trabecular vBMD beginning before midlife in men.(19,21)
DXA measures of aBMD are one of the most important predictors of osteoporotic fracture risk. Fewer studies have examined pQCT or QCT measures of vBMD and fracture in men, particularly among men of African ancestry. Among men and women, those with vertebral fracture have lower cortical and trabecular vBMD compared with controls.(22) Each SD decrease in cortical, but not trabecular, vBMD at the radius was associated with a 16-fold risk of fracture among 52 hemodialysis patients.(23) QCT measures of proximal femur trabecular vBMD was related to subsequent fracture risk largely among white men ≥65 yr of age.(24) In the Health Aging and Body Composition Study, the age-adjusted hazard ratio (HR) of nonspine fractures per SD decrease in BMD was highest among black men, particularly for QCT measures of spinal trabecular vBMD (HR = 3.00, 95% CI = 1.29, 7.00).(25) In this study, men with a personal history of fracture had significantly lower trabecular, but not cortical, vBMD at the radius compared with men without a history of fracture.
Previous studies using ancestry informative molecular markers in this Afro-Caribbean population have indicated that the ancestral proportions are 94.0% African, 4% European, and 1% Native American.(26) Despite the low level of non-African ancestry in this population, we still observed that mixed African ancestry was associated with lower cortical vBMD at the radius. These results are consistent with previous studies that have observed lower aBMD among blacks who have greater European ancestry.(27) The high proportion of African ancestry and negative impact of admixture on vBMD raises the possibility that this population may benefit from an increased prevalence of alleles of African origin that confer a skeletal advantage. The identification of these beneficial alleles may provide fundamental insight on bone biology and suggest new therapeutic targets for increasing bone mass and strength.
Previous studies have found that greater body weight is associated with higher aBMD in whites and blacks.(9,10,16) In this study, greater body weight, fat mass, and lean mass were all associated with higher trabecular vBMD. In contrast, each of these variables was associated with significantly lower cortical vBMD. Our results are consistent with the recent findings of Lorentzon et al.,(28) who showed that greater fat mass is associated with lower cortical vBMD at the tibia. The potential physiological mechanisms underlying the fat–bone connection are unclear, but increased adipocytes in the bone marrow compartment among heavier subjects may suppress osteoblastogenesis and contribute to decreased cortical vBMD by producing adipokines in the local bone microenvironment.(29) Additional research is needed to better understand the possible link between increased adiposity and decreased cortical vBMD.
Diabetes was associated with greater cortical and trabecular vBMD even after adjustments for age, body weight, and other covariates. The association was stronger for trabecular than cortical vBMD. A higher trabecular vBMD was observed in diabetic than nondiabetic black women and white men in the Health, Aging, and Body Composition Study.(30) However, a similar association was not observed among white women or black men in that study.(30) In the Diabetes Heart Study, trabecular vBMD was not associated with diabetes independently from BMI.(31) The mechanisms underlying the increased BMD in diabetes remain unclear but may involve the osteogenic effects of hyperinsulinemia.(32,33)
Prostate cancer remains the most common cancer in American men and disproportionately afflicts more men of African ancestry worldwide than other ethnic and racial groups.(18,34,35) ADT, achieved surgically through orchidectomy or through gonadotropin-releasing hormone agonists, is the traditional first-line treatment for metastatic prostate cancer and is increasingly being used in the treatment of localized, high-risk prostate cancer.(36) The prevalence of ADT may be increasing: by 2000, ∼3% of men in the U.S. Medicare fee-for-service program alone were androgen deprived.(37) Approximately 5% (n = 94) of the men in this analysis had a history of ADT. These men had significantly reduced cortical vBMD independent of other variables. The magnitude of the effect on cortical vBMD was similar to or greater than the effect of 10 yr of age on cortical vBMD in our study. Androgens are important for the development and maintenance of bone mass.(38,39) ADT for prostate cancer has been associated with a rapid and marked loss of DXA measures of aBMD(40–47) and with an increased risk of fracture.(36,48–53) The vast majority of men in these past studies were white. To our knowledge, this is the first study to document the association of ADT with BMD exclusively among men of African ancestry. These findings are of potential clinical importance given the higher risk of prostate cancer among African ancestry men.
The absence of an association between ADT and trabecular vBMD in the radius and tibia in this study was somewhat surprising given the established influence of androgens on trabecular bone. The effects of androgens on trabecular vBMD may differ for the axial and appendicular skeleton. For instance, Zhang et al.(54) found that orchiectomy in growing male rats produced a 24% reduction in bone volume and a 43% decrease in BMC of the femur. Because the decrease in bone mineral was greater than bone volume, femoral vBMD was significantly reduced (−30%). In contrast, vBMD at the lumbar spine did not change significantly in orchiectomized rats because bone volume (−26%) and BMC (−26%) decreased proportionately. Thus, bone fragility in androgen-deficient male rats results from decreased bone size and mass in the femur and from decreased size but not mass in the lumbar spine. Nonetheless, we may have had limited power to detect a difference in trabecular vBMD in our cross-sectional analysis.
Cigarette smoking was associated with lower trabecular but not cortical vBMD in our study. The Gothenburg Osteoporosis and Obesity Determinants study also did not observe a difference in cortical vBMD between white European men who did and did not smoke.(55) The effect of smoking on trabecular vBMD in this study was greater among current smokers but was also evident among past smokers. This suggests that smoking may have a long-lasting effect on trabecular vBMD. The association with vBMD was also observed in the multivariable models, indicating that the association was independent of other potentially intervening variables such as body weight and diabetes. The mechanisms by which cigarette smoking influences BMD are unclear but nicotine may directly affect bone metabolism and BMD by inhibiting the proliferation of osteoprogenitor cells in a concentration-dependent manner.(56,57)
Our multivariate models explained up to 16% of the variance in cortical vBMD and 10% of the variance in trabecular vBMD. Although modest, these estimates compare well with other reports of DXA measures of aBMD in men and women(11,12,58–60) and trabecular vBMD in men.(15) Nonetheless, our analysis suggests that many other undetermined variables, including inherited factors, may contribute to cortical and trabecular vBMD in men of African ancestry.
In multivariate analyses, we found no association of vBMD with several lifestyle and behavioral variables including physical activity and intake of alcohol, caffeine, and dietary calcium. Use of diuretics was also not associated with vBMD in this study. Although dietary calcium intake was not associated with vBMD, we did observe an association between chewing bones and high trabecular vBMD in multivariable models. The absence of an association with some variables could reflect low statistical power to detect an association or the inherent difficulty in quantifying some variables with questionnaires.
This study has several potential limitations. First, the cross-sectional design limits our ability to establish temporal relationships with trabecular and cortical vBMD. Confirmation of our results with longitudinal evaluations would be useful, particularly for establishing the magnitude, tempo and natural history of trabecular and cortical bone loss in this population. We also examined Afro-Caribbean men who were volunteers and their characteristics may differ from those of other groups. Information collected by questionnaires depended on participants' recall and this may have limited our ability to detect relationships. We also did not have measures of serum vitamin D or other hormones that may contribute to cortical and trabecular vBMD.
In summary, our study of a large population sample of middle-aged and older men of African ancestry supports the importance of several factors associated with trabecular and/or cortical volumetric BMD. Trabecular vBMD may decline before middle-age whereas cortical volumetric BMD may decline later in life among men of African ancestry. From a clinical and public health perspective, our findings reinforce the need to avoid cigarette smoking, and identify an inverse impact of androgen deprivation on cortical bone density. Our study also revealed a potentially negative impact of elevated body weight and adiposity on cortical vBMD. Moreover, longitudinal studies are needed to confirm these cross-sectional relationships.
ACKNOWLEDGMENTS
This study was supported in part by National Institutes of Health Grants R01-AR049747 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and R01-CA84950 from the National Cancer Institute.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Looker AC, Orwoll ES, Johnston CC, Jr, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 2.Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, Tosteson T. Racial differences in fracture risk. Epidemiology. 1994;5:42–47. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Hip fracture incidence among the old and very old: A population-based study of 745,435 cases. Am J Public Health. 1990;80:871–873. doi: 10.2105/ajph.80.7.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman SL, Madison RE. Decreased incidence of hip fracture in Hispanics, Asians, and blacks: California Hospital Discharge Data. Am J Public Health. 1988;78:1482–1483. doi: 10.2105/ajph.78.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin MR, Ray WA, Fought RL, Melton LJ., III Black-white differences in fracture rates. Am J Epidemiol. 1992;136:1378–1385. doi: 10.1093/oxfordjournals.aje.a116450. [DOI] [PubMed] [Google Scholar]
- 6.Cooper C, Campion G, Melton LJ., III Hip fractures in the elderly: A world-wide projection. Osteoporos Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 7.Hannan MT, Felson DT, Anderson JJ. Bone mineral density in elderly men and women: Results from the Framingham osteoporosis study. J Bone Miner Res. 1992;7:547–553. doi: 10.1002/jbmr.5650070511. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen TV, Eisman JA, Kelly PJ, Sambrook PN. Risk factors for osteoporotic fractures in elderly men. Am J Epidemiol. 1996;144:255–263. doi: 10.1093/oxfordjournals.aje.a008920. [DOI] [PubMed] [Google Scholar]
- 9.Bendavid EJ, Shan J, Barrett-Connor E. Factors associated with bone mineral density in middle-aged men. J Bone Miner Res. 1996;11:1185–1190. doi: 10.1002/jbmr.5650110818. [DOI] [PubMed] [Google Scholar]
- 10.Glynn NW, Meilahn EN, Charron M, Anderson SJ, Kuller LH, Cauley JA. Determinants of bone mineral density in older men. J Bone Miner Res. 1995;10:1769–1777. doi: 10.1002/jbmr.5650101121. [DOI] [PubMed] [Google Scholar]
- 11.Cauley JA, Fullman RL, Stone KL, Zmuda JM, Bauer DC, Barrett-Connor E, Ensrud K, Lau EM, Orwoll ES. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005;16:1525–1537. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 12.Hill DD, Cauley JA, Sheu Y, Bunker CH, Patrick AL, Baker CE, Beckles GL, Wheeler VW, Zmuda JM. Correlates of bone mineral density in men of African ancestry: The Tobago Bone Health Study. Osteoporos Int. 2008;19:227–234. doi: 10.1007/s00198-007-0450-9. [DOI] [PubMed] [Google Scholar]
- 13.Taaffe DR, Simonsick EM, Visser M, Volpato S, Nevitt MC, Cauley JA, Tylavsky FA, Harris TB. Lower extremity physical performance and hip bone mineral density in elderly black and white men and women: Cross-sectional associations in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2003;58:M934–M942. doi: 10.1093/gerona/58.10.m934. [DOI] [PubMed] [Google Scholar]
- 14.Marshall LM, Zmuda JM, Chan BK, Barrett-Connor E, Cauley JA, Ensrud KE, Lang TF, Orwoll ES. Race and ethnic variation in proximal femur structure and bone density among older men. J Bone Miner Res. 2008;23:121–130. doi: 10.1359/JBMR.070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruffing J, Cosman F, Zion M, Tendy S, Garrett P, Lindsay R, Nieves J. Determinants of bone mass and bone size in a large cohort of physically active young adult men. Nutr Metab (Lond) 2006;3:14. doi: 10.1186/1743-7075-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George A, Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in bone mineral density in older men. J Bone Miner Res. 2003;18:2238–2244. doi: 10.1359/jbmr.2003.18.12.2238. [DOI] [PubMed] [Google Scholar]
- 17.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18:943–953. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 18.Bunker CH, Patrick AL, Konety BR, Dhir R, Brufsky AM, Vivas CA, Becich MJ, Trump DL, Kuller LH. High prevalence of screening-detected prostate cancer among Afro-Caribbeans: The Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev. 2002;11:726–729. [PubMed] [Google Scholar]
- 19.Riggs BL, Melton Iii LJ, III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 20.Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: Evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo CR, Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Volpato S, Guralnik JM, Harris T, Ferrucci L. Aging bone in men and women: Beyond changes in bone mineral density. Osteoporos Int. 2003;14:531–538. doi: 10.1007/s00198-002-1322-y. [DOI] [PubMed] [Google Scholar]
- 22.Kaji H, Kosaka R, Yamauchi M, Kuno K, Chihara K, Sugimoto T. Effects of age, grip strength and smoking on forearm volumetric bone mineral density and bone geometry by peripheral quantitative computed tomography: Comparisons between female and male. Endocr J. 2005;52:659–666. doi: 10.1507/endocrj.52.659. [DOI] [PubMed] [Google Scholar]
- 23.Jamal SA, Gilbert J, Gordon C, Bauer DC. Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res. 2006;21:543–548. doi: 10.1359/jbmr.060105. [DOI] [PubMed] [Google Scholar]
- 24.Black DM, Bouxsein ML, Marshall LM, Cummings SR, Lang TF, Cauley JA, Ensrud KE, Nielson CM, Orwoll ES. Proximal femoral structure and the prediction of hip fracture in men: A large prospective study using quantitative computed tomography. J Bone Miner Res. 2008;23:1326–1333. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackey DC, Eby JG, Harris F, Taaffe DR, Cauley JA, Tylavsky FA, Harris TB, Lang TF, Cummings SR. Prediction of clinical non-spine fractures in older black and white men and women with volumetric BMD of the spine and areal BMD of the hip: The Health, Aging, and Body Composition Study. J Bone Miner Res. 2007;22:1862–1868. doi: 10.1359/jbmr.070807. [DOI] [PubMed] [Google Scholar]
- 26.Miljkovic-Gacic I, Ferrell RE, Patrick AL, Kammerer CM, Bunker CH. Estimates of African, European and Native American ancestry in Afro-Caribbean men on the island of Tobago. Hum Hered. 2005;60:129–133. doi: 10.1159/000089553. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer JR, Kammerer CM, Reich D, McDonald G, Patterson N, Goodpaster B, Bauer DC, Li J, Newman AB, Cauley JA, Harris TB, Tylavsky F, Ferrell RE, Zmuda JM. Genetic markers for ancestry are correlated with body composition traits in older African Americans. Osteoporos Int. 2007;18:733–741. doi: 10.1007/s00198-006-0316-6. [DOI] [PubMed] [Google Scholar]
- 28.Lorentzon M, Landin K, Mellstrom D, Ohlsson C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res. 2006;21:1871–1878. doi: 10.1359/jbmr.060814. [DOI] [PubMed] [Google Scholar]
- 29.Rosen CJ, Bouxsein ML. Mechanisms of disease: Is osteoporosis the obesity of bone. Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 30.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Zmuda JM, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study. J Bone Miner Res. 2004;19:1084–1091. doi: 10.1359/JBMR.040311. [DOI] [PubMed] [Google Scholar]
- 31.Register TC, Lenchik L, Hsu FC, Lohman KK, Freedman BI, Bowden DW, Carr JJ. Type 2 diabetes is not independently associated with spinal trabecular volumetric bone mineral density measured by QCT in the Diabetes Heart Study. Bone. 2006;39:628–633. doi: 10.1016/j.bone.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Stolk RP, Van Daele PL, Pols HA, Burger H, Hofman A, Birkenhager JC, Lamberts SW, Grobbee DE. Hyperinsulinemia and bone mineral density in an elderly population: The Rotterdam Study. Bone. 1996;18:545–549. doi: 10.1016/8756-3282(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 33.Barrett-Connor E, Kritz-Silverstein D. Does hyperinsulinemia preserve bone. Diabetes Care. 1996;19:1388–1392. doi: 10.2337/diacare.19.12.1388. [DOI] [PubMed] [Google Scholar]
- 34.Bunker CH, Patrick AL, Maharaj G, Keenan HA, Ramnarine S, Belle A, Richard JR, Dhir R. Prostate cancer risk is three-fold higher among men, aged 50-64, of African descent compared with men of Asian-Indian descent in Trinidad and Tobago. Ethn Dis. 2002;12:S30–S33. [PubMed] [Google Scholar]
- 35.Reddy S, Shapiro M, Morton R, Jr, Brawley OW. Prostate cancer in black and white Americans. Cancer Metastasis Rev. 2003;22:83–86. doi: 10.1023/a:1022216119066. [DOI] [PubMed] [Google Scholar]
- 36.Higano CS. Androgen-deprivation-therapy-induced fractures in men with nonmetastatic prostate cancer: What do we really know. Nat Clin Pract Urol. 2008;5:24–34. doi: 10.1038/ncpuro0995. [DOI] [PubMed] [Google Scholar]
- 37.Barry MJ, Delorenzo MA, Walker-Corkery ES, Lucas FL, Wennberg DC. The rising prevalence of androgen deprivation among older American men since the advent of prostate-specific antigen testing: A population-based cohort study. BJU Int. 2006;98:973–978. doi: 10.1111/j.1464-410X.2006.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiren KM, Orwoll ES. Androgens and bone: Basic aspects. In: Orwoll ES, editor. Osteoporosis in Men: The Effect of Gender and on Skeletal Health. San Diego, CA, USA: Academic Press; 1999. pp. 211–245. [Google Scholar]
- 39.Orwoll ES. Androgens and bone: Clinical aspects. In: Orwoll ES, editor. Osteoporosis in Men: The Effect of Gender on Skeletal Health. San Diego, CA, USA: Academic Press; 1999. pp. 247–274. [Google Scholar]
- 40.Conde FA, Sarna L, Oka RK, Vredevoe DL, Rettig MB, Aronson WJ. Age, body mass index, and serum prostate-specific antigen correlate with bone loss in men with prostate cancer not receiving androgen deprivation therapy. Urology. 2004;64:335–340. doi: 10.1016/j.urology.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 41.Kiratli BJ, Srinivas S, Perkash I, Terris MK. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology. 2001;57:127–132. doi: 10.1016/s0090-4295(00)00895-5. [DOI] [PubMed] [Google Scholar]
- 42.Preston DM, Torrens JI, Harding P, Howard RS, Duncan WE, McLeod DG. Androgen deprivation in men with prostate cancer is associated with an increased rate of bone loss. Prostate Cancer Prostatic Dis. 2002;5:304–310. doi: 10.1038/sj.pcan.4500599. [DOI] [PubMed] [Google Scholar]
- 43.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–1222. [PubMed] [Google Scholar]
- 44.Goldray D, Weisman Y, Jaccard N, Merdler C, Chen J, Matzkin H. Decreased bone density in elderly men treated with the gonadotropin-releasing hormone agonist decapeptyl (D-Trp6-GnRH) J Clin Endocrinol Metab. 1993;76:288–290. doi: 10.1210/jcem.76.2.7679397. [DOI] [PubMed] [Google Scholar]
- 45.Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–6417. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 46.Wei JT, Gross M, Jaffe CA, Gravlin K, Lahaie M, Faerber GJ, Cooney KA. Androgen deprivation therapy for prostate cancer results in significant loss of bone density. Urology. 1999;54:607–611. doi: 10.1016/s0090-4295(99)00301-5. [DOI] [PubMed] [Google Scholar]
- 47.Mittan D, Lee S, Miller E, Perez RC, Basler JW, Bruder JM. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab. 2002;87:3656–3661. doi: 10.1210/jcem.87.8.8782. [DOI] [PubMed] [Google Scholar]
- 48.Krupski TL, Foley KA, Baser O, Long S, Macarios D, Litwin MS. Health care cost associated with prostate cancer, androgen deprivation therapy and bone complications. J Urol. 2007;178:1423–1428. doi: 10.1016/j.juro.2007.05.135. [DOI] [PubMed] [Google Scholar]
- 49.Lopez AM, Pena MA, Hernandez R, Val F, Martin B, Riancho JA. Fracture risk in patients with prostate cancer on androgen deprivation therapy. Osteoporos Int. 2005;16:707–711. doi: 10.1007/s00198-004-1799-7. [DOI] [PubMed] [Google Scholar]
- 50.Abrahamsen B, Nielsen MF, Eskildsen P, Andersen JT, Walter S, Brixen K. Fracture risk in Danish men with prostate cancer: A nationwide register study. BJU Int. 2007;100:749–754. doi: 10.1111/j.1464-410X.2007.07163.x. [DOI] [PubMed] [Google Scholar]
- 51.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 52.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, Brandman J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–139. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 53.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 54.Zhang XZ, Kalu DN, Erbas B, Hopper JL, Seeman E. The effects of gonadectomy on bone size, mass, and volumetric density in growing rats are gender-, site-, and growth hormone-specific. J Bone Miner Res. 1999;14:802–809. doi: 10.1359/jbmr.1999.14.5.802. [DOI] [PubMed] [Google Scholar]
- 55.Lorentzon M, Mellstrom D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab. 2007;92:497–503. doi: 10.1210/jc.2006-1294. [DOI] [PubMed] [Google Scholar]
- 56.Kamer AR, El-Ghorab N, Marzec N, Margarone JE, III, Dziak R. Nicotine induced proliferation and cytokine release in osteoblastic cells. Int J Mol Med. 2006;17:121–127. [PubMed] [Google Scholar]
- 57.Liu XD, Zhu YK, Umino T, Spurzem JR, Romberger DJ, Wang H, Reed E, Rennard SI. Cigarette smoke inhibits osteogenic differentiation and proliferation of human osteoprogenitor cells in monolayer and three-dimensional collagen gel culture. J Lab Clin Med. 2001;137:208–219. doi: 10.1067/mlc.2001.113066. [DOI] [PubMed] [Google Scholar]
- 58.Lau EM, Leung PC, Kwok T, Woo J, Lynn H, Orwoll E, Cummings S, Cauley J. The determinants of bone mineral density in Chinese men—results from Mr. Os (Hong Kong), the first cohort study on osteoporosis in Asian men. Osteoporos Int. 2006;17:297–303. doi: 10.1007/s00198-005-2019-9. [DOI] [PubMed] [Google Scholar]
- 59.McGuigan FE, Murray L, Gallagher A, Davey-Smith G, Neville CE, Van't Hof R, Boreham C, Ralston SH. Genetic and environmental determinants of peak bone mass in young men and women. J Bone Miner Res. 2002;17:1273–1279. doi: 10.1359/jbmr.2002.17.7.1273. [DOI] [PubMed] [Google Scholar]
- 60.Bauer DC, Browner WS, Cauley JA, Orwoll ES, Scott JC, Black DM, Tao JL, Cummings SR. Factors associated with appendicular bone mass in older women. The Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1993;118:657–665. doi: 10.7326/0003-4819-118-9-199305010-00001. [DOI] [PubMed] [Google Scholar]