Abstract
The source of conscious experience has fueled scientific and philosophical debates for centuries. In auditory and motor domains, it is yet unknown how consciously and unconsciously obtained information may combine to enable the production and perception of speaking and singing. Both forms of vocalizations rely upon the interaction of brain networks responsible for perception and action. While perceptual experience and executed actions are usually well coupled, dissociations between perception and action can be informative of their underlying neural systems. Here we report a dissociation between production and perception: tone-deaf individuals, who cannot consciously perceive pitch differences, can paradoxically reproduce pitch intervals in correct directions. Our results suggest that multiple neural pathways have evolved for sound perception and production, so that pitch information sufficient for intact speech can be obtained separately from pathways necessary for conscious perception.
Perceiving pitch is a central function of the human and animal auditory system (1, 2). Humans are generally able not only to consciously perceive pitch differences, but also to produce pitch intervals accurately via the interaction of perceptual and motor neural systems (3), thus enabling the communication of musical and linguistic information. However, this ability is selectively impaired in one special population. People affected by tone-deafness, also known as congenital amusia, report musical difficulties or have been told that they sing out of tune, but have normal audiometry and no obvious language problems (4, 5). Psychophysically, one hallmark of tone-deaf individuals is their inability to consciously discriminate pitches less than one semitone apart (5, 6). This dichotomy offers a unique model to test the relationship between conscious perception and unconscious actions, and between brain mechanisms responsible for action and perception. We tested the hypothesis that tone-deaf individuals might have dissociated abilities in pitch perception and production. Using psychophysics combined with sound analysis of singing in tone-deaf and control samples, we provide evidence for intact but imprecise abilities in pitch production despite impaired perception in tone-deafness. Tone-deaf listeners were identified based on their significantly below-average performance on the Montreal Battery of Evaluation of Amusia (MBEA) (see Supplemental Data). In a first experiment assessing perception and production, pairs of pure tones, forming different small intervals, were presented to tone-deaf and normal control individuals. Listeners reproduced the tone intervals by humming (production task), and then indicated verbally whether the second tone was higher or lower than the first (perception task).
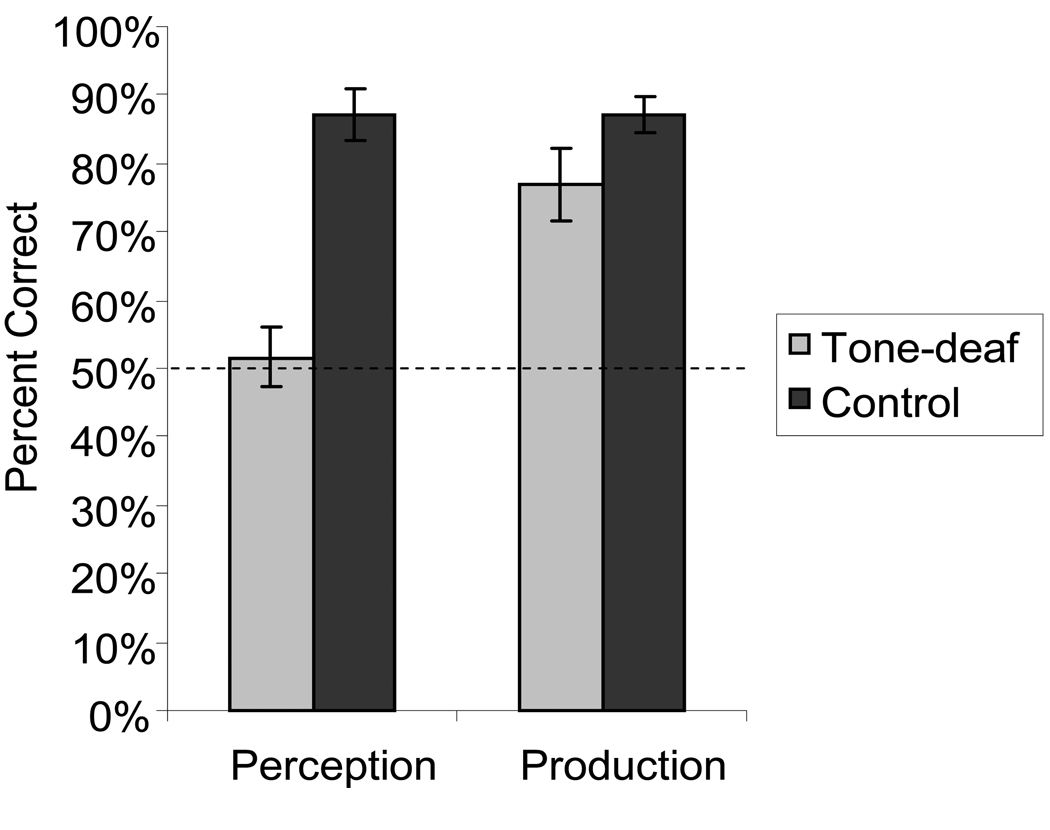
Tone-deaf listeners performed at chance for the perception task, and were significantly worse than controls. However, in the production task tone-deaf individuals were above chance at producing the correct pitch direction, with performance being indistinguishable from controls (Fig. 1). This dichotomy between perception and production points to a dissociation between action and perception pathways in the auditory system (7), possibly analogous to action-blindsight in the visual system (8).
Figure 1.
Perception and production accuracy in tone-deaf and control listeners. Each trial was scored as correct if the direction of verbal report (in the perception task) or the direction of produced fundamental frequencies (in the production task) was the same as target stimuli. Perception was significantly worse in tone-deaf individuals than controls, t(1,10) = 5.6, p = 0.0002. However, direction of pitch production was above chance in tone-deaf subjects, with performance being statistically indistinguishable from controls, t(1,10) = 1.05, p = 0.3. A two-way ANOVA with factors of group (tone-deaf vs. control) and task (perception vs. production) showed a significant interaction (F(1,20) = 13.2, p < 0.01), confirming differential performance in perception but not production in tone-deaf versus control listeners.
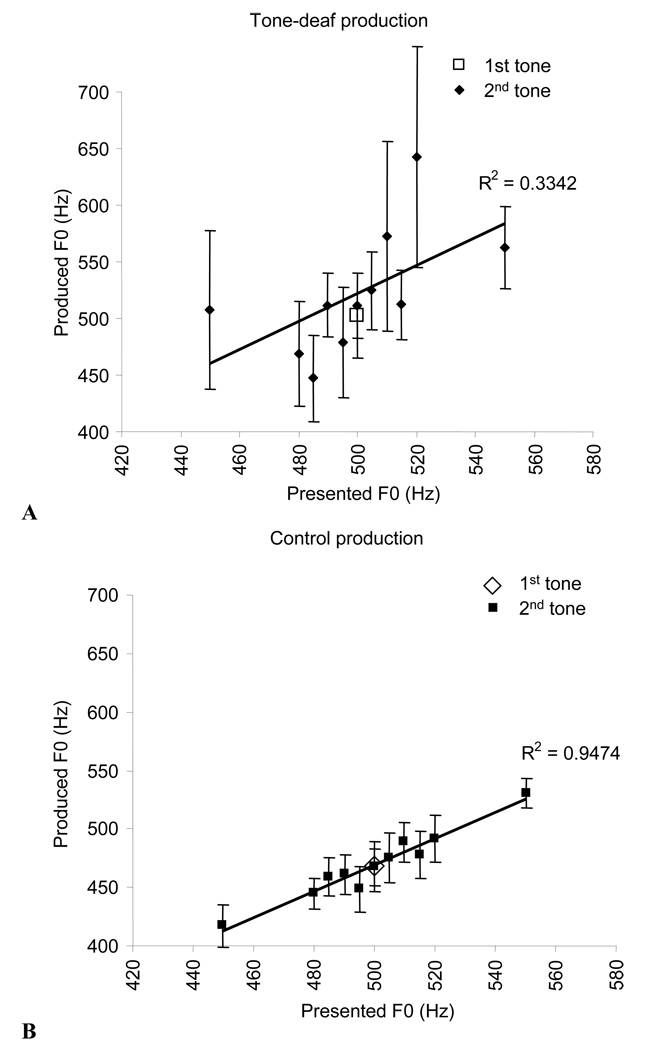
Pitch extraction of recorded sung intervals showed that while directions of interval production were intact, variability in produced pitches compared to target pitches was significantly higher in tone-deaf listeners than in controls (Fig. 2A,B). Thus, although tone-deaf listeners could produce pitch intervals in target directions, the pitches they produce are imprecise and highly variable. This leads to the common observation that tone-deaf individuals are unable to sing in tune.
Figure 2.
A. Mean fundamental frequencies of the two produced tones in tone-deaf listeners. The first tone had a target frequency at a constant 500Hz, whereas the second tone ranged from 450–550Hz. B. Same as A in normal controls. While both groups show a significant positive correlation between target and produced fundamental frequency, the correlation is significantly lower in the tone-deaf group (t(1,10) = 2.3, p = 0.046) and variability in pitch production is higher for the tone-deaf group, as indicated by a t-test comparing standard error across different subjects producing the same pitch: t(1,20) = 3.6, p = 0.0015. Error bars indicate between-subject standard error.
To further characterize the dichotomy between production and perception, we conducted adaptive staircase procedures on a subset of subjects (tone-deaf group: n = 3; control group: n = 3) to assess the psychophysical thresholds of production and perception (see Supplemental Data for details on methods). For tone-deaf individuals, production thresholds (Mean = 12.3Hz, SD = 2.5Hz) were much smaller than perception thresholds (Mean = 36.2Hz, SD = 2.0Hz). In contrast, control subjects showed nearly-identical thresholds for production and perception, with a slightly larger production threshold (Mean = 2.5 Hz, SD = 1.0Hz) than perception threshold (Mean = 2.0Hz, SD = 0.8Hz). Paired t-tests comparing thresholds for perception and production were conducted for each individual subject using confidence intervals obtained from the reversal points in the adaptive staircase procedure (in Hz). Results show significantly different perception and production thresholds for every tone-deaf subject (subject1: t(5) = 5.9, p = 0.002; s2: t(5) = 2.6, p = 0.047; s3: t(5) = 3.6, p = 0.02) but no such dissociation between perception and production thresholds for every control subject (s1: t(5) = 1.3, p = 0.3; s2: t(5) = 2.0, p = 0.1; s3: t(5) = 1.0, p = 0.4; see Supplemental Data Figure S1). These individual statistics confirm that perception and production are mismatched in tone-deaf individuals but not in controls.
Models of vocal communication generally involve interactions between the perception and production systems that allow the tuning of motor commands to achieve sound targets (3). Our results shed further light on these models by indicating that the auditory pathways necessary for vocal performance are, to some degree at least, distinct from those necessary for conscious perception. The fact that tone-deaf individuals show no clear impairment in perceiving and producing speech provides further support for this conclusion. The distinction between auditory streams for production and perception demonstrated here may be analogous to separate visual streams for action and perception (9). Further studies may aim to identify the precise neural correlates of this perception-action mismatch, and relate behavioral manifestations of tone-deafness to observed neurobiological anomalies in this unique population (10).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the NINDS (R01 NS045049), NIDCD (RO1 DC008796) and NSF (BCS0518837) to G.S. and NIDCD (R01 DC002852) to F.H.G. We thank three anonymous reviewers for helpful comments and suggestions.
Footnotes
SUPPLEMENTAL DATA
Supplemental data are available at http://www.current-biology.com
REFERENCES
- 1.Pantev C, Hoke M. Tonotopic organization of the auditory cortex: Pitch versus frequency representation. Science. 1989;246:486–488. doi: 10.1126/science.2814476. [DOI] [PubMed] [Google Scholar]
- 2.Bendor D, Wang X. The neuronal representation of pitch in primate auditory cortex. Nature. 2005;436:1161–1165. doi: 10.1038/nature03867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuddy LL, Balkwill LL, Peretz I, Holden RR. Musical difficulties are rare: a study of "tone deafness" among university students. Ann N Y Acad Sci. 2005;1060:311–324. doi: 10.1196/annals.1360.026. [DOI] [PubMed] [Google Scholar]
- 5.Peretz I, Ayotte J, Zatorre RJ, Mehler J, Ahad P, Penhune VB, Jutras B. Congenital amusia: a disorder of fine-grained pitch discrimination. Neuron. 2002;33:185–191. doi: 10.1016/s0896-6273(01)00580-3. [DOI] [PubMed] [Google Scholar]
- 6.Foxton JM, Dean JL, Gee R, Peretz I, Griffiths TD. Characterization of deficits in pitch perception underlying 'tone deafness'. Brain. 2004;127:801–810. doi: 10.1093/brain/awh105. [DOI] [PubMed] [Google Scholar]
- 7.Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proceedings of the National Academy of Sciences. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowey A, Stoerig P. The neurobiology of blindsight. Trends in Neurosciences. 1991;14:140–145. doi: 10.1016/0166-2236(91)90085-9. [DOI] [PubMed] [Google Scholar]
- 9.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 10.Mandell J, Schulze K, Schlaug G. Congenital amusia: An auditory-motor feedback disorder? Restorative Neurology and Neuroscience. 2007;25:323–334. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.