Abstract
Microbial nucleic acids are critical for the induction of innate immune responses, a host defense mechanism against infection by microbes. Recent studies have indicated that double-stranded DNA (dsDNA) induces potent innate immune responses via the induction of type I IFN (IFN) and IFN-inducible genes. However, the regulatory mechanisms underlying dsDNA-triggered signaling are not fully understood. Here we show that the translocation and assembly of the essential signal transducers, stimulator of IFN genes (STING) and TANK-binding kinase 1 (TBK1), are required for dsDNA-triggered innate immune responses. After sensing dsDNA, STING moves from the endoplasmic reticulum (ER) to the Golgi apparatus and finally reaches the cytoplasmic punctate structures to assemble with TBK1. The addition of an ER-retention signal to the C terminus of STING dampens its ability to induce antiviral responses. We also show that STING co-localizes with the autophagy proteins, microtubule-associated protein 1 light chain 3 (LC3) and autophagy-related gene 9a (Atg9a), after dsDNA stimulation. The loss of Atg9a, but not that of another autophagy-related gene (Atg7), greatly enhances the assembly of STING and TBK1 by dsDNA, leading to aberrant activation of the innate immune response. Hence Atg9a functions as a regulator of innate immunity following dsDNA stimulation as well as an essential autophagy protein. These results demonstrate that dynamic membrane traffic mediates the sequential translocation and assembly of STING, both of which are essential processes required for maximal activation of the innate immune response triggered by dsDNA.
Keywords: autophagy-related gene, double-stranded DNA, interferon, membrane traffic
Innate immunity is initiated following the recognition of pathogen-associated molecular patterns and functions as a first line of host defense against infectious agents (1–3). Increasing evidence indicates that pattern recognition receptors, such as Toll-like receptors and RIG-like helicases, detect microbial nucleic acids and mediate innate immune responses (1–3). However, the system responsible for double-stranded DNA (dsDNA)-induced innate immune responses, although extensively analyzed, is still unclear. Microbial dsDNA derived from bacteria and DNA viruses, as well as synthetic dsDNA, induce both type I IFN (IFN) production and other pro-inflammatory cytokines, such as interleukin IL-1β and IL-6 (1–9). It is also known that undigested host genomic DNA accumulated in phagocytes can also induce aberrant activation of the immune response (10, 11). These findings clearly indicate that analysis of the dsDNA-induced immune response is important to understand the mechanism underlying both anti-microbial responses and the development of autoimmune disease. While absent in melanoma (AIM) 2 has been identified as a cytoplasmic dsDNA sensor required for activation of inflammasome, a caspase-1-containing complex responsible for the production of IL-1β, the sensor(s) responsible for the production of type I IFN and IFN-inducible genes by dsDNA have not yet been identified (10–11). We and others have shown that IFN regulatory factor (IRF)-3 (a transcription factor for type I IFN and IFN-inducible genes) and TANK-binding kinase (TBK) 1 (an IRF3 kinase) both play critical roles in the innate immune response initiated by dsDNA (1–7). Stimulator of IFN genes (STING), which is also called MPYS/MITA/ERIS, has recently been shown to be a multispanning membrane protein and a mediator of dsDNA-induced type I IFN production, although the mechanism responsible for the regulation of dsDNA-induced innate immune responses by STING is not known (12–15). Here we attempted to demonstrate the mechanism underlying the dsDNA-triggered innate immune response by visualization of STING translocation.
Results
STING Assembles with TBK1 After dsDNA Stimulation.
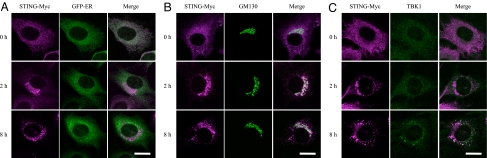
We first examined the localization of STING and TBK1 both before and after dsDNA stimulation. Although previous studies have indicated the importance of STING in the innate immune response, subcellular localization of this protein, in either the ER or in mitochondria, has been controversially reported (12–15). Hence, we decided to re-examine the localization of STING in unstimulated cells. We avoided using plasmid transient transfection methods to examine the localization of STING because the plasmid itself is a dsDNA and has been shown to induce immune responses. Our results show that STING localizes to the ER, but not to mitochondria, in unstimulated MEFs (Fig. 1A and Fig. S1A). Following dsDNA stimulation, STING rapidly translocates from the ER to the Golgi apparatus and finally assembles with TBK1 in cytoplasmic punctate structures where p62/Sequestosome 1, an aggregate marker, forms puncta (Fig. 1 B and C and Fig. S1B) (16). STING does not localize to endosomes, lysosomes or peroxisomes (Fig. S1 C–F). Even in MEFs lacking both TBK1 and IκB kinase (IKK)-i, STING forms puncta following dsDNA stimulation, indicating that the translocation of STING is independent of activation of downstream regulators (Fig. S2). Translocation of STING is specifically driven by dsDNA, such as poly (dA-dT), poly (dG-dC) and bacterial DNA, since localization of STING is not altered following stimulation with ssDNA, dsRNA, LPS, or IFNβ (Fig. S3A). We also found that puncta formation by TBK1 is also specifically induced by dsDNA, but not by dsRNA (Fig. S3B). These results indicate that the assembly of STING and TBK1 in cytoplasmic punctate structures is specifically driven by dsDNA stimulation.
Fig. 1.
Double-stranded DNA triggers the assembly of STING and TBK1. Wild-type MEFs stably expressing STING-Myc were transfected with poly (dA-dT) (1 μg/mL) for the indicated periods. The localization of STING and the indicated proteins was observed by confocal laser scanning microscopy. (Scale bars, 20 μm.)
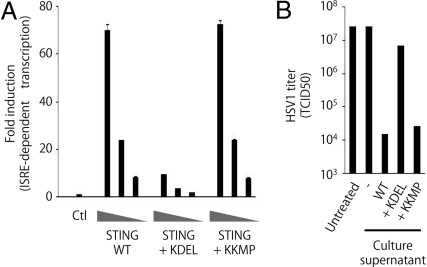
The translocation of STING in response to dsDNA prompted us to examine whether this translocation is required for mediation of the signaling pathway. It is well known that proteins which have ER-retention signal peptides on their C terminus localize to the ER (17). We found that the addition of ‘KDEL’ signal peptides, but not ‘KKMP’ signal peptides, to the C terminus of STING reduces its ability to induce IFN-stimulation responsive element (ISRE)-dependent transcription (Fig. 2A). Consistent with this result, the culture supernatant from 293 cells transfected with wild-type STING, but not that of the cells transfected with STING-KDEL, suppresses replication of Herpes simplex virus (HSV)-1 (Fig. 2B). These results indicate that translocation of STING is necessary for activation of downstream events.
Fig. 2.
The translocation of STING from ER facilitates to induce antiviral response. (A) Luciferase assay of lysates from 293 cells transfected with various amounts of the indicated STING expression vectors along with pISRE-Luc and pRL-TK. ISRE, IFN-stimulation responsive element. TK, thymidine kinase. Firefly luciferase activity was normalized to renilla luciferase activity to measure ISRE-dependent transcriptional activity. The results shown are means ± SD. (n = 3). (B) Anti-HSV1 activity in culture supernatants from 293 cells transfected with the indicated expression vectors for 24 h. HSV1 production [in 50% tissue culture infectious dose (TCID50)] was measured 24 h after infection of Vero cells either untreated or treated with the indicated culture supernatants.
STING Colocalizes with Autophagy-Related Proteins After dsDNA Stimulation.
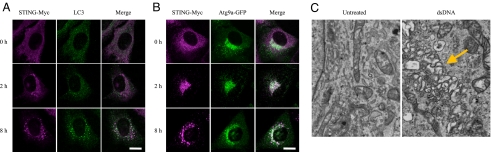
Next we attempted to analyze the cytoplasmic punctate structures of STING induced by dsDNA. As shown in Fig. S1B, p62, which is selectively degraded by autophagy, co-localizes with STING after dsDNA-stimulation, suggesting regulation of the response by autophagy, a bulk degradation system that delivers cytoplasmic constituents into lysosomes (16, 18–21). Autophagy-related proteins function as essential components of the membrane traffic system required for autophagy and are critical for the degradation of long-lived proteins, insoluble protein aggregates and invading microbes (18–21). We examined the co-localization of STING with the autophagy-related protein (Atg), ULK1 (a homologue of Atg1), Atg5, microtubule-associated protein 1 light chain (LC) 3 (a homologue of yeast Atg8), Atg9a, and Atg14L, since these are representative autophagy-related proteins in the four Atg complexes involved in the induction of autophagy (18–21). Among them, LC3 and Atg9a both show co-localization with STING after sensing dsDNA (Fig. 3 A and B and Fig. S4 A–C). These results were unexpected, since the autophagy-related proteins tested here have been reported to localize at isolation membranes and autophagosomes (18–21). Surprisingly, subsequent characterization by electron microscopy revealed that the STING-positive puncta induced by dsDNA stimulation did not have morphological characteristics of autophagosomes, double-membrane-bound structures, but were unidentified membrane-bound compartments (Fig. 3C and Fig. S5). However, in unstimulated MEFs, STING-positive puncta were hardly observed and STING localized to the ER (Fig. 3C and Fig. S5). Atg7-mediated ubiquitin-like conjugation, an essential step for autophagosome formation, was not important for regulation of dsDNA-induced innate immune responses because cytokine production and translocation of STING normally occurred in Atg7-knockout MEFs following dsDNA stimulation (Fig. S6 A and B). IFN-β production was also normal in Atg16L1-knockout MEFs stimulated with dsDNA (Fig. S6B). These results clearly demonstrate that dsDNA stimulation drives the formation of STING-positive unique punctate structures and suggests that several autophagy-related proteins regulate dsDNA-induced innate immune responses independently of autophagy.
Fig. 3.
STING colocalizes with Atg9a and assembles at unique membrane-bound compartments after dsDNA stimulation. (A and B) Wild-type MEFs stably expressing STING-Myc were transfected with poly (dA-dT) (1 μg/mL) for the indicated periods. The localization of STING and the indicated proteins was observed by confocal laser scanning microscopy. (Scale bars, 20 μm.) (C) Wild-type primary MEFs stably expressing STING-GFP were transfected with poly (dA-dT) for 8 h and then fixed. The cells were subjected to fluorescence-coupled electron microscopy. Yellow arrow indicates the STING -positive membrane-bound compartments.
Atg9a Is Required for Autophagy.
Atg9a is the only multispanning membrane protein identified as an Atg protein in mammals (18–22). Although the precise molecular functions of the Atg9a protein are still unclear, Atg9a is proposed to mediate membrane transport to generate autophagosomes (18–22). It has been reported that Atg9a locates to the Golgi apparatus and to late endosomes and that Atg9a does not steadily reside at one site, but rather dynamically cycles between these organelles under starvation conditions (22). Since Atg9a co-localizes with STING after sensing dsDNA, we generated Atg9a mutant mice and examined the role of Atg9a in the regulation of dsDNA-induced innate immune responses as well as in autophagosome formation (Fig. S7 A and B). All Atg9a-knockout mice died within 1 day of delivery, indicating that Atg9a is essential for survival during neonatal starvation (Fig. S7D). This phenotype is similar to that observed in Atg5-, Atg7-, and Atg16L1-deficient mice (18–23). Although Atg9a is not necessary for Atg12 conjugation to Atg5, it is required for efficient LC3 conjugation to PE, an essential process for the formation of autophagosomes (Fig. S8A) (18–21). Consistently, formation of LC3 dots and autophagosomes was hardly observed in Atg9a-knockout MEFs under nutrient-starved conditions (Fig. S8 B and D). The loss of Atg9a results in a decrease in the bulk degradation of long-lived proteins and the massive accumulation of p62 (Fig. S8 E and F). Although Atg9a knockout MEFs express a deleted form of Atg9a mRNA lacking exon 6 to exon 11 (Fig. S7C), the expression of such a mutant mRNA seems not to affect the induction of autophagy in Atg9a-knockout MEFs because complementation of Atg9a-knockout MEFs with the Atg9a-GFP protein restores the ability to induce autophagy (Fig. S8C). These results indicate that Atg9a is an essential requirement for autophagy.
Atg9a Regulates dsDNA-Induced Innate Immune Response.
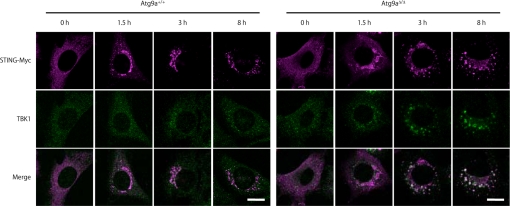
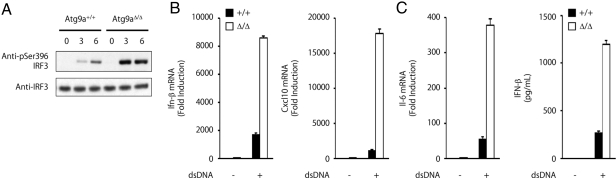
We examined the role of Atg9a in regulating the localization of STING. While dsDNA-induced translocation of STING from the ER to the Golgi apparatus occurs normally in Atg9a-knockout MEFs, translocation of STING from the Golgi apparatus to the punctate structures, and the assembly of STING and TBK1 is greatly enhanced in Atg9a-knockout MEFs (Fig. 4). Consistently, dsDNA-induced phosphorylation of IRF3 is enhanced in Atg9a-knockout MEFs (Fig. 5A). Our data show that both the transcription of Ifn-β, Il-6, and Cxcl10, and the production of IFN-β are enhanced in Atg9a-knockout MEFs stimulated with dsDNA (Fig. 5 B and C). The enhanced production of IFN-β in Atg9a-knockout MEFs is normalized by retroviral transduction of Atg9a-GFP (Fig. S9). These results indicate that Atg9a limits dsDNA-induced innate immune responses by regulating the assembly of STING and TBK1.
Fig. 4.
Atg9a controls the assembly of STING and TBK1 after dsDNA stimulation. Atg9a+/+ and Atg9aΔ/Δ primary MEFs stably expressing STING-Myc were transfected with poly (dA-dT) (1 μg/mL) for the indicated periods. The cells were fixed, immunostained with anti-Myc and anti-TBK1 antibodies and then observed by confocal laser scanning microscopy. (Scale bars, 20 μm.)
Fig. 5.
Loss of Atg9a enhances dsDNA-induced innate immune response. (A) Immunoblots of lysates from Atg9a+/+ and Atg9aΔ/Δ primary MEFs transfected with poly (dA-dT) for the indicated periods. The membranes were probed with the indicated antibodies. (B) Quantitative-RT-PCR of the indicated RNAs from Atg9a+/+ and Atg9aΔ/Δ primary MEFs transfected with poly (dA-dT) for 8 h. The results shown are means ± SD. (n = 3). (C) Production of IFN-β by Atg9a+/+ and Atg9aΔ/Δ primary MEFs transfected with poly (dA-dT) for 24 h. The results shown are means ± SD. (n = 3).
Discussion
Increasing evidence has shown the critical role of autophagy-related proteins in the physiological and pathological conditions (18–21, 23–27). This study highlights a unique role for Atg9a in the regulation of the dsDNA-induced innate immune response. Although all autophagy-related proteins are necessary for the induction of autophagy, each autophagy-related protein acts as a distinct regulator of membrane traffic and is required for the differential steps of autophagosome formation (18–21). Therefore, it is possible that the distinct function of each autophagy-related protein contributes to certain types of cellular response in addition to autophagy. Indeed, the selective involvement of Atg9a in dsDNA-induced innate immune responses discloses that each autophagy-related protein does not always play the same role in immunity, but has distinct functions that regulate the immune system.
Recent studies have shown that the poly (dA-dT), B-form of dsDNA used in this study, induces RNA polymerase III-dependent innate immune responses both in human cells and in immortalized mouse cells (28, 29). However, it also has been reported that in primary mouse cells, B-form dsDNA induces innate immune responses independently of the RNA polymerase III-IFN-β promoter stimulator (IPS)-1 signaling axis (28–30). Enhanced B-form dsDNA-induced innate immune responses in Atg9a-knockout MEFs might be independent of the function of RNA polymerase III because we used primary MEFs in the experiments. We confirmed that the translocation of STING is induced by B-form dsDNA in both primary and immortalized mouse MEFs as well as in human cells (Figs. 1 and 4, and Fig. S3C). These results indicate that both the immortalization and species differences are unrelated to the translocation of STING driven by B-form dsDNA. Further studies are still needed to identify the dsDNA sensor responsible for the translocation of STING, and to understand the precise molecular mechanisms underlying dsDNA-induced signal transduction.
Materials and Methods
Reagents.
Anti-phosphoSer396-IRF3 and anti-Myc antibodies were purchased from Cell Signaling Technology. Anti-IRF3 antibody was purchased from Santa Cruz Biotechnology. Recombinant mouse IFN-β and the ELISA kit for mouse IFN-β were purchased from PBL InterferonSource. ELISA kits for mouse CXCL10 and IL-6 were purchased from R&D Systems. Poly (dA-dT) was purchased from Sigma. Lipofectamine 2000 was purchased from Invitrogen.
Plasmids.
pcDNA3.1 (+) was purchased from Invitrogen. pISRE-luc was purchased from Stratagene. pRL-TK was purchased from Promega. The expression constructs pMRX-ires-puro and pMRX-ires-bsr (kindly donated by Dr. S. Yamaoka) were derivatives of pMX (kindly donated by Dr. T. Kitamura), and have been described previously (31).
Complementary DNA encoding mouse Sting was inserted into pcDNA3, pMRX-ires-bsr and pMRX-GFP-ires-bsr, generating pcDNA3-Sting, pMRX-Sting-ires-bsr, and pMRX-Sting-GFP-ires-bsr, respectively. Complementary DNA encoding the transmembrane domain of Cyb5 was inserted into pMRX-GFP-MCS-ires-bsr, generating pMRX-GFP-ER-ires-bsr. Complementary DNA encoding the GFP and Atg9a were inserted into pMRX-ires-puro, generating pMRX-Atg9a-GFP-ires-puro.
Generation of Atg9a-Deficient Mice.
The fragment of the Atg9a gene was isolated from genomic DNA extracted from wild-type ES cells by PCR. A targeting vector was constructed by replacing exons 6, 7, 8, 9, 10, and 11 with a neomycin-resistance gene cassette (neo), and a herpes simplex virus thymidine kinase driven by a PGK promoter was inserted into the genomic fragment to enable negative selection. After the targeting vector was transfected into ES cells, G418, and gancyclovir doubly resistant colonies were selected and screened by PCR and Southern blotting. Homologous recombinants were microinjected into C57BL/6 female mice, and heterozygous F1 progenies were intercrossed to obtain Atg9a-deficient mice. The Atg9a-deficient mice used were on a 129Sv × C57BL/6 background.
Mice were maintained in our animal facility and treated in accordance with the guidelines of Osaka University.
Mice, Cells, and Viruses.
Mice deficient in Atg16L1 have been previously described15. Atg7-deficient mice were kindly donated by Drs. M. Komatsu and K. Tanaka. The Plat-E cells used for the generation of recombinant retrovirus were kindly donated by Dr. T. Kitamura (University of Tokyo, Tokyo, Japan). Retroviral infection was performed as previously described (31). Cells (293 and HeLa) were purchased from ATCC. Herpes simplex virus 1 was kindly donated by Dr. Kawaguchi (University of Tokyo, Tokyo, Japan).
Preparation of Mouse Embryonic Fibroblasts.
Mouse embryonic fibroblasts were prepared as previously described (23).
RT-PCR.
Total RNA was isolated using RNAeasy Mini kits (Qiagen) according to the manufacturer's instructions. Reverse transcription was performed using ReverTra Ace (TOYOBO) according to the manufacturer's instructions.
For quantitative PCR, cDNA fragments were amplified by RealTime PCR Master Mix (TOYOBO) according to the manufacturer's instructions. Fluorescence from the TaqMan probe for each cytokine was detected by a 7500 Real-Time PCR System (Applied Biosystems). To determine the relative induction of cytokine mRNA in response to dsDNA stimulation, the mRNA expression level of each gene was normalized to the expression level of 18S RNA. The experiments were repeated at least twice. The results were reproducible.
Immunoblotting.
Immunoblotting were performed as previously described (23). The experiments were repeated at least three times. The results were reproducible.
ELISA.
The level of cytokine production was measured by ELISA according to manufacturer's instructions. The experiments were repeated at least three times. The results were reproducible.
Fluorescence Microscopy.
Immunocytochemistry analysis was performed as previously described (23, 32). Samples were examined under a fluorescence laser scanning confocal microscope, FV1000 (Olympus). The experiments were repeated at least twice. The results were reproducible.
Electron Microscopy Analysis.
Electron microscopy analysis was performed as previously described (23). The experiments were repeated at least twice. The results were reproducible.
Materials and methods for SI figures are described in SI Text.
Supplementary Material
Acknowledgments.
We thank Drs. K. Tanaka, M. Komatsu, N. Mizushima, T. Kitamura, and Y. Kawaguchi for providing invaluable materials; the members of the Laboratory of Host Defense for discussion; M. Kumagai, N. Kitagaki, R. Abe, and Y. Fujiwara for technical assistance, and E. Kamada for secretarial assistance. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Ministry of Health, Labor, and Welfare of Japan; and the 21st Century Center of Excellence Program of Japan, and by National Institutes of Health Grant AI070167 (to S.A).
Note.
While our manuscript was in submission, Ishikawa et al. (33) reported the translocation of STING from ER to EXOC2-positive compartments after dsDNA stimulation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911267106/DCSupplemental.
References
- 1.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 5.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 7.Charrel-Dennis M, et al. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe. 2008;4:543–554. doi: 10.1016/j.chom.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder K, Muruve DA, Tschopp J. Innate immunity: Cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol. 2009;19:R262–R265. doi: 10.1016/j.cub.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawane K, et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 11.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin L, et al. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signaling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci USA. 2009;106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komatsu M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Stornaiuolo M, et al. KDEL and KKXX retrieval signals appended to the same reporter protein determine different trafficking between endoplasmic reticulum, intermediate compartment, and Golgi complex. Mol Biol Cell. 2003;14:889–902. doi: 10.1091/mbc.E02-08-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z, Klionsky DJ. Autophagosome formation: Core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 19.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 20.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webber JL, Young AR, Tooze SA. Atg9 trafficking in mammalian cells. Autophagy. 2007;3:54–56. doi: 10.4161/auto.3419. [DOI] [PubMed] [Google Scholar]
- 23.Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 24.Cadwell K, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 26.Jounai N, et al. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci USA. 2007;104:14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tal MC, et al. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci USA. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ablasser A, et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009 doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu YH, Macmillan JB, Chen ZJ. RNA Polymerase III detects cytosolic DNA and induces Type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Q, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Saitoh T, et al. TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem. 2003;278:36005–36012. doi: 10.1074/jbc.M304266200. [DOI] [PubMed] [Google Scholar]
- 32.Matsunaga K, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa H, Ma Z, Barber GN. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.