Abstract
Joubert syndrome is an autosomal recessive disorder that is characterized by a variable combination of central nervous system, respiratory and eye anomalies. It is a syndrome with a variable phenotype: partial or complete absence of the cerebellar vermis is seen in all patients, while other cardinal findings include episodic tachypnea and apnea in the neonatal period, jerky eye movements, hypotonia, severe mental handicap, developmental delay, ataxia and impaired equilibrium. Even within sibships the phenotype may vary, making it difficult to establish the exact clinical diagnostic boundaries of Joubert syndrome. A case of Joubert syndrome in a newborn is reported and the importance of recognizing the syndrome in the neonatal period so that specific and effective supportive measures can be started as soon as possible is stressed.
Keywords: Abnormal respiratory pattern, Ataxia, Cerebellar hypoplasia, Joubert syndrome, Newborn
Abstract
Le syndrome de Joubert est un trouble récessif autosomique caractérisé par une association variable d’anomalies des systèmes nerveux central, respiratoire et oculaire. C’est un syndrome au phénotype variable : tous les patients présentent une absence partielle ou complète du vermis cérébelleux et, parmi les autres observations primordiales, une tachypnée et une apnée épisodiques pendant la période néonatale, de brusques mouvements oculaires, une hypotonie, un handicap intellectuel marqué, un retard du développement, une ataxie et une atteinte de l’équilibre. Même au sein de la fratrie, le phénotype peut varier, ce qui rend difficile l’établissement de balises diagnostiques cliniques exactes pour le syndrome de Joubert. Le cas d’un syndrome de Joubert chez un nouveau-né est présenté, et est soulignée l’importance de dépister le syndrome pendant la période néonatale, pour que des mesures de soutien précises et efficaces puissent être mises en place le plus rapidement possible.
The association of developmental defects of the cerebellar vermis with mental retardation, episodic hyperpnea and abnormal eye movements was recognized by Joubert et al (1) in four siblings and one sporadic case. Later, Boltshauser and Isler (2) reported three more cases with variable degrees of clinical symptoms and suggested the syndrome be called Joubert syndrome (JS). Saraiva and Baraitser (3) reviewed 101 cases with the possible diagnosis of JS and proposed a diagnostic criteria: vermian hypoplasia, hypotonia, developmental delay and abnormal breathing or eye movements. In 1997, Steinlin and colleagues (4) developed new criteria that included neuroimaging findings reported by Maria and colleagues (5). Magnetic resonance imaging shows characteristic brainstem and cerebellar vermis abnormalities, including the presence of the molar tooth sign.
JS is a disorder characterized by episodic panting tachypnea alternating with periods of apnea and jerky eye movements in the neonatal period associated with ataxia, psychomotor retardation and cerebellar vermis agenesis or hypoplasia (6). A variety of other abnormalities have been described in children with JS, including a characteristic facial appearance, delayed language, hypersensitivity to noise, autism, ocular and oculomotor abnormalities, meningoencephaloceles, microcephaly, low-set ears, polydactyly, retinal problems, kidney abnormalities, soft tissue tumour of the tongue, liver disease and duodenal atresia (7). Even within sibships the phenotype may vary, making it difficult to establish the exact clinical diagnostic boundaries of JS (8). The biochemical and genetic basis of the disease is unknown.
JS is a well-known but rare, autosomal recessive condition. From a review of the literature, it does appear that, even though the clinical features of the disorder are present in the newborn period, the correct diagnosis is often not made for several months or years after birth. JS is a nonprogressive disease and early diagnosis would positively affect the subsequent management and outcome. This report shows that with the availability of neonatal magnetic resonance imaging, it is quite feasible to make a diagnosis in the neonatal period.
CASE PRESENTATION
A four-day-old girl was admitted to hospital for evaluation of abnormal respiratory pattern and hypotonia. She was born at term after an uneventful pregnancy. She was the first child of a healthy 20-year-old mother and a healthy 22-year-old father. The infant was the product of a first-degree consanguineous marriage. The baby was born by vaginal delivery and did not cry immediately after birth. The Apgar scores were four at 1 min and seven at 5 min. Oxygen therapy had been given by head box. A striking respiratory abnormality was apparent shortly after birth. On admission to the hospital the baby was found to have episodes of panting with a respiratory rate of up to 100 breaths/min, alternating with apnea lasting for 15 to 20 s with cyanosis.
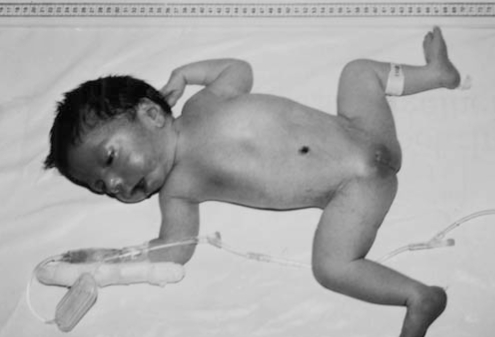
On physical examination, her weight was 3250 g (50th percentile), her height was 48 cm (25th percentile) and her head circumference was 34 cm (25th to 50th percentile). She had a broad nasal bridge, anteverted nostrils and a triangular-shaped open mouth. She was hypotonic and had intermittent jerky horizontal eye movements. Bilateral ptosis and optic atrophy were detected. Other physical examination findings were normal (Figure 1).
Figure 1).
Picture of the patient in the neonatal period (15 days old)
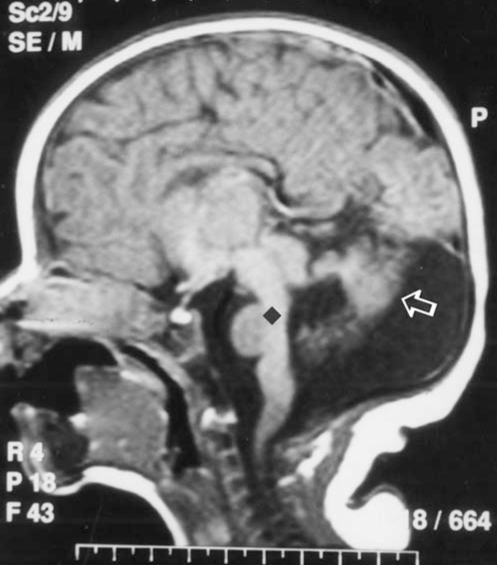
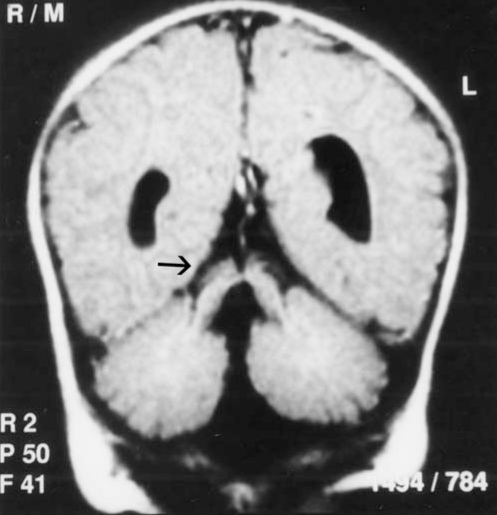
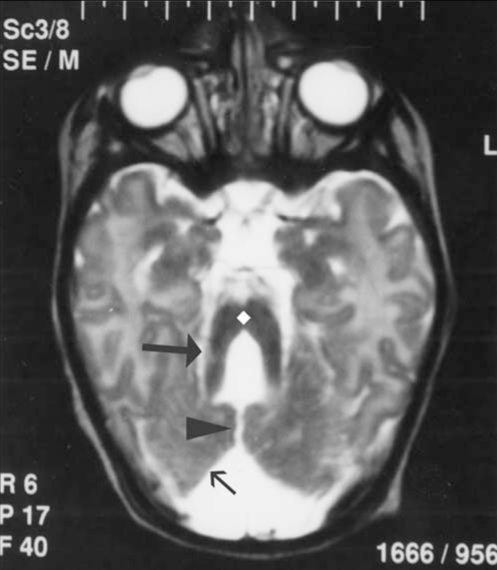
Laboratory investigations, including hemoglobin, hematocrit, serum calcium, phosphorus, alkaline phosphatase, blood urea nitrogen, creatinine, electrolytes, blood sugar, lactate, ammonia, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, acid-base values, cerebrospinal fluid examination, blood and urine amino acid, chromosome analysis, and urine analysis were normal. Magnetic resonance imaging showed hypoplasia of the cerebellar hemispheres and vermis, and a narrowed isthmus of the mid brain with a characteristic ‘molar tooth appearance’ (Figures 2–4).
Figure 2).
Sagittal T1 weighted magnetic resonance image reveals absence of vermian folia (arrow) and thin brainstem (diamond)
Figure 4).
Coronal fluid attenuated inversion recovery image reveals thickened superior cerebellar peduncles (arrow)
The infant was followed-up by the author’s neurology outpatient clinic. At the age of nine months (last follow-up control), the child’s development was obviously retarded: she did not smile, follow with her eyes or grasp objects, control her head, or sit independently. The muscle tone was floppy, but reflexes were normal.
DISCUSSION
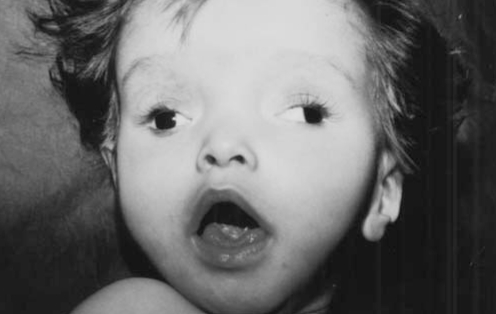
The clinical presentation of children with JS can include non-specific features such as hypotonia, ataxia and developmental delay. Careful examination of the face shows a characteristic appearance. The features include a large head, a prominent forehead, high rounded eyebrows, epicanthal folds, ptosis (occasionally), an upturned nose with evident nostrils, an open mouth (the mouth tends to have an oval shape early on, a ‘rhomboid’ appearance later, and finally can appear triangular with downturned mouth angles), tongue protrusion and rhythmic tongue motions, and occasionally low-set and tilted ears (7). Our patient has a dysmorphic facial appearance with a prominent forehead, high rounded eyebrows, ptosis, an upturned nose with evident nostrils, an open mouth and low-set ears (Figure 5).
Figure 5).
Facial characteristics of the case at the age of nine months
One of the hallmark features of JS is a paucity of postural muscle tone. Infants have moderately severe hypotonia, lack spontaneous movements and lie with legs fully abducted with the lateral surface against the examination table (frog-leg position) (7). Severe hypotonia was present in our patient.
One of the key clinical features of JS is developmental delay, which is usually severe and present across a variety of domains, including adaptive behaviours, motor, language and general development. Previous reports suggest that developmental outcome is independent of sex, siblings’ outcomes and severity of gross central nervous system malformations (4,9). Our patient was severely developmentally delayed; she could not control her head, follow objects, roll over or sit up.
Respiratory abnormalities have been considered a ‘classic hallmark’ of the condition, present in all four siblings initially described by Joubert and colleagues (1). However, it is not a consistent feature; it was reported in 44% of the cases by Kendal and colleagues (10), 68% by Pellegrino et al (11) and 71% by Maria and colleagues (12). Abnormal respirations are typically seen in the neonatal period and improve with age (2,3). On admission, our patient had episodes of a panting type of breathing alternating with apnea and cyanosis. Soon after hospitalization, the baby’s abnormal breathing became very frequent, but short apneic periods lasted without cyanosis or alteration in heart rate. Tachypnea could be triggered by handling and subsided during sleep.
Ocular and oculomotor abnormalities are common in JS, and are helpful in making a diagnosis. Seventy per cent to 100% of the patients have such abnormalities (4,5,11). Abnormal eye movements are varied and include nystagmus, strabismus, oculomotor ataxia and vertical gaze palsy (7). Retinal problems include congenital retinal dystrophy, pigmentary retinopathy, fundus flavus, chorioretinal coloboma, perimacular and retinal blindness (4,5,11). In our patient, abnormal eye movements were horizontal nystagmus, exotropia, and oculomotor apraxia. Bilateral pitosis and optic atrophy were detected.
Magnetic resonance imaging studies of patients with JS show a constellation of abnormalities in the central nervous system. Key radiological features include deeper-than-normal posterior interpeduncular fossa, prominent or thickened superior cerebellar peduncles and vermian hypoplasia or dysplasia. These abnormalities can be grouped together to show the ‘molar tooth sign’ on axial magnetic resonance imaging, as Maria and colleagues first described (5). The radiological findings were evaluated further in a detailed study conducted by Maria and colleagues that showed the diagnostic value of the molar tooth sign (12). Further detailed descriptions were reviewed by Quisling and colleagues (13). In our patient, the radiological findings included dysgenetic cerebellum, complete aplasia of vermis and narrowed isthmus of the midbrain. The superior cerebellar peduncles bilaterally appeared enlarged and horizontal which was consistent with a molar tooth malformation (Figures 2–4).
Since the first report by Joubert and colleagues, approximately 200 cases have been published in the literature. The molecular basis of JS is unknown. The apparent autosomal recessive pattern of inheritance is suggested by the observation of pedigrees with multiple affected offspring from normal parents. In addition, consanguinity has been documented in a few cases (3). A locus (or loci) for this disorder has not been identified previously and cytogenetic studies, where described, have been normal in patients with JS (14). Saar et al (15) conclude that JS is clinically and genetically heterogeneous and that one locus maps to chromosome 9q.
Developmental outcome in JS is variable. Steinlin et al (4) suggested that outcomes in JS can be divided in three courses: first, children who die young; second, patients who survive but are severely developmentally delayed and have a variety of visual and motor handicaps; and third, patients whose developmental quotients fall within the mildly delayed range (70 to 80). Therefore, in view of the variability of the clinical outcome, the authors stress the importance of recognizing the syndrome in the neonatal period so that specified and prompt supportive measures can be instituted.
Figure 3).
Axial T2 weighted image shows dysgenetic cerebellar hemispheres (small arrow) with a prominent vermian cleft (arrowhead) and narrowed isthmus of the mid brain (diamond). The superior cerebellar peduncles bilaterally appear enlarged and horizontal, resulting in characteristic ‘molar tooth appearance’ (big arrow)
REFERENCES
- 1.Joubert M, Eisenring JJ, Robb JP, Anderman F. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia and mental retardation. Neurology. 1969;19:813–25. doi: 10.1212/wnl.19.9.813. [DOI] [PubMed] [Google Scholar]
- 2.Boltshauser E, Isler W. Episodic hyperpnea, abnormal eye movements, retardation, ataxia, associated with dysplasia of the cerebellar vermis. Neuropadiatrie. 1977;8:57–66. doi: 10.1055/s-0028-1091505. [DOI] [PubMed] [Google Scholar]
- 3.Saraiva JM, Baraitser M. JS Joubert syndrome: A review. Am J Med Genet. 1992;43:726–31. doi: 10.1002/ajmg.1320430415. [DOI] [PubMed] [Google Scholar]
- 4.Steinlin M, Schmid M, Landau K, Boltshauser E. Follow-up in children with Joubert syndrome. Neuropediatrics. 1997;28:204–11. doi: 10.1055/s-2007-973701. [DOI] [PubMed] [Google Scholar]
- 5.Maria BL, Hoang KB, Tusa RJ, et al. ‘Joubert syndrome’ revisited: Key ocular motor signs with magnetic resonance imaging correlation. J Child Nerol. 1977;12:423–30. doi: 10.1177/088307389701200703. [DOI] [PubMed] [Google Scholar]
- 6.Cantani A, Lucenti P, Ronzani GA, Santoro C. Joubert syndrome. Review of the fifty-three cases so far published. Ann Genet. 1990;33:96–8. [PubMed] [Google Scholar]
- 7.Maria BL, Boltshauser E, Palmer SC, Tran TX. Clinical features and revised diagnostic criteria in Joubert syndrome. J Child Neurol. 1999;14:583–91. doi: 10.1177/088307389901400906. [DOI] [PubMed] [Google Scholar]
- 8.Raynes HR, Shanske A, Goldberg S, Burde R, Rapin I. Joubert syndrome: Monozygotic twins with discordant phenotypes. J Child Neurol. 1999;14:649–54. doi: 10.1177/088307389901401005. [DOI] [PubMed] [Google Scholar]
- 9.Gitten J, Dede D, Fennell E, et al. Neurobehavioral development in Joubert syndrome. J Child Neurol. 1998;13:391–7. doi: 10.1177/088307389801300806. [DOI] [PubMed] [Google Scholar]
- 10.Kendall B, Kingsley D, Lambert SR, et al. Joubert syndrome: A clinico-radiological study. Neuroradiology. 1990;31:502–6. doi: 10.1007/BF00340131. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrino JE, Lensch MW, Muenki M, Chance PF. Clinical and molecular analysis in Joubert syndrome. Am J Med Genet. 1997;72:59–62. doi: 10.1002/(sici)1096-8628(19971003)72:1<59::aid-ajmg12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Maria BL, Quisling RG, Rosainz LC, et al. Molar tooth sign in Joubert syndrome: Clinical, radiologic, and pathologic significance. J Child Neurol. 1999;14:368–76. doi: 10.1177/088307389901400605. [DOI] [PubMed] [Google Scholar]
- 13.Quisling RG, Barkovich AJ, Maria BL. Magnetic resonance imaging features and classification of central nervous system malformations in Joubert syndrome. J Child Neurol. 1999;14:628–35. doi: 10.1177/088307389901401002. [DOI] [PubMed] [Google Scholar]
- 14.Chance PF, Cavalier L, Satran D, et al. Clinical nosologic and genetic aspects of Joubert syndrome and related syndromes. J Child Neurol. 1999;14:660–6. doi: 10.1177/088307389901401007. [DOI] [PubMed] [Google Scholar]
- 15.Saar K, Al-Gazali L, Sztriha L, et al. Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am J Hum Genet. 1999;65:1666–71. doi: 10.1086/302655. [DOI] [PMC free article] [PubMed] [Google Scholar]