Abstract
It has long been known that heavy alcohol consumption leads to neuropathology and neuronal death. While the response of neurons to an ethanol insult is strongly influenced by genetic background, the underlying mechanisms are poorly understood. Here, we show that even a single intoxicating exposure to ethanol causes non-cell-autonomous apoptotic death specifically of Drosophila olfactory neurons, which is accompanied by a loss of a behavioral response to the smell of ethanol and a blackening of the third antennal segment. The Drosophila homolog of glycogen synthase kinase-3 (GSK-3)β, Shaggy, is required for ethanol-induced apoptosis. Consistent with this requirement, the GSK-3β inhibitor lithium protects against the neurotoxic effects of ethanol, indicating the possibility for pharmacological intervention in cases of alcohol-induced neurodegeneration. Ethanol-induced death of olfactory neurons requires both their neural activity and functional NMDA receptors. This system will allow the investigation of the genetic and molecular basis of ethanol-induced apoptosis in general and provide an understanding of the molecular role of GSK-3β in programmed cell death.
Keywords: ethanol-induced apoptosis, lithium, olfactory system, NMDA receptors
Heavy alcohol consumption leads to neuropathological changes and neuronal cell death (1–4). Brains of alcoholics are reduced in weight and volume, and ≈10% of alcoholics develop a severe cognitive disorder, such as alcoholic dementia or Wernicke-Korsakoff Syndrome (1, 2, 4). Chronic ethanol consumption in humans and rats leads to cholinergic neuron loss in the basal forebrain, which causes impairment of memory (4). In addition, alcoholics display diminished olfactory sensitivity, with one study finding that more than half of alcohol-dependent patients are hyposmic (5). This result is mirrored in rodent models, where 2 days of acute ethanol exposure causes the death of olfactory neurons, followed by retrograde degradation in the temporal dentate gyrus and regions of the hippocampus known to be involved in olfaction and memory (6, 7).
Several mechanisms have been proposed to explain ethanol-induced brain damage (4). One of these mechanisms involves thiamine deficiency (4, 8), another involves the induction of reactive oxygen species and increased production of polyamines (4, 9). Many of ethanol's neurotoxic effects are mediated through cellular excitability and interactions of ethanol with NMDA receptors. Ethanol binds to and inhibits the function of NMDA receptors in many types of neurons (10, 11), although chronic ethanol exposure results in a compensatory increase in glutamatergic neurotransmission (4). Upon ethanol withdrawal, neurons are hyper-excitable, and the resultant excess of intracellular Ca2+ can lead to mitochondrial damage and activation of apoptotic pathways (4). Finally, ethanol can induce acute excitotoxic cell death, as shown by enhancement of ethanol cytotoxicity in cortical neurons treated with the NMDA receptor agonist MK-801 (12) and enhancement of NMDA excitotoxicity in aminergic neurons treated with ethanol (13).
Glycogen synthase kinase 3β (GSK-3β) is a multifunctional protein that can both inhibit and activate apoptosis (14). This protein has been implicated as a mediator of cell death in a variety of systems, including tau-mediated neurodegeneration (15), β-amyloid-associated neurotoxicity (16), excitotoxic cell death (17, 18), and, most intriguingly for the present work, ethanol-induced apoptosis of cultured neurons (17).
Here we describe a model for ethanol-induced neuronal apoptosis in Drosophila melanogaster. We show that a single sedating dose of ethanol causes widespread apoptosis in the antennae. This apoptosis is dependent on shaggy (sgg), the Drosophila homolog of GSK-3. Ethanol-induced neuronal death requires electrical activity and is mediated by NMDA receptors in olfactory receptor neurons (ORNs). Our system will allow the use of powerful genetic tools available in Drosophila to begin identifying the genes and mechanisms involved in predisposition to ethanol-induced neuronal death.
Results and Discussion
Ethanol Vapor Causes Death of Olfactory Receptor Neurons.
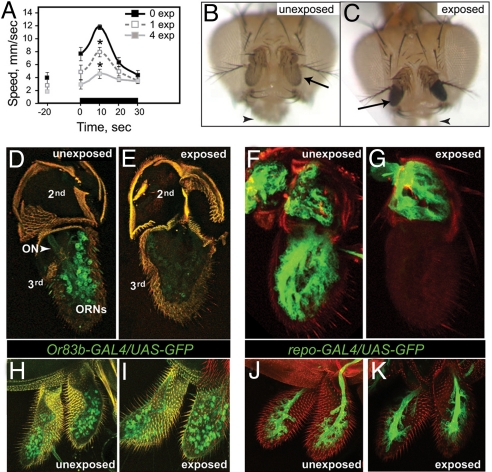
When Drosophila are exposed to ethanol vapor, they display an immediate and transient increase in locomotor activity (19). We found that preexposure to a sedating dose of ethanol vapor diminished this startle response (Fig. 1A) and was accompanied by an obvious and specific phenotype: blackening of the third antennal segments, the primary olfactory organs of the fly (Fig. 1 B and C). Visible damage was restricted to third antennal segments. The maxillary palps, secondary olfactory organs that do not respond to the odor of ethanol (19), were unaffected (see Fig. 1 B and C).
Fig. 1.
Ethanol causes death of olfactory cells. (A) Locomotor activity profile showing olfactory startle of unexposed and ethanol preexposed flies. Ethanol exposure started at time 0. Male flies were preexposed to a 100:50 ratio of ethanol vapor to air flow rate (E/A) for 25 min once or four times (once per day). Olfactory behavior was measured 2 days after ethanol preexposure. Preexposed flies showed a significantly reduced startle (n = 6, *P < 0.01 at 10 s). (B and C) Heads from unexposed (B) and ethanol-exposed (C, single exposure) flies. The third antennal segments (arrows) are blackened in the ethanol-exposed fly, while the maxillary palps (arrowheads) are unaffected. (D and E) Confocal reconstructions of antennae from flies expressing GFP (green) in ORNs under the control of Or83b-GAL4. In unexposed flies (D), ORN nuclei and the olfactory nerve (ON) are clearly visible. Antennae from ethanol-exposed flies (E) show a strong reduction in ORN GFP expression, and the ON is no longer detectable. (F and G) Confocal reconstructions of antennae from flies expressing GFP in glia under the control of repo-GAL4. Glial expression of GFP is seen in both the second and third antennal segments in unexposed flies (F), while expression in the third antennal segment is specifically lost in ethanol-exposed flies (G). (H–K) Confocal reconstructions of maxillary palps from flies expressing GFP (green) in ORNs (H, I) or glia (J, K). The maxillary palps of ethanol-exposed flies (I, K) are unaffected by ethanol exposure, as compared with unexposed maxillary palps (H, I).
To investigate this ethanol-induced olfactory damage, we visualized ORNs and glia by expression of GFP using the GAL4-UAS system (20). Analysis of flies that express GFP under the control of Or83b-GAL4, which is expressed in ≈80% of ORNs (21), revealed that in antennae from unexposed flies, the nuclei of the ORNs were clearly visible, as was the olfactory nerve (ON) (Fig. 1D). In contrast, antennae from flies preexposed to ethanol displayed strongly reduced or undetectable GFP expression and the ON was not visible (Fig. 1E). Analysis of flies that express GFP in glia under the control of the repo-GAL4 driver (22) revealed similar cellular loss upon ethanol exposure (Fig. 1 F and G). By contrast, maxillary palp ORNs and glia were unaffected (Fig. 1 H–K).
The Drosophila olfactory system includes three morphologically distinct types of sensory hairs, basiconic, coeloconic, and trichoid sensilla (23), which are distinguished by the expression of subsets of odorant receptor genes (24). The antenna contains all three types, whereas the maxillary palp contains only basiconic sensilla. To ask if the sensitivity of antennae, compared to maxillary palps, can be explained by the differential sensitivity of different hair types to ethanol, we generated flies expressing GFP in basiconic or trichoid sensilla (using Or22a-GAL4 or Or67d-GAL4, respectively (Fig. S1) (25). Analysis of GFP expression after ethanol preexposure revealed that basiconic and trichoid sensilla survived in 53 and 56% of exposed antennae, respectively. For comparison, the ORN population at large, as defined by Or83b-GAL4-driven expression, survived in 55% of exposed antennae. Because the sensory hair subtypes are equally sensitive to ethanol, the differential response of antennae and maxillary palps cannot be attributed to the presence of different sensillar subtypes.
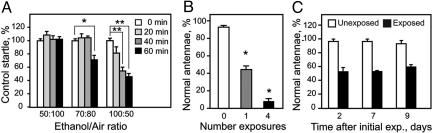
The extent of the behavioral and anatomical damage was dependent on the dose of ethanol, as well as the number of exposures (Fig. 2). Neither a moderate concentration of ethanol (70:80 ethanol:air ratio, E/A), nor a relatively low concentration (50:100 E/A) led to changes in the olfactory startle response after a 20-min preexposure, while a high ethanol concentration (100:50 E/A) caused a consistent but not statistically significant decrease in startle (see Fig. 2A). Longer exposure times led to a stronger phenotype: at the high concentration, 40- and 60-min exposures reduced startle magnitude to 54 and 46% of control levels, respectively (see Fig. 2A). Furthermore, with longer exposure times, the moderate concentration of ethanol caused olfactory damage.
Fig. 2.
Parametric analyses. (A) Ethanol preexposure-induced loss of olfactory startle increases with both ethanol concentration and length of a single exposure. Flies were exposed to increasing E/A of 50:100, 70:80, or 100:50 for 0, 20, 40, or 60 min, respectively, and the degree of startle in response to ethanol was measured 2 days later. (*, P < 0.01, **, P < 0.001). (B) The degree of morphological damage of antennae also increased with exposure number. Flies were given a single 100:50 E/A exposure once, or once per day, for 4 consecutive days and analyzed 2 days later (*, P < 0.01 for all comparisons). (C) Olfactory damage is permanent. Flies were assayed 2, 7, and 9 days after a single exposure to ethanol. Recovery was never observed.
The cumulative effect of multiple ethanol exposures was greater than a single dose (see Fig. 1A and Fig. 2B). On average, 45% of flies subjected to a single high-concentration preexposure showed blackening of at least one-third antennal segment (see Fig. 2B), whereas 92% of flies that received four doses over the course of 2 days showed blackened antennae (see Fig. 2B). Similarly, a single ethanol exposure reduced startle by 30%, while four exposures reduced it by 53% (see Fig. 1A). Finally, the damage caused by high-concentration ethanol exposure was irreversible (see Fig. 2C). The proportion of flies with black antennae did not change in up to 9 days of recovery time, and flies did not recover their olfactory startle within this time period (Fig. S2).
Ethanol-Induced Death of ORNs Occurs by Apoptosis.
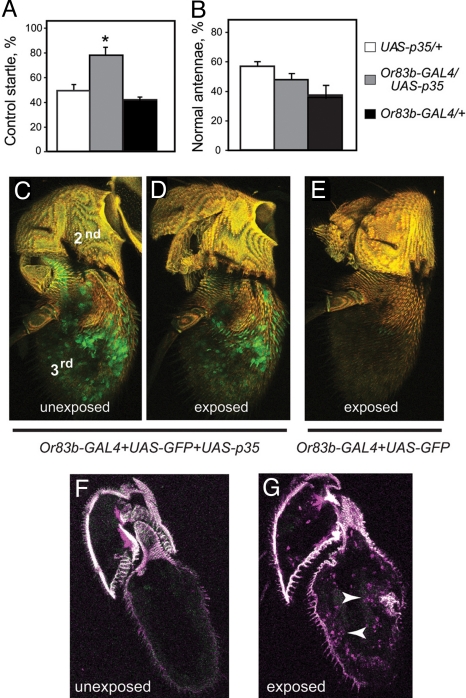
Acute ethanol exposure can cause neuronal death by either apoptotic or necrotic mechanisms, depending on the experimental conditions (3, 7, 26). To determine if ethanol-induced ORN death was caused by apoptosis, we asked if protection could be achieved by expressing baculovirus p35, a caspase inhibitor (27). We used Or83b-GAL4 to drive expression of UAS-p35 in ORNs, exposed the flies to ethanol, and examined them after 2 days. Flies expressing p35 in ORNs (Or83b-GAL4/UAS-p35) retained 81% of their unexposed startle response, whereas genetic controls retained only 48 to 54% (Fig. 3A). Analysis of GFP expression confirmed that the ORNs survive and appear normal in 80% of ethanol-exposed antennae expressing p35, while surviving ORNs were seen in only 40% of control antennae (Fig. 3 C–E). To confirm that cell death occurs by apoptosis, we undertook TUNEL, which labels apoptotic cells. Control antennae showed no TUNEL staining, whereas antennae dissected from flies 0 to 3 h after ethanol exposure exhibited TUNEL-positive nuclei (Fig. 3 F and G). Together, these results indicate that ethanol-induced ORN death occurs by apoptosis.
Fig. 3.
Ethanol-induced olfactory damage occurs by apoptosis. (A) Expression of p35 in ≈80% of ORNs using the Or83b-GAL4 driver protects flies from loss of olfactory startle caused by a single ethanol preexposure delivered 2 days before testing (n = 8, *, P < 0.01). Data are presented as the % of maximum startle response in preexposed flies relative to that of unexposed controls of the same genotype. (B) Expression of p35 in ORNs does not rescue antennal morphology in flies exposed to ethanol as in (A) (n = 8). (C and D) Confocal reconstruction of antennae from flies carrying Or83b-GAL4, UAS-GFP, and UAS-p35. Ethanol exposure did not cause loss of ORNs (D) in the third antennal segment as is seen in control flies not expressing p35 (E). Green, GFP; yellow, autofluorescence of the cuticle. (F and G) Sections of antennae from a fly preexposed to ethanol shows an increase in TUNEL-positive nuclei (arrowheads in G) when compared to the unexposed control (F).
Curiously, the flies that were protected by expression of p35 in ORNs still displayed ethanol-induced antennal blackening (Fig. 3B), indicating that olfaction can be rescued independently of antennal morphology. It may be that antennal blackening in flies that were protected by expression of p35 in ORNs is a result of the death of nonneuronal cells, such as glia.
Ethanol-Induced ORN Death Requires the GSK3β Homolog Shaggy.
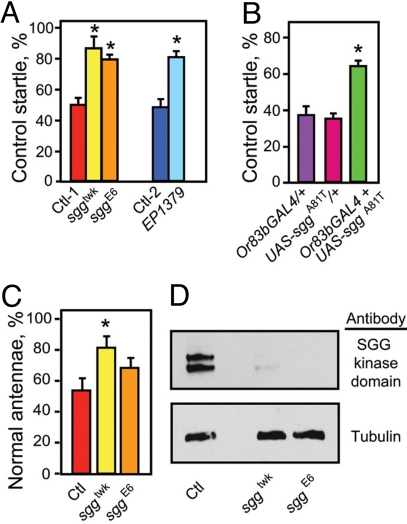
To investigate the mechanism by which ethanol kills olfactory cells, we carried out a genetic screen for mutations that cause flies to be resistant or hypersensitive to the toxic effects of a single ethanol exposure. We identified shaggy (sgg), encoding the Drosophila homolog of GSK-3β. The allele of sgg isolated in our screen, sggtwk (28), was resistant to the toxic effects of ethanol on both olfactory startle and antennal morphology (Fig. 4 A and C). Two additional loss-of-function alleles of sgg showed similar effects on the startle response, and one of these, sggE6, was modestly protective against antennal blacking (see Fig. 4 A and C). Immunoblotting revealed that both sggtwk and sggE6 reduced antennal expression of adult-specific Sgg proteins to <10% of control values (Fig. 4D). Expression of a dominant-negative form of Sgg, SggA81T (29), in ORNs produced resistance to the effects of ethanol (Fig. 4B). Together, these data suggest that Sgg functions in ORNs to promote ethanol-induced damage.
Fig. 4.
Ethanol-induced ORN death requires Sgg/GSK3β. (A) Reduction of sgg function results in protection of the olfactory startle. Three loss-of-function alleles of sgg (twk, E6, and EP1379), all of which have a ≈90% reduction in the adult-specific forms of SGG (28), are resistant to ethanol-induced startle loss when compared with controls (Ctl-1 is a precise excision of the twk transposon and Ctl-2 is EP1576) (n = 7, *, P < 0.01 for twk and E6; *, P = 0.0013 for EP1379, Student's t test). (B) Expression of a dominant-negative allele of sgg (UAS-sggA81T) in the ORNs (using the Or83b-GAL4 driver) results in significant protection against ethanol-induced loss of olfactory startle (n = 4, *, P < 0.05). (C) sggtwk also demonstrates resistance to the ethanol-induced antennal-blackening phenotype (n = 7, *, P < 0.01), while sggE6 shows a trend toward resistance, although the data did not achieve statistical significance. (D) Western analysis demonstrates that adult-specific Sgg proteins are expressed in antennae, and that both sggtwk and sggE6 result in a strong reduction of these proteins. Each lane was loaded with protein from 30 dissected antennae.
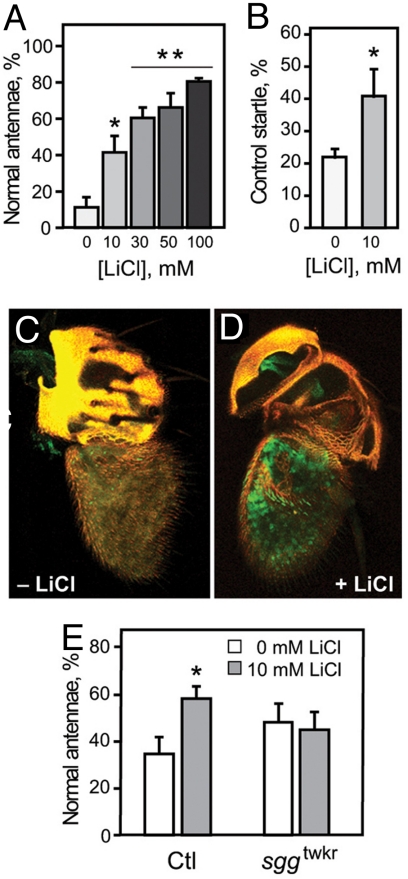
To test whether the effects of sgg were caused by developmental requirements or, alternatively, because of a change in acute response to ethanol, we tested if adult flies could be protected from the damaging effects of ethanol with the GSK-3β inhibitor lithium. We fed adult wild-type flies food containing a range of lithium chloride (LiCl) concentrations for 7 days, exposed them to ethanol on day 5, and examined them for olfactory startle and antennal morphology on day 7. LiCl had a dose-dependent protective effect on ethanol-induced antennal blackening (Fig. 5A). Ten millimolar LiCl also had a protective effect on the olfactory startle (Fig. 5B), although this effect was not increased further at higher LiCl concentrations, perhaps because of pleiotropic effects on behavior. Feeding flies equivalent concentrations of other salts, such as KCl or NaCl, had no effect, indicating that protection is specific to LiCl (Fig. S3). Consistent with the behavioral data, there were surviving ORNs in 62% of antennae from ethanol-exposed flies fed 10 mM LiCl, compared to only 22% in control antennae (Fig. 5 C and D). Thus, both the behavioral and anatomical effects of ethanol exposure can be ameliorated by acute pharmacological intervention with a GSK-3β inhibitor.
Fig. 5.
Lithium prevents ethanol-induced programmed cell death in adult flies. (A) Flies fed LiCl-containing food for 5 days before ethanol exposure show a dose-dependent resistance to the damaging effects of ethanol on antennal morphology (n = 6, *, P < 0.05 for 0 vs. 10 mM; **, P < 0.01 for 0 vs. 30, 50, or 100 mM). (B) LiCl feeding also protects against startle loss caused by a single ethanol preexposure (n = 6, *, P = 0.038, Student's t test). (C and D) Confocal reconstructions of antennae from flies expressing GFP in ORNs under the control of the Or83b-GAL4 driver. Ethanol exposure did not cause loss of ORNs in the third antennal segment in flies that were fed 10-mM LiCl for 5 days before exposure (D), as is seen in control flies that were not fed LiCl (C). GFP, green; yellow, autofluorescence of the cuticle. (E) The protective effect of LiCl requires sgg. sggtwk and control (Ctl) flies were fed food containing 10-mM LiCl for 5 days and exposed to ethanol vapor for 45 min (all other experiments involved 25-min exposures). The low dose of LiCl and the longer exposure to ethanol were chosen to minimize a potential “ceiling effect” that might be expected in the already-resistant sggtwk allele. Controls showed the expected protective effect of LiCl (n = 7, *, P = 0.005, Student's t test), while sggtwk was not protected by LiCl (, P = 0.39, Student's t test).
To confirm that the protective effect of LiCl was a result of inhibition of Sgg/GSK-3β, rather than another target, we tested the effect of LiCl on sggtwk mutant flies. While sggtwk was itself resistant to the damaging effects of ethanol, significant antennal blackening was produced in the mutant by increasing the dose of ethanol, thus allowing us to assay protection by LiCl. LiCl had no effect on ethanol-induced antennal damage in sggtwk mutant flies, while control flies were significantly protected (Fig. 5E). These data show that at least some of the protective effects of LiCl are mediated by inhibition of Sgg.
Electrical Silencing Protects the ORNs From Ethanol-Induced Apoptosis.
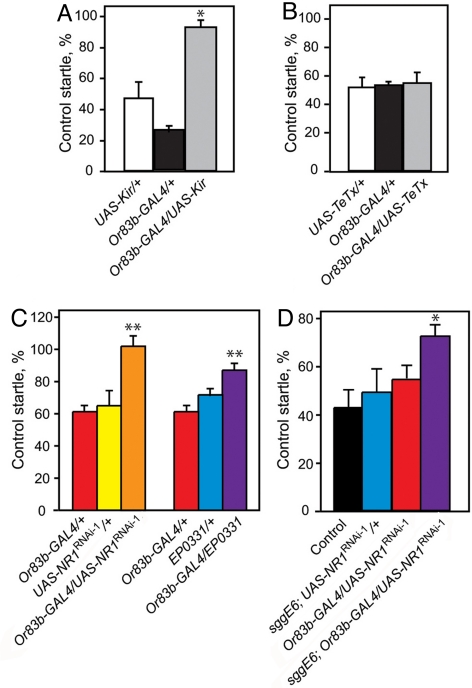
GSK3β has been implicated in cell death in response to a variety of insults, including excitotoxicity (18, 30). To test if excessive neural activity mediates ethanol-induced apoptosis of ORNs, we silenced the majority of ORNs by expression of the mammalian inward rectifying K+ channel Kir2.1 (31) using Or83b-GAL4. This manipulation fails to silence all neurons required for ethanol startle (see below), most likely because of the fact that there are two families of ORNs that do not express Or83b. Nevertheless, it almost completely abolished the loss of olfactory startle induced by ethanol preexposure. Flies expressing Kir2.1 in Or83b-positive ORNs retained 96% of their startle after exposure to ethanol, compared with 41 to 61% in control flies (Fig. 6A), an effect that was not observed upon synaptic silencing of ORNs by expression of tetanus-toxin light chain (32) (Fig. 6B). Thus, ethanol-induced apoptosis of ORNs requires their activity.
Fig. 6.
Electrical silencing protects ORNs from ethanol-induced damage. (A) Expression of Kir2.1 (UAS-Kir) in ORNs using the Or83b-GAL4 driver protects flies from ethanol-induced loss of olfactory startle (n = 6, *, P < 0.01). Data are presented as % startle retained by preexposed flies compared to unexposed flies of the same genotype. (B) Synaptic silencing by expression of tetanus-toxin light chain (TeTx) had no protective effect (n = 4). (C) Double-stranded RNA interference (UAS-NR1RNAi) and antisense dNR1 (EP0331) expression protects flies against ethanol-induced startle loss (n = 5, **, P < 0.01, *, P < 0.05). (D) Combining mutation of sgg with UAS-NR1RNAi driven by Or83b-GAL4 results in a supra-additive protective effect. While sggE6 and Or83b-GAL4/UAS-NR1RNAi flies both demonstrate weak resistance, the combination results in a synergistic effect (n = 3, *, P < 0.05).
Ethanol-Induced Neuronal Death is Not Cell-Autonomous.
To ask if ethanol-induced apoptosis is cell-autonomous, we used Or67d-GAL4 and Or22a-GAL4 to drive expression of UAS-p35 in trichoid or basiconic sensilla, respectively. The flies also carried UAS-GFP to mark p35-expressing cells. We exposed the flies to ethanol and examined the survival of GFP-labeled ORNs. Expression of p35 under the control Or67d-GAL4 was not protective (47% antennal survival, comparable to controls). Expression under the control of Or22a-GAL4 was modestly protective, although lower than when p35 was expressed under the control of Or83b-GAL4 (Table S1). Thus, expression of p35 in Or67d-GAL4-expressing cells is not sufficient to protect those cells, strongly suggesting that ethanol-induced cell death is not cell-autonomous. The greater protective effect seen with Or22a-GAL4, compared to Or67d-GAL4, is interesting, given that the former is expressed in a far greater number of ORNs (see Fig. S1). Thus, the protective effect is proportional to the fraction of cells expressing p35, which strengthens the hypothesis that ethanol-induced ORN death is nonautonomous.
Curiously, flies are still able to startle in response to ethanol even when ≈80% of their ORNs are inactivated by Kir2.1 or tetanus toxin expression. Similarly, Or83b null mutants, in which dendritic localization of OR proteins is disrupted, leading to severe defects in odor-responsive behaviors (21), display a normal startle in response to the smell of ethanol (Fig. S4). These results indicate that the Or83b-expressing ORNs are not responsible for the olfactory response to ethanol. The fact that we are nevertheless able to protect the startle behavior through expression of p35 or Kir2.1 in the Or83b-expressing ORNs further implies that the protective effect (and, by extension, the widespread ORN death caused by ethanol) is not cell-autonomous. Finally, we never observe ethanol-exposed antennae in which only a subset of the ORNs have died; thus, ethanol-induced ORN death is an all-or-none phenomenon.
Excitotoxic Death of ORNs Is Mediated By NMDA Receptors.
Because many of ethanol's toxic effects on neurons are mediated by interactions with NMDA receptors, and because acute ethanol exposure can lead to NMDA receptor-mediated excitotoxic cell death in cortical (12) and aminergic (13) neurons, we hypothesized that the acute neurotoxic effects of ethanol on Drosophila ORNs might be caused by over-stimulation via NMDA receptors. To test this hypothesis, we examined the effects of genetic manipulation of the NR1 subunit of the NMDA receptor in ORNs.
We down-regulated dNR1 expression with two, independently generated double-stranded RNA interference constructs, UAS-NR1RNAi-1 and UAS-NR1RNAi-2 (33). These constructs reduce dNR1 transcript (by 47% when driven in neurons) and protein levels (33), respectively. Flies expressing either UAS-NR1RNAi transgene under the control of the Or83b-GAL4 driver showed significant protection of olfactory startle after ethanol exposure (Fig. 6C and Fig. S5). We confirmed this result by driving expression from EP0331, a UAS-containing P element inserted in the 3′ end of dNR1. EP0331 is inserted in an orientation to drive antisense expression (34), and it has been shown that driving EP0331 with hsp70-GAL4 results in a significant reduction in dNR1 (35). Or83b-GAL4/+; EP (3)0331/+ flies, like those expressing UAS-NR1RNAi, were resistant to startle loss caused by ethanol exposure (see Fig. 6C).
To establish that the protective effects on olfactory startle were caused by neuroprotective effects of inhibiting dNR1 expression, we examined the ORNs of flies coexpressing UAS-NR1RNAi (or antisense) and GFP in ORNs. Down-regulation of dNR1 in ORNs led to enhanced survival after ethanol exposure: on average, 56% of antennae from flies with reduced dNR1 retained GFP expression, compared with only 35% of antennae from control flies.
Finally, it is known that lithium can protect against NMDA-receptor-mediated excitotoxic neuronal death in both cell culture and rodent models (36, 37). Thus, mutation of sgg and down-regulation of dNR1 may exert their protective effects by impairing the same cellular pathway. If this were the case, the combination of sgg mutation and dNR1 down-regulation should result in a synergistic effect. To test this hypothesis, we subjected flies of genotype sggE6; Or83b-GAL4/UAS-NR1RNAi-1, as well as control flies bearing only one or neither of the two genetic manipulations, to a high dose of ethanol (to overcome the baseline resistance phenotypes). The experimental flies retained 74% of control startle, compared to the 65% predicted if the effects of the two manipulations were independent. This greater-than-additive resistance suggests that Sgg and dNR1 are conveying resistance by way of a common pathway.
Summary.
We show that exposure of adult flies to a single sedating dose of ethanol vapor results in widespread death of cells in the third antennal segment and a consequent loss of olfaction. The effect is dose-dependent, requires Sgg/GSK-3β, and can be prevented by treatment with the GSK-3β inhibitor LiCl. Ethanol-induced death of the antennal ORNs is non- cell-autonomous, apoptotic, and dependent on electrical activity and function of the NMDA receptor. This system will allow the study of ethanol-induced neuronal apoptosis in an organism that is amenable to rapid and complex genetic manipulations, likely leading to insights into the genes involved in sensitivity to ethanol neurotoxicity and a greater understanding of the molecular processes of neuronal death in alcoholic dementia. The system will also allow screening for drugs that can prevent ethanol-induced neuronal apoptosis. Finally, neurons were protected from ethanol-induced apoptosis by inhibiting Sgg/GSK3β with LiCl, indicating the possible utility of GSK3β as a target for preventative therapy in alcoholic neurodegeneration.
Experimental Procedures
Drosophila Strains and Culture.
Flies were raised at 25 °C and 70% humidity on standard cornmeal/molasses medium. All experiments were carried out in a white1118 Berlin genetic background. Behavioral assays used 20 to 25 male flies aged 2 to 4 days after eclosion at the start of the experiment. Flies analyzed for behavior were subjected to brief (<5 min) CO2 anesthesia no <24 h before behavioral assays. For source of fly strains, see the SI Text.
Olfactory Startle.
To assay olfactory startle, we used the locomotor tracking system (19) (see the SI Text for details).
LiCl Feeding.
Standard cornmeal molasses medium was supplemented with LiCl to the desired final concentration (0 to 100 mM). Twenty to twenty-five 2-day-old male flies were placed on the medium and allowed to feed for 5 days. In initial experiments, 0.5% FD&C blue #1 was added to the food to verify consumption. After 5 days, flies were exposed for 30 min to 100:50 E/A, then placed back on the lithium-containing food for 2 more days. Flies were then subjected to behavioral and visual assays, as described above.
Western Blots and Immunohistochemistry.
Western blots and immunohistochemistry were carried out using standard procedures (see SI Text for details).
Statistical Analyses.
All analyses are one-way ANOVA with Tukey HSD posthoc analysis, unless otherwise indicated.
Supplementary Material
Acknowledgments.
We thank L. Vosshall for technical advice and fly stocks, G. Ophir-Shohat for technical advice, Fred Wolf for sharing unpublished information about sgg alleles, and A. Rothenfluh, I. King, and D. Tran for helpful discussions. This work was supported by National Institutes of Health/National Institute of Alcohol Abuse and Alcoholism Grants F32 AA015000 (to R.L.F.), and AA10035 and AA13105 (to U.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910813106/DCSupplemental.
References
- 1.Martin PR, Adinoff B, Weingartner H, Mukherjee AB, Eckardt MJ. Alcoholic organic brain disease: Nosology and pathophysiologic mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10(2):147–164. doi: 10.1016/0278-5846(86)90069-2. [DOI] [PubMed] [Google Scholar]
- 2.Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56(4):356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- 3.Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26(4):547–557. [PubMed] [Google Scholar]
- 4.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 5.Rupp CI, et al. Reduced olfactory sensitivity, discrimination, and identification in patients with alcohol dependence. Alcohol Clin Exp Res. 2003;27(3):432–439. doi: 10.1097/01.ALC.0000057945.57330.2C. [DOI] [PubMed] [Google Scholar]
- 6.Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: Possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20(2):284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 7.Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav. 2002;72(3):521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- 8.Gibson GE, Zhang H. Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem Int. 2002;40(6):493–504. doi: 10.1016/s0197-0186(01)00120-6. [DOI] [PubMed] [Google Scholar]
- 9.Sun AY, et al. Ethanol and oxidative stress. Alcohol Clin Exp Res. 2001;25(5) Suppl ISBRA:237S–243S. doi: 10.1097/00000374-200105051-00038. [DOI] [PubMed] [Google Scholar]
- 10.Faingold CL, N′Gouemo P, Riaz A. Ethanol and neurotransmitter interactions–from molecular to integrative effects. Prog Neurobiol. 1998;55(5):509–535. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 11.Wirkner K, et al. Ethanol-induced inhibition of NMDA receptor channels. Neurochem Int. 1999;35(2):153–162. doi: 10.1016/s0197-0186(99)00057-1. [DOI] [PubMed] [Google Scholar]
- 12.Corso TD, Mostafa HM, Collins MA, Neafsey EJ. Brain neuronal degeneration caused by episodic alcohol intoxication in rats: Effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801. Alcohol Clin Exp Res. 1998;22(1):217–224. [PubMed] [Google Scholar]
- 13.Crews FT, Waage HG, Wilkie MB, Lauder JM. Ethanol pretreatment enhances NMDA excitotoxicity in biogenic amine neurons: protection by brain derived neurotrophic factor. Alcohol Clin Exp Res. 1999;23(11):1834–1842. [PubMed] [Google Scholar]
- 14.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79(4):173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas JJ, et al. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20(1–2):27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takashima A, Noguchi K, Sato K, Hoshino T, Imahori K. Tau protein kinase I is essential for amyloid beta-protein-induced neurotoxicity. Proc Natl Acad Sci USA. 1993;90(16):7789–7793. doi: 10.1073/pnas.90.16.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takadera T, Ohyashiki T. Glycogen synthase kinase-3 inhibitors prevent caspase-dependent apoptosis induced by ethanol in cultured rat cortical neurons. Eur J Pharmacol. 2004;499(3):239–245. doi: 10.1016/j.ejphar.2004.07.115. [DOI] [PubMed] [Google Scholar]
- 18.Facci L, Stevens DA, Skaper SD. Glycogen synthase kinase-3 inhibitors protect central neurons against excitotoxicity. Neuroreport. 2003;14(11):1467–1470. doi: 10.1097/00001756-200308060-00012. [DOI] [PubMed] [Google Scholar]
- 19.Wolf FW, Rodan AR, Tsai LT, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22(24):11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brand AH, Manoukian AS, Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- 21.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Xiong WC, Okano H, Patel NH, Blendy JA, Montell C. Repo encodes a glial-specific homeo domain protein required in the Drosophila nervous system. Genes Dev. 1994;8(8):981–994. doi: 10.1101/gad.8.8.981. [DOI] [PubMed] [Google Scholar]
- 23.Shanbhag SR, Muller B, Steinbrecht RA. Atlas of olfactory organs of Drosophila melanogaster 2. Internal organization and cellular architecture of olfactory sensilla. Arthropod Struct Dev. 2000;29(3):211–229. doi: 10.1016/s1467-8039(00)00028-1. [DOI] [PubMed] [Google Scholar]
- 24.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15(17):1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37(5):827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 26.Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5(6):515–521. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- 27.Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 28.Wolf FW, Eddison M, Lee S, Cho W, Heberlein U. GSK-3/Shaggy regulates olfactory habituation in Drosophila. Proc Natl Acad Sci USA. 2007;104(11):4653–4657. doi: 10.1073/pnas.0700493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourouis M. Targeted increase in shaggy activity levels blocks wingless signaling. Genesis. 2002;34(1–2):99–102. doi: 10.1002/gene.10114. [DOI] [PubMed] [Google Scholar]
- 30.Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-d-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci USA. 1998;95(5):2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johns DC, Marx R, Mains RE, O'Rourke B, Marban E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19(5):1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 33.Wu CL, et al. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci. 2007;10(12):1578–1586. doi: 10.1038/nn2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rørth P, et al. Systematic gain-of-function genetics in Drosophila. Development. 1998;125:1049–1057. doi: 10.1242/dev.125.6.1049. [DOI] [PubMed] [Google Scholar]
- 35.Xia S, et al. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol. 2005;15(7):603–615. doi: 10.1016/j.cub.2005.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: Involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem. 2002;80(4):589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- 37.Cappuccio I, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci. 2005;25(10):2647–2657. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.