Abstract
Better assessment of the association between cardiovascular disease and osteoporosis in older men may help identify shared etiologies for bone and heart health in this population. We assessed the association of BMD and bone turnover markers (BTMs) with risk of cardiovascular events (myocardial infarction or stroke) in 744 men ≥50 yr of age. During the 7.5-yr prospective follow-up, 43 strokes and 40 myocardial infarctions occurred in 79 men. After adjustment for confounders (age, weight, height, smoking, education, physical activity, self-reported history of diabetes, hypertension, and prevalent ischemic heart disease), men in the lowest quartile of BMD at the spine, whole body, and forearm had a 2-fold increased risk of cardiovascular events. Men in the highest quartile of bone resorption markers (deoxypyridinoline [DPD], C-telopeptide of type I collagen) had a 2-fold increased risk of cardiovascular events (e.g., multivariable-adjusted hazard ratio [including additional adjustment for BMD] was 2.11 [95% CI: 1.26–3.56], for the highest quartile of free DPD relative to the lowest three quartiles). The results were similar for men without prevalent ischemic heart disease and for myocardial infarction and stroke analyzed separately. Our data suggest that men with low BMD or high bone resorption may be at increased risk of myocardial infarction and stroke in addition to fracture. Thus, men with osteoporosis may benefit from screening for cardiovascular disease. Further study to elucidate the biological mechanism shared by bone and vascular disease may help efforts to identify men at risk or develop treatment.
Key words: osteoporosis, cardiovascular disease, bone resorption, BMD, men, cohort study
INTRODUCTION
Cardiovascular disease and osteoporosis are two major public health problems. Both diseases may share common risk factors such as aging, smoking, or sedentary lifestyle(1–3) and may have common pathophysiological mechanisms (e.g., age-related sex steroid hormone deficit or proinflammatory cytokines such as C-reactive protein [CRP] and interleukin-6 [IL6]).(4–10)
Several studies have shown a positive association in postmenopausal women between decreased BMD and increased cardiovascular risk or severity of aortic calcification.(11–14) In contrast, findings have been conflicting in men. In the Framingham study, men with low metacarpal cortical area did not have greater incidence of ischemic heart disease.(12) In the NHANES cohort, men with low phalangeal BMD did not have greater risk of stroke.(15) Severity of aortic calcifications was associated with a 2- to 3-fold higher risk of clinical fracture during a 10-yr follow-up(16) but not with the risk of hip fracture during a 21-yr follow-up.(17) In the Framingham study, metacarpal bone loss and progression of aortic calcification correlated in women but not in men.(18)
Cross-sectional data are also conflicting. Men who reported cardiovascular disease had lower volumetric BMD (vBMD) at the spine but not the hip.(14) Men who reported myocardial infarction had lower hip BMD than those who did not.(19) Presence of coronary calcifications has been associated with a nonsignificant 8% lower lumbar spine vBMD.(20) In 18 men with peripheral artery disease, BMC was lower in the leg with more severe disease.(21) Subclinical peripheral arterial disease diagnosed on the basis of the ankle-arm index has been associated with slightly lower hip BMD in some,(14) but not all, studies.(22,23) No association has been found between BMD and stroke prevalence in men.(24,25)
To our knowledge, no studies have evaluated bone turnover markers (BTMs) with respect to cardiovascular risk in older home-dwelling men. Whereas a single BMD measurement reflects skeletal health from birth, through growth, acquisition of peak BMD, young adult maintenance, and bone loss during aging, BTMs may represent the current state of bone remodeling, reflecting recent lifestyle factors, nutrition, chronic diseases, and treatment. If osteoporosis and cardiovascular diseases share common risk factors, BMD may reflect long-acting risk factors, whereas BTM levels may better reflect the current status of bone metabolism.
Assessment of the relation of both BMD and BTM to risk of cardiovascular disease may provide a comprehensive approach to help identify potential shared etiologies in bone and heart health in older men. We therefore evaluated the association of BMD and BTM levels with the risk of myocardial infarction and stroke in a cohort of men ≥50 yr of age followed prospectively for 7.5 yr. To assess whether bone status may reflect subclinical cardiovascular abnormalities, we carried out similar analysis in men without overt ischemic heart disease at baseline. Finally, we carried out analyses separately for myocardial infarction and stroke to verify if the trend was similar for both types of cardiovascular events.
MATERIALS AND METHODS
Cohort and study design
MINOS is a prospective cohort study of male osteoporosis with a primary aim to assess predictors of bone loss and fragility fractures in men.(26) Participants were recruited in 1995–1996 from the Société de Secours Minière de Bourgogne (SSMB) rolls in Montceau les Mines (Saône et Loire). The study was accepted by the local ethics committee and performed in accord with the Helsinki Declaration of 1975 as revised in 1983. Letters inviting participation in the study were sent to a randomly selected sample of 3400 men 50–85 yr of age living in Montceau les Mines and adjacent villages. Eight hundred forty-one men agreed to participate and provided informed consent.
This study was carried out in 743 men who had had BMD measurements, lateral radiographs of the spine, blood and urine collection at the baseline examination in 1995–1996, and were followed for 7.5 yr. Ninety-eight men were excluded (4 men died of unexplained sudden death, 67 men refused bone densitometry, 4 had radiographs of poor quality, 26 refused 24-h urinary collection). Participants were followed from recruitment to the first of the following: myocardial infarction, stroke, last contact, death, or end of study (90 mo of follow-up). For men who died during the follow-up, dates of death were provided by the SSMB administration. For two men, we did not obtain data on their life status after 5 yr of follow-up. All other survivors were followed up for 7.5 yr.
BMD
BMD was measured at the lumbar spine (L2–L4), right hip (femoral neck, trochanter, and total hip), and whole body by DXA (QDR-1500; Hologic, Waltham, MA, USA).(20) For the lumbar spine, the CV was 0.33% using a commercial phantom and 0.62% using a human lumbar spine phantom embedded in methyl methacrylate. For the total hip and its components (femoral neck, trochanter), the CV was 0.81–0.94% using a commercial hip phantom. BMD of two regions of interest (ROIs) of the distal forearm was measured using single energy X-ray absorptiometry (DTX100; Osteometer, Rodovre, Denmark). The distal region includes 20 mm of ulna and radius situated proximally to the site where the spacing between the two bones is 8 mm. The ultradistal radius ROI is situated distally to the previous one. For the distal forearm BMD, the CV was 0.47% using a commercial calibration standard. All scans were analyzed manually. Three hip scans and three forearm scans with positioning errors were excluded.
Biochemical BTMs
Fasting serum and 24-h urine samples were collected at baseline and stored at −80°C until assayed in 1996–1997. Bone formation was assessed by serum levels of osteocalcin (OC), bone-specific alkaline phosphatase (BALP), and N-terminal propeptide of type I procollagen (P1NP).(27) Bone resorption was assessed using 24-h urinary excretion of total and free deoxypyridinoline (DPD), as well as by serum and urinary levels of C-terminal telopeptide of type I collagen (CTX-I).(27) Urinary BTM levels were expressed per mmoles creatinine (cr). Measurements of serum levels of 25-hydroxycholecalciferol [25(OH)D] and PTH were performed as described previously.(28)
Covariates
At baseline, participants completed questionnaires administered by an interviewer to assess age, smoking, alcohol, education, physical activity, medical history, and medication use. Tobacco smoking was assessed as current smoker, former smoker >25 packet-years, former smoker ≤25 packet-years, and never-smoker. Alcohol intake was assessed as sum of current average weekly intakes of wine, beer, and spirits, expressed in grams per week and divided into quartiles. Education level was assessed as >8 versus ≤8 yr at school. Current and past physical activity at work was evaluated according to a self-reported four-level scale (low, medium, hard, and very hard). Current leisure physical activity was calculated on the basis of the overall amount of time (h/wk) spent walking, gardening, and participating in leisure sport activity including seasonal activities. Assessment of comorbidities present at baseline were self-reported including ischemic heart disease (history of myocardial infarction or angina pectoris or taking medications used for the treatment of the ischemic heart disease at the period of recruitment; e.g., nitrates, aspirin, and β-blockers), hypertension, type I and II diabetes, Parkinson's disease, and history of stroke. In addition, we also assessed the use of thiazides and use of vitamin K–inhibiting anticoagulants.
Body weight and height were measured by standard devices. Osteoarthritis of the lumbar spine (L2–L4) was assessed on the lateral radiographs of the spine performed at baseline using a 27-point severity scale described previously.(26)
Myocardial infarction and stroke
Self-reported myocardial infarction and stroke, herein referred to as “cardiovascular events,” were recorded by questionnaire administered by an interviewer at five study visits conducted every 18 mo during follow-up. Men who did not return for a visit were contacted by telephone to assess myocardial infarction and stroke. For nine cardiovascular events in 136 men who died, information was obtained from proxy respondents, usually a wife. Cardiovascular events (reported by the participant or his proxy) were confirmed by review of medical records by the staff of the MINOS study. Men who died of sudden death were included in the analysis when the autopsy had been performed or when it was possible to obtain clinical data indicating a cardiovascular event (e.g., electrocardiogram performed by the emergency unit). Four men with sudden unexplained death were not included in the analysis.
Statistical methods
Bivariate comparisons of covariates between men who did or did not sustain a cardiovascular event were performed using a t-test for variables with Gaussian distribution, Mann-Whitney's test for variables with skewed distribution, a χ2 test for 2 × 2 class variables, and the Cochrane-Armitage test for trend for smoking habits. For age-adjusted comparisons, we used analysis of covariance for continuous variables (log-transformed for variables with skewed distribution) and the Cochran-Mantel-Haenszel test for class variables. Age-adjusted incidence of cardiovascular events was calculated in 10-yr age groups.
We used Cox regression to calculate hazard ratios (HRs) and 95% CIs for the association between risk of cardiovascular events and baseline BMD and BTM, each categorized by quartiles. To account for seasonal variation, quartiles of BTM, 25(OH)D, and PTH were created separately according to season of blood draw (summer versus the three other seasons).(28) The fourth (highest) quartile of BMD and the first (lowest) quartile of BTM were used as referent groups. Because results indicated a threshold effect for both BMD and BTM on risk of cardiovascular events, we dichotomized BMD as the highest versus the lower three quartiles and BTM as the lowest versus the higher three quartiles. Proportional hazard assumptions were met by the studied variables. We used backward selection to identify potential confounders. Covariates that significantly differed between men who did and did not sustain a cardiovascular event were evaluated as were those factors suggested by previous studies to be risk factors for osteoporosis or cardiovascular disease. These included age, weight, height, smoking (four classes), alcohol intake (g/wk, highest versus three lower quartiles), education (>8 versus ≤8 yr school), leisure physical activity (h/wk), physical activity at work (low to medium versus high to very high), prevalent ischemic heart disease (yes/no), hypertension, diabetes (yes/no), stroke (yes/no), and Parkinson's disease (yes/no). Analyses for spine BMD were adjusted for lumbar arthritis. Our final models included age, weight, height, education level, leisure and occupational physical activity, smoking, prevalent ischemic heart disease, hypertension, diabetes, and 25(OH)D (lowest quartile versus other quartiles). Former stroke, Parkinson's disease, and PTH (highest quartile versus lower quartiles, continuous) were not significant and not retained in the final models. Multivariable models evaluating the relation between BTM levels and cardiovascular events additionally included whole body BMD because this skeletal site had the strongest association with the outcome. To assess the contribution of low BMD and high BTM to the cardiovascular risk, we assessed the risk on four groups: normal BMD and BTM levels (referent), low BMD and normal BTM, normal BMD and elevated BTM, and low BMD and elevated BTM. Homogeneity of HR was assessed by the log-rank test. We conducted further analysis excluding men with ischemic heart disease at baseline. We repeated our analysis examining myocardial infarction as the outcome and then using stroke as the outcome. In the models considering one outcome, the follow-up time of participants who sustained the second outcome was measured until the end of study or death. Finally, we assessed risk for myocardial infarction and stroke introduced as separate variables in the same model using multivariate generalized logistic regression where both outcomes were assessed versus the reference group. Analyses were performed using SAS 8.2 software (Cary, NC, USA).
RESULTS
Descriptive analyses
During the 7.5-yr follow-up, we recorded 40 myocardial infarctions and 43 strokes (defined as cardiovascular events) in 79 men. The median time to the first cardiovascular event was 4.5 yr (range, 125–2730 days). Men who sustained cardiovascular events were, at baseline, older, shorter, and smoked more than those who did not have cardiovascular events (Table 1). Cases had greater occupational physical activity but less leisure physical activity than those who did not sustain cardiovascular events. Prevalent ischemic heart disease was more frequent in men with cardiovascular events; however, no differences were seen between men who did or did not sustain cardiovascular events in body mass index (BMI), alcohol intake, prevalence of hypertension, diabetes, Parkinson's disease, or former stroke. In contrast, men with cardiovascular event had lower BMD (at all skeletal sites) and higher BTM levels.
Table 1.
Baseline Characteristics of Men Who Did and Did Not Sustain a Cardiovascular Event
| Baseline characteristic |
Major cardiovascular event |
p* | p† | |
| No (n = 664) | Yes (n = 79) | |||
| Age (yr) | 65 ± 7 | 68 ± 8 | <0.0001 | |
| Body weight (kg) | 80 ± 13 | 78 ± 12 | 0.13 | |
| Body height (cm) | 169 ± 6 | 166 ± 7 | <0.0001 | <0.005 |
| Body mass index (kg/m2) | 28.0 ± 3.7 | 28.2 (3.8) | 0.67 | |
| Smoking (n, %) current | 69 (10.4) | 13 (15.6) | <0.02 | <0.01 |
| Former >25 packet-years | 68 (10.2) | 12 (15.6) | ||
| Former ≤25 packet-years | 307 (46.2) | 33 (42.8) | ||
| Never-smoker | 221 (33.2) | 21 (26.0) | ||
| Alcohol intake (g/d) | 37.5 [10.8; 75.0] | 37.5 [5.4; 64.3] | 0.59 | |
| Education (n, %) ≤8 yr | 176 (26.3) | 18 (22.8) | 0.49 | |
| >8 yr | 489 (73.7) | 61 (77.2) | ||
| Leisure physical activity (h/wk) | 20 [14; 29] | 17 [12; 25] | <0.05 | 0.35 |
| Occupational physical activity | <0.01 | <0.01 | ||
| Low/medium (n, %) | 102 (15.3) | 5 (6.3) | ||
| Hard/very hard | 563 (84.7) | 74 (93.7) | ||
| Ischemic heart disease (n, %) | 87 (13.1) | 26 (32.9) | <0.0001 | <0.0001 |
| Hypertension (n, %) | 172 (25.9) | 21 (26.6) | 0.84 | |
| Diabetes (n, %) | 48 (7.2) | 7 (8.9) | 0.51 | |
| Parkinson's disease (n, %) | 11 (1.7) | 2 (2.5) | 0.61 | |
| Former stroke (n, %) | 19 (2.9) | 3 (3.8) | 0.65 | |
| Thiazide use (n, %) | 40 (6.0) | 3 (3.8) | 0.42 | |
| Vitamin K–inhibiting anticoagulants (n, %) | 22 (3.3) | 8 (10.1) | <0.005 | <0.05 |
| Lumbar spine (g/cm2) | 1.037 ± 0.185 | 0.994 ± 0.180 | <0.05 | <0.05 |
| Femoral neck (g/cm2) | 0.846 ± 0.120 | 0.818 ± 0.130 | 0.05 | 0.26 |
| Trochanter (g/cm2) | 0.741 ± 0.108 | 0.704 ± 0.120 | <0.005 | <0.05 |
| Total hip (g/cm2) | 0.968 ± 0.128 | 0.930 ± 0.140 | <0.02 | 0.15 |
| Whole body (g/cm2) | 1.213 ± 0.109 | 1.164 ± 0.112 | <0.001 | <0.001 |
| Distal forearm (g/cm2) | 0.525 ± 0.064 | 0.497 ± 0.076 | <0.001 | <0.02 |
| Ultradistal radius (g/cm2) | 0.432 ± 0.063 | 0.399 ± 0.076 | <0.0001 | <0.002 |
| 25(OH)D (ng/ml) | 27.4 ± 11.6 | 24.5 ± 11.1 | <0.05 | 0.26 |
| PTH (pg/ml) | 40 ± 18 | 41 ± 19 | 0.55 | |
| Osteocalcin (ng/ml) | 19.05 ± 6.81 | 21.33 ± 13.27 | <0.02 | 0.25 |
| BALP (U/liter) | 16.71 ± 6.20 | 18.74 ± 8.18 | <0.01 | <0.05 |
| P1NP (ng/ml) | 36.01 ± 17.41 | 40.22 ± 21.81 | <0.05 | <0.05 |
| Total DPD (nmol/mmol creat) | 6.88 ± 2.68 | 8.79 ± 4.27 | <0.0001 | <0.0001 |
| Free DPD (nmol/mmol creat) | 3.40 ± 1.10 | 4.23 ± 1.85 | <0.0001 | <0.0001 |
| Urinary CTX-I (μg/mmol creat) | 123.4 ± 78.2 | 146.5 ± 88.2 | <0.02 | <0.02 |
| Serum CTX-I (mM) | 2.42 ± 1.20 | 2.86 ± 2.07 | <0.01 | 0.24 |
Data are presented as mean ± SD, median [interquartile range], or number (percentage).
* Unadjusted p value.
† Age-adjusted p value.
25(OH)D, 25-hydroxyxholecalciferol; BALP, bone alkaline phosphatase; DPD, deoxypyridinoline.
BMD and cardiovascular risk
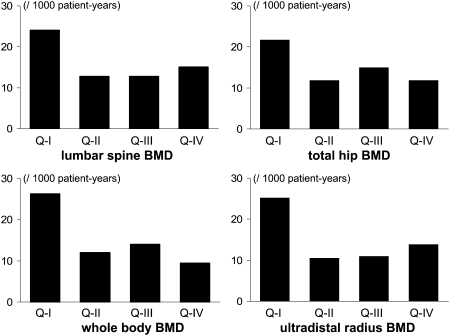
For whole body, distal forearm, ultradistal radius, lumbar spine, and trochanter, age-adjusted incidence of cardiovascular events was similar in the three upper quartiles of BMD and higher only in the lowest quartile (Fig. 1). A similar, but weaker pattern, suggesting a threshold with respect to the risk of cardiovascular event for men in the lowest quartile of BMD, was found for the fully adjusted models controlling for age, weight, height, smoking, occupational physical activity, education level, ischemic heart disease, hypertension, diabetes, 25(OH)D concentrations, and vitamin K–inhibiting anticoagulants and thiazides treatment: HR (95% CI) was 2.33 (1.13–4.81) for men in the lowest quartile of whole body BMD, whereas HRs did not differ from 1.00 for men in the higher quartiles (Q2: 1.03 [0.45–2.36], Q3: 1.27 [0.59–2.73], Q4: 1.00 [referent]). Similar results were found when the lowest BMD quartile was compared versus three upper quartiles in the age- and fully adjusted models (Table 2).
FIG. 1.
Age-adjusted incidence of the major cardiovascular event in 744 men from the MINOS cohort according to the quartiles of BMD of the lumbar spine (top left graph), total hip (top right graph), whole body (bottom left graph), and ultradistal radius (bottom right graph).
Table 2.
Risk of Cardiovascular Event According to Baseline BMD (Lowest Quartile vs. Higher Three Quartiles) in 743 Men, MINOS Study, 1995–2003
| BMD |
HR (95% CI) comparing lowest quartile vs. three upper quartiles |
|
| Age-adjusted | Fully adjusted | |
| Spine* (<0.910 g/cm2) | 1.95 (1.17–3.25)† | 1.60 (0.94–2.72) |
| Femoral neck (<0.756 g/cm2) | 1.19 (0.71–1.99) | 1.33 (0.77–2.27) |
| Trochanter (<0.657 g/cm2) | 1.65 (1.03–2.64)† | 1.56 (0.94–2.60) |
| Total hip (<0.872 g/cm2) | 1.58 (0.98–2.53) | 1.59 (0.93–2.74) |
| Whole body (<1.131 g/cm2) | 2.20 (1.40–3.46)‡ | 1.78 (1.05–3.03)† |
| Distal forearm (<0.477 g/cm2) | 1.79 (1.16–2.87)† | 1.51 (0.88–2.55) |
| Ultradistal radius (<0.386 g/cm2) | 2.23 (1.41–3.53)§ | 1.65 (0.99–2.75) |
Adjusted for age, weight, height (all continuous), education (>8 vs. ≤8 yr), occupational physical activity (low to medium vs. high to very high), smoking (current smoker, former smoker >25 packet-years, former smoker ≤25 packet-years, never smoker), ischemic heart disease, hypertension, diabetes (all yes/no), use of thiazides, use of vitamin K–inhibiting anticoagulants, and lowest 25(OH)D quartile.
Values in parentheses represent the cut-off value of the first quartile.
* Adjusted additionally for lumbar osteoarthritis.
† p < 0.05.
‡ p < 0.01.
§ p < 0.001.
Biochemical BTMs and cardiovascular risk
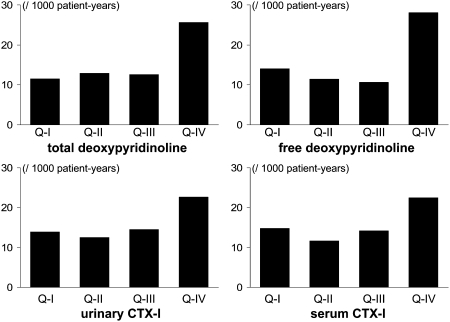
Age-adjusted incidence of cardiovascular events was similar for men in the three lowest quartiles and higher for those in the highest quartile of bone resorption markers (Fig. 2). The pattern was similar for the fully adjusted models; for free DPD, multivariable HRs (95% CI) were as follows: Q1: 1.00 (referent), Q2: 0.95 (0.44–1.92), Q3: 0.90 (0.39–1.90), Q4: 2.04 (1.10–3.79). Similarly, for urinary CTX-I, multivariable HRs (95% CI) were as follows: Q1: 1.00, Q2: 0.99 (0.50–1.99), Q3: 0.98 (0.49–1.93), Q4: 1.97 (1.06–3.64). In the fully adjusted models, men in the highest quartile of total and free DPD as well as serum and urinary CTX-I had twice the risk of cardiovascular events compared with men in the lower three quartiles, and the association was attenuated only slightly after additional adjustment for whole body BMD (Table 3). HRs for OC, BALP, and P1NP were >1.0 but did not attain statistical significance. The interactions between BMD and BTM levels were not significant.
FIG. 2.
Age-adjusted incidence of the major cardiovascular event in 744 men from the MINOS cohort according to the quartiles of the urinary excretion of total DPD (top left graph), free DPD (top right graph), urinary CTX-I (bottom left graph), and serum CTX-I (bottom right graph).
Table 3.
Risk of Cardiovascular Events According to Baseline Levels of Biochemical Bone Turnover Markers in 743 Men, MINOS Study, 1995–2003
| Bone turnover marker |
Multivariable-adjusted HR comparing highest quartile vs. three lower quartiles |
Additional adjustment for whole body BMD |
||
| HR | 95% CI | HR | 95% CI | |
| Osteocalcin (>22.3/22.5 ng/ml) | 1.64 | 0.96–2.83 | 1.55 | 0.90–2.68 |
| BAP (>18.5/19.3 U/liter) | 1.51 | 0.88–2.58 | 1.37 | 0.80–2.36 |
| P1NP (>39.1/43.5 ng/ml) | 1.36 | 0.78–2.34 | 1.20 | 0.68–2.11 |
| T-DPD (>6.87/8.64 nmol/mmol cr) | 1.99 | 1.18–3.34* | 1.90 | 1.12–3.25† |
| F-DPD (>3.60/4.19 nmol/mmol cr) | 2.19 | 1.30–3.66‡ | 2.11 | 1.26–3.56‡ |
| U CTX-I (>135.1/160.1 μg/mmol cr) | 2.14 | 1.26–3.65‡ | 2.05 | 1.19–3.51* |
| Serum CTX-I (>2.83/3.16 mM) | 1.91 | 1.11–3.28† | 1.79 | 1.03–3.10† |
Adjusted for age, weight, height (all continuous), education (>8 vs. ≤8 yr), occupational physical activity (low to medium vs. high to very high), smoking (current smoker, former smoker >25 packet-years, former smoker ≤25 packet-years, never smoker), ischemic heart disease, hypertension, diabetes (all yes/no), use of thiazides, use of vitamin K–inhibiting anticoagulants, and lowest 25(OH)D quartile.
Values in parentheses represent the cut-off value of the highest quartile in summer/highest quartile in other seasons.
* p < 0.01.
† p < 0.05.
‡ p < 0.005.
T-DPD, total deoxypyridinoline; F-DPD, free deoxypyridinoline.
In men with low BMD and high bone resorption, risk of cardiovascular event was two to three times higher compared with men with normal BMD and BTM levels (Table 4). Men with elevated CTX-I levels and normal BMD had a 2-fold higher risk of cardiovascular event compared with the reference group. Men with high free or total DPD and normal BMD had slightly (not significantly) higher cardiovascular risk (data not shown). In men with low BMD and normal BTM levels, HR for cardiovascular event did not attain statistical significance.
Table 4.
Risk of Cardiovascular Events According to Baseline Levels of BMD and Biochemical Markers of Bone Resorption in 742 Men, MINOS Study, 1995–2003
| BMD |
Serum CTX-I |
Urinary CTX-I |
||
| Q1–3 ≤ 2.83/3.16 mM [n/N HR (95%CI)] | Q4 > 2.83/3.161 mM [n/N HR (95%CI)] | Q1–3≤135.1/160.1 μg/mmol cr [n/N HR (95%CI)] | Q4 >135.1/160.1 μg/mmol cr [n/N HR (95%CI)] | |
| Whole body | ||||
| Q2–4 ≥1.131 g/cm2 | 33/445 1.00 | 13/125 2.12 (1.05–4.26)* | 33/456 1.00 | 14/109 2.39 (1.19–4.82)* |
| Q1 <1.131 g/cm2 | 17/116 1.86 (0.95–3.60) | 16/ 57 2.75 (1.30–5.83)† | 18/115 1.57 (0.81–3.05) | 14/ 63 2.81 (1.38–5.75)† |
| Ultradistal radius | ||||
| Q2–4 ≥0.386 g/cm2 | 33/452 1.00 | 12/113 2.04 (0.98–4.27) | 32/458 1.00 | 12/105 2.10 (1.04–4.23)* |
| Q1 <0.386 g/cm2 | 19/110 1.59 (0.83–2.06) | 15/ 68 2.28 (1.11–4.66)* | 19/113 1.33 (0.70–2.56) | 16/ 67 2.73 (1.39–5.37)† |
The numbers present the cut-off value of the first quartile of BMD and of the fourth quartile of bone resorption markers.
Adjusted for age, weight, height (all continuous), education (>8 vs. ≤8 yr), occupational physical activity (low to medium vs. high to very high), smoking (current smoker, former smoker >25 packet-years, former smoker ≤25 packet-years, never smoker), ischemic heart disease, hypertension, diabetes (all yes/no), use of thiazides, use of vitamin K–inhibiting anticoagulants, and lowest 25(OH)D quartile.
Log-rank test for the homogeneity in HR: whole body and serum CTX-I, p < 0.002; whole body and urinary CTX-I, p < 0.001; ultradistal radius and serum CTX-I, p < 0.002; ultradistal radius and urinary CTX-I, p < 0.001.
* p < 0.05 and † p < 0.005 for the comparison vs. the reference group.
Relationship of the BTM levels with the cardiovascular risk in subgroups
In 631 men who did not report ischemic heart disease at baseline, 51 men had cardiovascular events (stroke, 30; myocardial infarction, 22). In the fully adjusted models, BMD was not predictive of cardiovascular events. In similar models, free and total DPD and urinary CTX-I were predictive of cardiovascular events (HR = 3.29, 95% CI: 1.72–6.28, p < 0.001; HR = 2.04, 95% CI: 1.06–3.93, p < 0.05; HR = 2.23, 95% CI: 1.15–4.24, p < 0.05; highest quartile versus three lower quartiles).
In the fully adjusted models including whole body BMD and analyzing myocardial infarction and stroke separately, total and free DPDs were predictive of myocardial infarction (HR = 2.44, 95% CI: 1.19–5.04, p < 0.05 and HR = 3.06, 95% CI: 1.50–6.27, p < 0.005; highest quartile versus three lower quartiles). In similar models, serum and urinary CTX-I predicted stroke (HR = 2.24, 95% CI: 1.09–4.59, p < 0.05 and HR = 2.06, 95% CI: 1.00–4.24, p = 0.05; highest quartile versus three lower quartiles). Both for myocardial infarction and stroke, HRs for other bone resorption markers were consistently >1.0 but did not attain statistical significance.
In fully adjusted polychotomous models including whole body BMD (considering myocardial infarction and stroke separately in the same model), urinary CTX-I was predictive of both myocardial infarction and stroke (OR = 2.18, 95% CI: 1.01–4.74 and OR = 2.19, 95% CI: 1.04–4.64; highest quartile versus three lower quartiles). In similar multivariate polychotomous models, free and total DPDs were predictive of myocardial infarction and showed a trend for stroke (free DPD: OR = 3.60, 95% CI: 1.72–7.52, p < 0.01 and OR = 1.78, 95% CI: 0.89–3.81; highest quartile versus three lower quartiles).
DISCUSSION
In a prospective study of a cohort of men, increased bone resorption (highest quartile) was associated with a 2-fold higher risk of cardiovascular events, such as myocardial infarction and stroke, independent of BMD and other confounders including history of ischemic heart disease. The threshold effect for the association between bone parameters and cardiovascular risk suggests that only men with abnormal bone status have a higher risk of cardiovascular event. To our knowledge, this is the first prospective study to examine both BMD and BTM as they relate to cardiovascular events in men.
Low BMD and high bone resorption may reflect poor general health status associated with higher cardiovascular risk. BTM levels depend largely on current lifestyle, nutrition, hormone secretion, diseases, and medication.(29) For instance, smoking was associated with a higher risk of cardiovascular disease and mortality, as well as with lower BMD and higher BTM levels.(1–3) However, the association between bone resorption rate and cardiovascular risk remained significant after adjustment for smoking and other potential confounders.
Several common risk factors need to be discussed. Low levels of bioavailable testosterone and 17β-estradiol are associated with higher BTM levels and lower BMD.(4,6,30) However, the overall association between testosterone level and cardiovascular risk is weak.(5,7) Similarly, data on the association between 17β-estradiol level and prospectively assessed cardiovascular risk in men are discordant.(31–34) In the elderly, vitamin D deficit is associated with a higher risk of osteoporosis and of myocardial infarction.(35–37) However, in our cohort, vitamin D level was only weakly correlated with bone resorption markers.(28)
Another link between bone and cardiovascular diseases may be osteoprotegerin (OPG),(38) but clinical and experimental data are discordant. OPG inhibits bone resorption(38); however, correlations of OPG with BMD and BTM levels are divergent and weak.(39–41) Increased OPG concentration was associated with favorable hip geometry measures in a large cohort of older men.(42) OPG inhibits bone resorption and vascular calcification(38,43); however, higher OPG level has been associated with increased cardiovascular risk.(44,45) Thus, the interpretation of the role of OPG in the association found in our study is not straightforward.
Another explanation of our findings may be low-grade systemic inflammation. Increased levels of cytokines (e.g., CRP, IL-6, or TNFα) may stimulate bone resorption, and their serum levels may correlate positively with BTM levels.(8,46,47) Moreover, their increased blood levels predicted cardiovascular events in previous studies.(9,10,48) Inflammatory cells secrete matrix metalloproteinases (MMPs), which degrade bone collagen and participate in bone resorption. In vivo, products of the degradation of elastin by MMP stimulated calcification of the arterial tunica media, whereas inhibition of MMP attenuated it.(49–52)
Another potential determinant of bone loss and cardiovascular disease is oxidative stress (i.e., a perturbation in the pro- and antioxidant balance). Oxidative stress markers are predictive of cardiovascular morbidity.(53,54) Oxidative stress is associated with a faster bone turnover driven by higher bone resorption not matched by a higher bone formation because oxidative stress inhibits osteoblastogenesis.(55,56) Interestingly, in our study, levels of bone resorption markers were more increased than bone formation markers in men with cardiovascular events and only increased levels of bone resorption, but not bone formation, markers predicted cardiovascular risk.
Other proteins can be involved in the regulation of bone and vascular metabolism (e.g., α-klotho or inhibitors of mineralization such as matrix GLA protein [MGP] and fetuin A).(57–59) It is possible that the above factors act through other ones (e.g., low-grade systemic inflammation is more frequent in smokers).(60) One risk factor may act through different mechanisms in bone and in arteries (e.g., vitamin D deficit may result in secondary hyperparathyroidism in bone and in a MGP deficit in the vascular wall).(35,61) Different factors may potentiate their effects in both systems (e.g., vitamin D deficit and inflammation). PTH and MMP increase bone resorption, whereas vascular calcification may be inhibited less strongly, if the MGP content is low.(62)
Our study has several limitations. First, inhabitants of Montceau les Mines may be not representative of the French population. Second, our study was not designed to investigate cardiovascular diseases, and information on these diseases has not been rigorously collected. Third, the baseline cardiovascular health status was assessed on the basis of data from health questionnaires. Evaluation of prevalent diseases analyzed as confounders was limited to self-report (yes/no, no data on the duration, severity or treatment). Fourth, some potential confounders were rare (e.g., former stroke) or had low average intensity (e.g., smoking—median 10 cigarettes/d), and their effect could not be correctly assessed. A number of potential confounders (e.g., severity and duration of cardiovascular disease, presence of subclinical ischemic heart disease, nutritional habits except current calcium intake) were not assessed such that residual confounding may still exist. Finally, the outcome variable (incident myocardial infarction and stroke) was self-reported or reported by a proxy in nine men (11%). All the reported events were confirmed using medical records but not verified by formal adjudication. This approach may have underestimated the incidence of the cardiovascular events but avoided the false-positive events. We studied stroke and myocardial infarction but not milder forms of incident cardiovascular disease. These two diseases are clear-cut events whose dates of occurrence and diagnosis can be easily confirmed and that reflect a severe degree of cardiovascular pathology. However, we did not use predefined diagnostic criteria for each disease; thus, men with a given type of cardiovascular event may not be a homogenous group with respect to severity of disease. The recruited volunteers may be healthier than the general population and have lower BTM levels and lower risk of cardiovascular events than the general population. A single measurement of BTM levels may not fully reflect the average bone turnover rate in an individual.
Our finding that increased bone resorption was associated with higher risk of cardiovascular event in older men regardless of other variables supports the hypothesis that cardiovascular diseases and osteoporosis may share common risk factors and biological mechanisms. Thus, older men with low BMD or high bone resorption may be at increased risk of myocardial infarction and stroke in addition to fracture. Jointly with our previous study,(16) these data suggest that osteoporotic men may benefit from screening for cardiovascular disease and vice versa. Further study of pathophysiological mechanisms underlying this association is needed to better understand shared etiologies in bone and heart health in older men to identify men at risk and to develop treatment.
ACKNOWLEDGMENTS
This study was supported by a contract INSERM/Merck-Sharp-Dohme Chibret. Dr. Kiel was supported by a grant from the National Institutes of Health (R01 AR/AG 41398).
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Ezzati M, Henley J, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112:489–497. doi: 10.1161/CIRCULATIONAHA.104.521708. [DOI] [PubMed] [Google Scholar]
- 2.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szulc P, Garnero P, Claustrat B, Marchand F, Duboeuf F, Delmas PD. Increased bone resorption in moderate smokers with low body weight: The Minos study. J Clin Endocrinol Metab. 2002;87:666–674. doi: 10.1210/jcem.87.2.8232. [DOI] [PubMed] [Google Scholar]
- 4.Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, Orwoll ES. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91:3908–3915. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 6.Szulc P, Claustrat B, Marchand F, Delmas PD. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: The MINOS study. J Clin Endocrinol Metab. 2003;88:5240–5247. doi: 10.1210/jc.2003-030200. [DOI] [PubMed] [Google Scholar]
- 7.Hak AE, Witteman JCM, de Jong FH, Geerlings MI, Hofman A, Pols HAP. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: The Rotterdam study. J Clin Endocrinol Metab. 2002;87:2632–2639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 8.Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: A longitudinal study. J Clin Endocrinol Metab. 2008;93:1952–1958. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GDO, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentrations of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 11.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 12.Samelson EJ, Kiel DP, Broe KE, Zhang Y, Cupples LA, Hannan MT, Wilson PWF, Levy D, Williams SA, Vaccarino V. Metacarpal cortical area and risk of coronary heart disease. The Framingham Study. Am J Epidemiol. 2004;159:589–595. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- 13.Tankó LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 14.Farhat GN, Strotmeyer ES, Newman AB, Dutton-Tyrrell K, Bauer DC, Harris T, Johnson KC, Taaffe DR, Cauley JA. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: The Health, Aging, and Body Composition Study. Calcif Tissue Int. 2006;79:102–111. doi: 10.1007/s00223-006-0052-0. [DOI] [PubMed] [Google Scholar]
- 15.Mussolino ME, Madans JH, Gillum RF. Bone mineral density and stroke. Stroke. 2003;34:e20–e22. doi: 10.1161/01.STR.0000065826.23815.A5. [DOI] [PubMed] [Google Scholar]
- 16.Szulc P, Kiel D, Delmas PD. Calcifications in the abdominal aorta predict fractures in men - MINOS study. J Bone Miner Res. 2008;23:95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 17.Samelson EJ, Cupples LA, Broe KE, Hannan MT, O'Donnell CJ, Kiel DP. Vascular calcification in middle-age and long term risk of hip fracture: The Framingham study. J Bone Miner Res. 2007;22:1449–1454. doi: 10.1359/jbmr.070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PWF. Bone loss and the progression of abdominal aortic calcification over a 25 year period: The Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 19.Magnus JH, Broussard DL. Relationship between bone mineral density and myocardial infarction in US adults. Osteoporos Int. 2005;16:2053–2062. doi: 10.1007/s00198-005-1999-9. [DOI] [PubMed] [Google Scholar]
- 20.Sinnott B, Syed I, Sevrukov A, Barengolts E. Coronary calcifications and osteoporosis in men and postmenopausal women are independent processes associated with aging. Calcif Tissue Int. 2006;78:195–202. doi: 10.1007/s00223-005-0244-z. [DOI] [PubMed] [Google Scholar]
- 21.Laroche M, Pouilles JM, Ribot C, Bendayan P, Bernard J, Boccalon H, Mazières B. Comparison of the bone mineral content of the lower limbs in men with ischemic atherosclerotic disease. Clin Rheumatol. 1994;13:611–614. doi: 10.1007/BF02243003. [DOI] [PubMed] [Google Scholar]
- 22.Wong SYS, Kwok T, Woo J, Lynn H, Griffith JF, Leung J, Tang YYN, Leung PC. Bone mineral density and the risk of peripheral arterial disease in men and women: Results from Mr. and Ms Os, Hong Kong. Osteoporos Int. 2005;16:1933–1938. doi: 10.1007/s00198-005-1968-3. [DOI] [PubMed] [Google Scholar]
- 23.van der Klift M, Pols HAP, Hak AE, Witteman JCM, Hofman A, de Laet CEDH. Bone mineral density and the risk of peripheral arterial disease: The Rotterdam study. Calcif Tissue Int. 2002;70:443–449. doi: 10.1007/s00223-001-2076-9. [DOI] [PubMed] [Google Scholar]
- 24.Mussolino ME, Gillum RF, Madans JH. Bone mineral density and stroke risk. Stroke. 2001;32:2956–2957. [PubMed] [Google Scholar]
- 25.Jørgensen L, Engstad T, Jacobsen BK. Bone mineral density in acute stroke patients. Low bone mineral density may predict first stroke in women. Stroke. 2001;32:47–51. doi: 10.1161/01.str.32.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Szulc P, Marchand F, Duboeuf F, Delmas PD. Cross-sectional assessment of age-related bone loss in men. Bone. 2000;26:123–129. doi: 10.1016/s8756-3282(99)00255-0. [DOI] [PubMed] [Google Scholar]
- 27.Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD. Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res. 2001;16:1642–1650. doi: 10.1359/jbmr.2001.16.9.1642. [DOI] [PubMed] [Google Scholar]
- 28.Szulc P, Munoz F, Marchand F, Chapuy MC, Delmas PD. Role of vitamin D and parathyroid hormone in the regulation of bone turnover and bone mass in men: The MINOS study. Calcif Tissue Int. 2003;73:520–530. doi: 10.1007/s00223-002-2103-5. [DOI] [PubMed] [Google Scholar]
- 29.Szulc P, Delmas PD. Biochemical markers of bone turnover: Potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008;19:1683–1704. doi: 10.1007/s00198-008-0660-9. [DOI] [PubMed] [Google Scholar]
- 30.Szulc P, Munoz F, Claustrat B, Garnero P, Marchand F, Duboeuf F, Delmas PD. Bioavailable estradiol may be an important determinant of osteoporosis in men: The MINOS study. J Clin Endocrinol Metab. 2001;86:192–199. doi: 10.1210/jcem.86.1.7126. [DOI] [PubMed] [Google Scholar]
- 31.Abbott RD, Launer LJ, Rodriguez BL, Ross GW, Wilson PW, Masaki KH, Strozyk D, Curb JD, Yano K, Popper JS, Petrovich H. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68:563–568. doi: 10.1212/01.wnl.0000254473.88647.ca. [DOI] [PubMed] [Google Scholar]
- 32.Tivesten A, Hulthe J, Wallenfeldt K, Wikstrand J, Ohlsson C, Fagenberg B. Circulating estradiol is an independent predictor of progression of carotid artery intima-media thickness in middle-aged men. J Clin Endocrinol Metab. 2006;91:4433–4437. doi: 10.1210/jc.2006-0932. [DOI] [PubMed] [Google Scholar]
- 33.Ärnlöv J, Pencina MJ, Amin S, Nam BH, Benjamin EJ, Murabito JM, Wang TJ, Knapp PE, D'Agostino RB, Bhasin S, Vasan RS. Endogenous sex hormones and cardiovascular disease incidence in men. Ann Intern Med. 2006;147:176–184. doi: 10.7326/0003-4819-145-3-200608010-00005. [DOI] [PubMed] [Google Scholar]
- 34.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78:539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 35.Dawson-Hughes B. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6th ed. Washington, DC, USA: American Society for Bone and Mineral Research; 2008. Calcium and vitamin D; pp. 257–259. [Google Scholar]
- 36.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giovanucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men. A prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 39.Oh KW, Rhee EJ, Lee WY, Kim SW, Baek KH, Kang MI, Yun EJ, Park CY, Ihm SH, Choi MG, Yoo HJ, Park SW. Circulating osteoprotegerin and rceptor activator of NF-κB ligand system are associated with bone metabolism in middle-aged males. Clin Endocrinol (Oxf) 2005;62:92–98. doi: 10.1111/j.1365-2265.2004.02179.x. [DOI] [PubMed] [Google Scholar]
- 40.Khosla S, Arrighi HM, Melton LJ, III, Atkinsn EJ, O'Fallon WM, Dunstan C, Riggs BL. Correlates of osteoprotegerin levels in women and men. Osteoporos Int. 2002;13:394–399. doi: 10.1007/s001980200045. [DOI] [PubMed] [Google Scholar]
- 41.Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD. Osteoprotegerin serum levels in men: Correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab. 2001;86:3162–3165. doi: 10.1210/jcem.86.7.7657. [DOI] [PubMed] [Google Scholar]
- 42.Samelson EJ, Broe KE, Demissie S, Beck TJ, Karasik D, Kathiresan S, Kiel DP. Increased plasma osteoprotegerin concentrations are associated with indices of bone strength of the hip. J Clin Endocrinol Metab. 2008;93:1789–1795. doi: 10.1210/jc.2007-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21:1616. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- 44.Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary heart disease. J Clin Endocrinol Metab. 2003;88:1024–1028. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- 45.Kiechl S, Schett G, Schwaiger J, Seppi K, Eder P, Egger G, Santer P, Mayr A, Xu Q, Willeit J. Soluble receptor activator of nuclear factor-κB ligand and risk for cardiovascular disease. Circulation. 2007;116:385–391. doi: 10.1161/CIRCULATIONAHA.106.686774. [DOI] [PubMed] [Google Scholar]
- 46.Bruunsgaard H. The clinical impact of systemic low-level inflammation in elderly populations. Dan Med Bull. 2006;53:285–309. [PubMed] [Google Scholar]
- 47.Kim BJ, Yu YM, Kim EN, Chung YE, Koh JM, Kim GS. Relationship between serum hsCRP concentration and biochemical bone turnover markers in healthy pre- and postmenopausal women. Clin Endocrinol (Oxf) 2007;67:152–158. doi: 10.1111/j.1365-2265.2007.02853.x. [DOI] [PubMed] [Google Scholar]
- 48.Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, Kuller LH, Cushman M. Association of carotid artery intime-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality. The Cardiovascular Health Study. Circulation. 2007;116:32–38. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 49.Simionescu A, Simionescu DT, Vyavahare NR. Osteogenic responses in fibroblasts activated by elastin degradation products and transforming growth factor-β1. Am J Pathol. 2007;171:116–123. doi: 10.2353/ajpath.2007.060930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouvet C, Moreau S, Blanchette J, de Blois D, Moreau P. Sequential activation of matrix metalloproteinase 9 and transforming growth factor β in arterial elastocalcinosis. Arterioscler Thromb Vasc Biol. 2008;28:856–862. doi: 10.1161/ATVBAHA.107.153056. [DOI] [PubMed] [Google Scholar]
- 51.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model. Role of matrix metalloproteinases. Circulation. 2004;110:3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–1516. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 53.Walter MF, Jacob JF, Jeffers B, Ghadanfar MM, Preston GM, Buch J, Masson MP. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: A longitudinal analysis of the PREVENT study. J Am Coll Cardiol. 2004;44:1996–2002. doi: 10.1016/j.jacc.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 54.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 55.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt MA, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting β-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282:27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- 57.Nabeshima Y, Imura H. α-Klotho: A regulator that integrates calcium homeostasis. Am J Nephrol. 2008;28:455–464. doi: 10.1159/000112824. [DOI] [PubMed] [Google Scholar]
- 58.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 59.Schäfer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Müller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein α2–Heremans-Schmid glycoprotein/ fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EFM. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 61.Fraser JD, Otawara Y, Price PA. 1,25-dihydroxyvitamin D3 stimulates the synthesis of matrix γ–carboxyglutamic acid protein by osteosarcoma cells. J Biol Chem. 1988;263:911–916. [PubMed] [Google Scholar]
- 62.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]