Abstract
The hippocampal formation is a brain region noted for its plasticity in response to stressful events and adrenal steroid hormones. Recent work has shown that chromatin remodeling in various brain regions, including the hippocampus, is associated with the effects of stress in a variety of models. We chose to examine the effects of stress, stress duration, corticosterone administration, and fluoxetine treatment on the levels of hippocampal histone H3 methylation at lysines 4, 9, and 27, marks associated, respectively, with active transcription, heterochromatin formation, and transcriptional repression. We found that acute stress increased the levels of H3K9 tri-methylation (H3K9me3) in the dentate gyrus (DG) and CA1, while it reduced levels of H3K9 mono-methylation (H3K9me1) and H3K27 tri-methylation (H3K27me3) in the same regions, and had no effect on levels of H3K4 tri-methylation (H3K4me3). Seven days of restraint stress reduced levels of H3K4me3 in the CA1 and H3K27me3 in the DG and CA1, while increasing basal levels of H3K9me3. Chronic restraint stress (CRS) for 21 days mildly increased levels of H3K4me3 and reduced H3K9me3 levels in the DG. Treatment with fluoxetine during CRS reversed the decrease in DG H3K9me3, but had no effect on the other marks. These results show a complex, surprisingly rapid, and regionally specific pattern of chromatin remodeling within hippocampus produced by stress and anti-depressant treatment that may open an avenue of understanding the interplay of stress and hippocampal gene expression, and reveal the outlines of a potential chromatin stress response that may be diminished or degraded by chronic stress.
Keywords: brain, chromatin, corticosteroids, fluoxetine
The hippocampus proper and the dentate gyrus comprise a brain region that is necessary not only for the formation of new declarative memories, but for emotional regulation, spatial processing, and neuroendocrine control (1). The hippocampal formation is a brain region that is particularly subject to structural, functional, and neurogenic change in response to stress and stress-related diseases, such as depression and post-traumatic stress disorder (PTSD) (1–3). Many of these alterations are mediated by changes in gene transcription, some of which may be quite persistent. At present, the genetic and epigenetic mechanisms by which an acute stressor induces PTSD or chronic stress leads to depression are incompletely understood. Further, an understanding of the temporal checkpoints which mark the transition from a stress to which an organism can adapt homeostatically, to one which it must adapt allostatically (i.e., adaptation through change) (4), is important in comprehending how stress produces brain plasticity and pathology.
Epigenetic information, defined as inherited states of gene activity not based on changes to the underlying DNA sequence, is established at least in part by alterations of the protein/DNA complex termed chromatin. The fundamental unit of chromatin is the nucleosome, which consists of DNA tightly associated with histone proteins. Recent work in the field of chromatin biology has established that covalent modifications of these histone proteins represent an intricate “histone code” that can influence chromatin structure and gene transcription (5). It has recently been suggested that neuronal plasticity may be controlled in part by such chromatin-remodeling events (6), and a number of studies have implicated chromatin modification in models of stress, memory, drug abuse, and depression (7–10).
The core histones of the nucleosome, H2A, H2B, H3, and H4 are subject to a variety of modifications to their tail regions, including acetylation, phosphorylation, ubiquitinylation, and methylation (5). In the hippocampus, evidence that histone modifications might play a significant role in the function of the structure came from the work of Weaver (11), who showed that differences in maternal behavior produced differences in the behavior of offspring, and that this difference was associated with changes in histone acetylation, and could be blocked by a histone deacetylase inhibitor. Subsequent work has connected acetylation of histone H4 (12) and acetylation, phosphorylation and methylation of H3 in the hippocampus with stress (13–15). Histone lysine methylation, unlike acetylation, for example, is remarkable for the site specificity of its associated machinery and its ability to recruit specific effector protein complexes (16–18).
Stress, particularly chronic stress, has a number of effects on the structure and function of the hippocampal formation. In humans, single severe acute stressors can result in posttraumatic stress disorder, while chronic stress can contribute to depression and a number of other chronic disease states; these disorders are associated with smaller hippocampal volumes (1, 2, 19). In animal models, chronic stress reduces hippocampal neurogenesis (20) and the complexity of dendritic arbors (21–23) as well as impairing function in assays of hippocampal dependent spatial memory and LTP (19, 24). Many of these changes can be blocked pharmacologically with anti-depressant drugs such as fluoxetine (25–28).
The hippocampus' susceptibility to stress is in part mediated by the fact it expresses high levels of receptors for corticosteroids (29). The hippocampus also appears to express a larger number of the enzymes responsible for chromatin remodeling than other brain regions (Allen Brain Atlas: http://www.brain-map.org/) and corticosteroid receptors have been shown to associate with a number of them (30, 31). It is, therefore, likely that the hippocampus will prove to be a region which is as plastic in terms of chromatin remodeling as it is in other respects. We chose to examine the methylation of histone H3 at lysines 4, 9, and 27, based on the diversity in apparent function of these marks (18). Tri-methylation of histone H3 K4 (H3K4me3) is consistently associated with the promoters of genes that are transcriptionally “on” (18, 32, 33). Tri-methylation of histone H3 K9 (H3K9me3), however, is associated with heterochromatin formation and gene silencing (18), while tri-methylation of H3 K27 (H3K27me3) has been associated with developmental repression of gene transcription (18, 34). Here, we examine the effects of stress, stress duration, and the anti-depressant fluoxetine on a sample of histone H3 methyl marks in the hippocampus and show a pattern of changes remarkable for their rapidity, magnitude, and regional specificity.
Results
We found that immunoreactivity for the histone marks examined was largely restricted to the granule cell and pyramidal cell layers of the hippocampal formation, although in the case of the H3K9me3 antibody, some immunoreactivity was found in the neuropil (see Fig. 2D below). All measurements were taken from the granule or pyramidal cell layers.
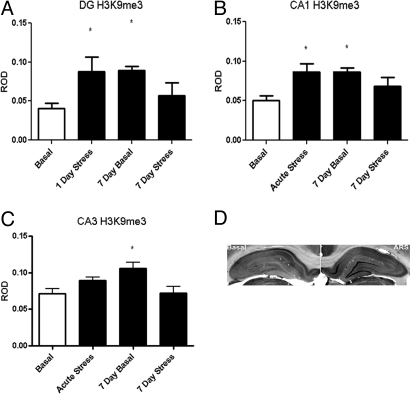
Fig. 2.
Levels of H3K9me3 immunoreactivity in hippocampus from rats either unstressed (basal), after acute stress, 24 h after 6 days of stress (7-day basal) or 7 days of restraint stress in the DG (A), CA1 (B), and CA3 (C). (D) Representative sections through the hippocampus of unstressed (basal) and acutely restrained (ARS) rats. *, P < 0.05 vs. basal.
Acute Restraint Stress.
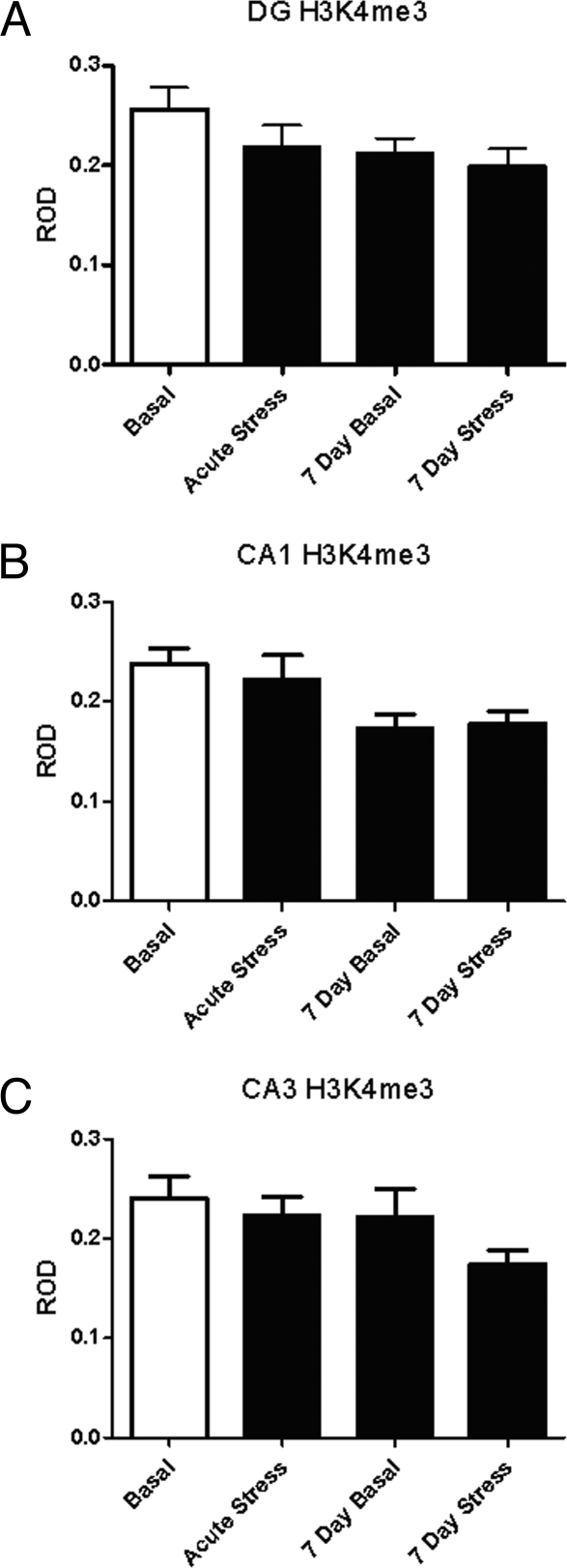
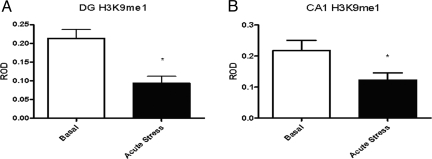
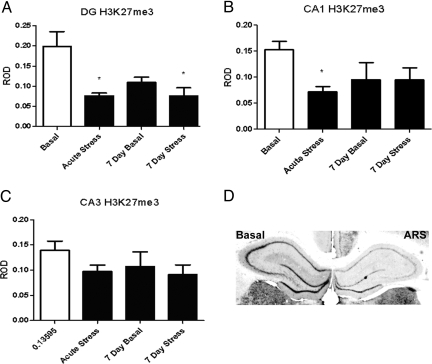
To assess the effects of acute stress on histone H3 methylation, we subjected rats to acute restraint stress and examined global levels of trimethylation at K4, K9, and K27, as well as mono-methylation at K9 (antibodies for mono-methylated K4 and K27 were not reliable for immunocytochemistry). Acute restraint stress produced no detectable change in levels of the transcriptionally active mark H3K4me3 immunoreactivity in the hippocampal formation (Fig. 1). It did, however, produce a 73% increase in the repressive, heterochromatin associated mark, H3K9me3 levels in the CA1 (±16%, P < 0.05, n = 7) (Fig. 2B) and DG (115 ± 16%, P < 0.003, n = 7) (Fig. 2A). No change in H3K9me3 levels was observed in the CA3 after acute stress (Fig. 2C). We also measured H3K9me1 levels, as a control for the observed changes in H3K9me3, after acute stress treatment and found a 44% decrease in mono-methylation in CA1 (±18%, P < 0.05, n = 5) (Fig. 3B) and DG (54 ± 16%, P < 0.005, n = 5) (Fig. 3A). Levels of the repressive mark, H3K27me3 decreased by 45% (±21%, P < 0.03, n = 5) (Fig. 4B) in the DG and 53% (±13%, P < 0.005, n = 5) (Fig. 4B) in the CA1, and was not significantly altered in the CA3 (Fig. 4C).
Fig. 1.
Levels of H3K4me3 immunoreactivity in hippocampus from rats either unstressed (basal), after acute stress, 24 h after 6 days of stress (7-day basal) or 7 days of restraint stress in the DG (A), CA1 (B), and CA3 (C).
Fig. 3.
Levels of H3K9me1 immunoreactivity after acute stress in the DG (A) and CA1 (B). *, P < 0.05 vs. basal.
Fig. 4.
Levels of H3K27me3 immunoreactivity in hippocampus from rats either unstressed (basal), after acute stress, 24 h after 6 days of stress (7-day basal) or 7 days of restraint stress in the DG (A), CA1 (B), and CA3 (C). (D) Representative sections through the hippocampus of unstressed (basal) and acutely restrained (ARS) rats. *, P < 0.05 vs. basal.
Subchronic 7-Day Restraint Stress.
Much like acute restraint, 7 days of repeated restraint had little significant effect on H3K4me3 levels (Fig. 1). Only in the CA1 (Fig. 1B) was there a main effect of treatment [P < 0.05, F (3, 16) = 3.393]. Although no significant group differences were observed, there is a mild decline in the 7-day groups. In contrast to acute stress, basal levels of H3K9me3 immunoreactivity were 49% higher (±12%, P < 0.05, n = 7) after 6 days of stress and reduced 40% (±4%, P < 0.05, n = 7) from the 7 day basal level by the seventh day of stress. Levels of H3K27me3 were reduced 62% (±9%, P < 0.5, n = 5) (Fig. 4A) in the dentate gyrus after 6 days of acute stress (7-day basal group), and the levels remained low after 7 days, with no significant reduction from subsequent stress (7-day stress group). No effect was observed in the CA1 or CA3 (Fig. 4 B and C).
Chronic Restraint Stress (CRS) and Fluoxetine Treatment.
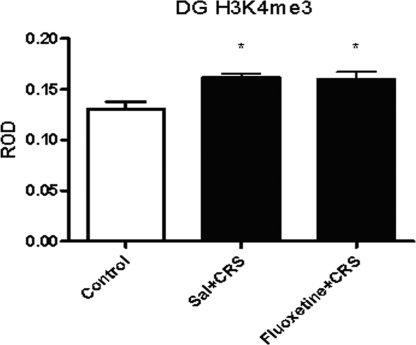
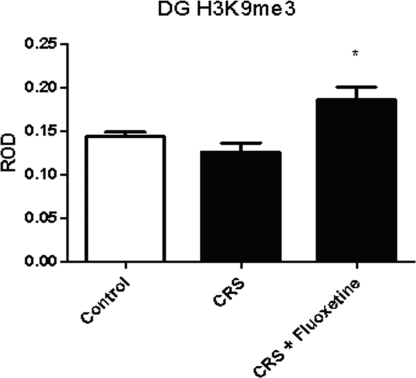
We also examined the effects of CRS and CRS coupled with fluoxetine treatment, which has been shown to reduce or block some of the effects of CRS on the hippocampus, on histone H3 methylation. Both CRS [24%; ±3%; df = 3,23; F (2, 30) = 6.743; P < 0.05; n = 6] and CRS with concurrent fluoxetine [22%; ±6%; df = 3,23, F (2, 30) = 6.743, P < 0.05; n = 6] produced a mild elevation in H3K4me3 in the DG (Fig. 5). There was a significant main effect of CRS on H3K9me3, which was decreased 15% [±8%, F (2, 30) = 6.743, P < 0.004, n = 8] in the DG by CRS and increased 30% (±8%, P < 0.05, n = 8) (Fig. 6) by concurrent treatment with fluoxetine; no change was detected in other subregions of the hippocampal formation. In addition, no significant changes were observed in H3K27me3 levels after CRS or CRS with fluoxetine. These changes suggest modest transcriptional activation after 21-day chronic stress and more substantial transcriptional repression by fluoxetine.
Fig. 5.
Levels of H3K4me3 immunoreactivity in the DG after CRS or CRS with daily 10 mg/kg fluoxetine treatment. *, P < 0.05 vs. basal.
Fig. 6.
Levels of H3K9me3 immunoreactivity in the DG after CRS or CRS with daily 10 mg/kg fluoxetine treatment. *, P < 0.05 vs. basal.
Discussion
The hippocampus is a brain region particularly susceptible to the effects of stress, and, in addition, appears to be a region subject to significant amounts of chromatin modification. Previous studies have shown changes in covalent histone modification in a variety of models, including seizures (35), psychostimulant treatment (36), exercise (37), and depression (13, 38). Epigenetic mechanisms are now thought to be potential contributors both to normal cognitive processes and to a variety of neuropsychiatric diseases (7, 9, 39). This study documents dramatic effects of acute stress and changes in three histone marks that are indicative of time dependent effects of repeated stress that are regionally localized within the hippocampal formation and suggest time-dependent changes in positive and negative effects on gene transcription.
A number of laboratories have shown interactions between stressors, the stress response, and histone modification. Chronic social defeat stress has been shown to alter acetylation and methylation of histones on the promoter regions of a number of genes in the nucleus accumbens (38, 40) and anti-depressant treatment in this model has been shown to reverse some of these effects. Acute stress has been shown to have an impact on histone acetylation and phosphorylation levels in the hippocampal formation (15, 41, 42). It, therefore, seems that chromatin remodeling in the brain is likely to play a significant role in normal and pathogenic responses to stress and in the treatment of stress-related diseases.
The present study chose to examine the effects of stress and SSRI treatment on levels of methylation of histone H3. We selected the K4, K9, and K27 sites as they have been associated with increased transcription, heterochromatin formation, and transcriptional repression, respectively (5, 33, 43), and as such, represent much of the functional range that histone methylation is thought to have with regard to the regulation of transcription.
We found that acute stress produced profound, rapid changes in the global levels of tri-methylation of histone H3 at lysines 9 and 27, but more minor changes at lysine 4. Chronic stress, on the other hand, produced only a small reduction in H3K9 tri-methylation and a small increase in the levels of H3K4 tri-methylation. These results are surprising given the magnitude and regional specificity of the changes, as well as the rapidity with which they occur after an acute stressor. It is also possible that there are significant changes occurring on a more gene specific level, but the fact that at least some methyl mark changes are detectable on a gross, whole-cell level is suggestive of a large-scale reordering of chromatin in the cell in response to stress. Methyl marks have been thought to be relatively stable as compared to phosphorylation and acetylation of histones. Indeed, until relatively recently, there were no proteins with known histone de-methylase activity (44, 45). However, recent studies have begun to show that they are as labile as other marks in proliferating cells (16). Our findings suggest that, even in terminally differentiated neurons, methyl marks may also be subject to rapid change.
It is interesting that acute stress produced much larger changes in levels of H3K9me3 and H3K27me3 immunoreactivity than chronic stress. This suggests that acute stress induces much larger epigenetic and transcriptional changes than chronic stress, a supposition supported by experiments comparing gene expression after acute stress or corticosterone and CRS (46–49). This may be due to the habituation of the hypothalamic-pituitary-adrenal axis after chronic homotypic stress (21, 23). That the direction of the change is different for the two marks is puzzling given that both are thought to be transcriptionally repressive. However, in some cases, the H3K9 mark can be associated with increased transcription when it is found in the coding region rather than the promoter region of a gene (50). The change in H3K9me3 levels admits other potential explanations, given the role of this mark in heterochromatin formation (51, 52); it seems possible that the increase in this mark represents stress-induced facultative heterochromatin formation, perhaps via the KAP-1 pathway, which is present in the mammalian hippocampus and seems to play a role in the transcriptional response to stress in that region (53).
We found that levels of H3K9me3 rose with acute stress and remained elevated after 6 days of stress (7-day basal condition), whereas chronic stress did not induce a significant change in H3K9me3 immunoreactivity. Also of interest was the fact that on the seventh day of stress, the ability of stress to elevate H3K9me3 has been lost, suggesting that the system may habituate to subsequent stress at some point between 1 and 6 days of repeated stress. Similarly, H3K27 tri-methylation declined with acute stress and remained low after 7 days, but showed no change after 21 days of CRS. Potentially, the loss of stress induced up-regulation of K9 methylation, and down-regulation of H3K27me3 may contribute to chronic stress induced remodeling of the hippocampal formation, which is not evident until chronic stress accumulates for 21 days (54).
Antidepressant treatment has been shown to block many of the hippocampal sequelae of chronic stress (25–27, 55–57), and therefore, we sought to determine if fluoxetine had a similar effect on histone H3 methylation. Chronic treatment with fluoxetine during CRS did produce elevated levels of K9 methylation. Consistent with observations of other stress-induced markers in humans and animal models, acute treatment with fluoxetine did not alter the levels of any of the three methyl marks we examined. Given that chronic, but not acute, fluoxetine treatment is associated with antidepressant effects, this finding suggests that histone H3 tri-methylation in response to stress may be an important factor in orchestrating the hippocampal response to stress and antidepressants.
The effects of high levels of chronic corticosterone produced small reductions in the levels of the H3K9me3 and H3K27me3, suggesting that chronic, high dose corticosteroids have an influence on chromatin structure, although of a lesser magnitude when compared to acute stress (Fig.S1 and Fig. S2) Acute stress and acute corticosteroid treatment (SI Text) did not produce the same effect. Indeed no effect was observed from acute corticosterone treatment. It would appear, therefore, that the changes in histone marks we observe after acute stress are mediated by other neurochemical events, most likely either glutamate or catecholamines, both of which are elevated by acute stressors (58, 59).
In summary, the effects of stress and fluoxetine on histone H3 tri-methylation, represent an aspect of the hippocampal response to stress which may represent an avenue of understanding how stress induces transcriptional and, by extension, structural and functional change in the hippocampus.
Methods
Animals.
Adult male Sprague–Dawley rats were obtained from Charles River Laboratories at 70 days of age. Animals were housed two to three per cage (same-age cage mates) in clear polycarbonate cages with wood chip bedding. All animals were maintained on a 12-h light–dark schedule (lights on at 8:00 AM) and the temperature was kept at 21 ± 2 °C. All animals had ad libitum access to food and water. All procedures were carried out in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Acute Restraint Stress.
Rats were stressed as below for 30 min, followed by a 45-min recovery in the home cage before sacrifice. All acute stress sessions were administered between 12:00 and 3:00 PM.
Seven-Day Restraint Stress.
Rats were stressed daily for 7 days, for 30 min as above and killed 45 min after the last stress (stress), or immediately before the time of the last stress (7-day basal). All stress sessions were administered between 12:00 and 3:00 PM. For further detail on the acute and 7-day stress paradigms see (60).
Chronic Restraint Stress and Fluoxetine Treatment.
Animals were left undisturbed after arrival for 1 week. Stressed animals were restrained in wire mesh restrainers, secured at the head and tail ends with large binder clips. Chronic stress was administered for 6 h daily for 21 days from 10:00 AM to 4:00 PM. Fluoxetine (10 mg/kg) or vehicle (0.9% saline, 1 mL/kg) was injected s.c. immediately before restraint. Animals were returned to their home cages immediately after termination of the stressor. These animals were killed by deep anesthesia with pentobarbital (100 mg/kg) and perfused roughly 24 h after the last stress (i.e., between 1:00 and 4:00 PM).
Immunohistochemistry.
All animals were killed by deep anesthesia with pentobarbital (100 mg/kg), followed by perfusion with 100 mL of heparinized saline then 200 mL of 4% paraformaldehyde. Brains were postfixed for 4 h in 4% paraformaldehyde before sinking in 30% sucrose and flash freezing on dry ice. Brains were stored at −80 °C until they were cut into 40-μm-thick sections which were then stored in cryoprotectant at −20 °C before being processed for immunohistochemistry.
For the purposes of the experiments described here, 2–3 dorsal hippocampal sections per animal (selected from −3.1 to −3.8 mm caudal to bregma) were picked and processed together as follows: 40-μm-thick brain sections were rinsed in 0.1 M Tris-saline (TS) 3 × 10 min each. Blocked in 0.5% BSA (BSA) in 0.1 M TS, with 0.25% Triton X-100, for 30 min and then incubated in primary antibody for H3K4me3, H3K9me3, H3K9me1, or H3K27me3, (Millipore) at a dilution of 1:8,000 (H3K9me3 and H3K27me3), 1:2,000 (H3K9me1) or 1:30,000 (H3K4me3) overnight at 4 °C in a solution of 0.1% BSA/0.25% Triton X-100 in 0.1 M TS. Tissue was rinsed in 0.1 M TS 3 ×10−15 min each. Sections were then incubated with secondary antibody (biotinylated anti-rabbit) diluted 1:400 with 0.1% BSA in 0.1 M TS (25 μL for each 10 mL) for 30 min at room temperature (RT). They were then washed in 0.1 M TS 3 × 10 min before incubation in ABC reagent (Vector Labs) for 30 min at RT followed by three more 10 min TS washes. Sections were then rinsed before incubation in DAB (Sigma) for 6 min, followed by three brief TS washes and three brief washes in 0.1 M PB. Sections were then dehydrated in ethanol and xylenes before cover-slipping with DPX and densitometry.
Data provided by the manufacturer shows that each antibody was at least 1,000-fold more selective for their specific trimethylation vs. other H3 trimethyl marks. All antibodies were tested by blocking with their respective immunizing peptides to confirm their specificity. Immunoreactivity was also confirmed using Western blots.
Densitometry and Statistics.
Densitometry was accomplished using MCID (Imaging Research) software and a light box with an attached CCD camera with a 60-mm Nikon lens. Two to three sections per animal were examined and each region was analyzed across its full length within each section (excluding transition zones between regions, e.g., CA1 subiculum) and, after subtracting background (we used the corpus callosum), the values for each region for each animal averaged to provide a single data point for that region and animal in relative optical density (arbitrary units). Data were then analyzed statistically using Graphpad Prism (Graphpad Software). Either ANOVA or Student's t test were used depending on the number of groups in the comparison. Tukey's test was used for post-hoc analysis of ANOVA results. A P value <0.05 was regarded as significant.
Supplementary Material
Acknowledgments.
We thank C. David Allis of Rockefeller University for reagents and constructive criticism, Elizabeth Waters of Rockefeller University and Katherine Maguire of Trinity College, Dublin for their assistance in characterizing antibodies for immunohistochemistry, and to Russell Romeo of Barnard College for providing tissue. This work was supported by National Institutes of Health Grants MH15125 and MH41256 (to R.G.H., K.J.M., and B.S.M.), and the Gary R. Helman Foundation (R.G.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911143106/DCSupplemental.
References
- 1.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 3.Conrad CD. Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 5.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 6.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nestler EJ. Epigenetic mechanisms in psychiatry. Biol Psychiatry. 2009;65:189–190. doi: 10.1016/j.biopsych.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reul JM, Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology. 2007;32:S21–25. doi: 10.1016/j.psyneuen.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 12.Sokolova NE, Shiryaeva NV, Dyuzhikova NA, Savenko YN, Vaido AI. Effect of long-term mental and pain stress on the dynamics of H4 histone acetylation in hippocampal neurons of rats with different levels of nervous system excitability. Bull Exp Biol Med. 2006;142:341–343. doi: 10.1007/s10517-006-0361-3. [DOI] [PubMed] [Google Scholar]
- 13.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 14.Bilang-Bleuel A, et al. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: Involvement in a glucocorticoid receptor-dependent behavioral response. Eur J Neurosci. 2005;22:1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 15.Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: Relevance for c-fos induction. J Neurochem. 2007;101:815–828. doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- 16.Ng SS, Yue WW, Oppermann U, Klose RJ. Dynamic protein methylation in chromatin biology. Cell Mol Life Sci. 2009;66:407–422. doi: 10.1007/s00018-008-8303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: Lessons from professional pocket pickers. Nat Struct Mol Biol. 2007;14:1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 22.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 23.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 25.Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 26.Czeh B, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: Hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 27.Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav. 2006;83:186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: Rapid actions of serotonergic and antidepressant agents. J Neurosci. 2002;22:3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- 30.Trotter KW, Archer TK. Nuclear receptors and chromatin remodeling machinery. Mol Cell Endocrinol. 2007;265–266:162–167. doi: 10.1016/j.mce.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trtkova K, Bouchal J, Kolar Z. Histone acetylation and methylation in the signaling of steroid hormone receptors. Cell Mol Biol. 2007;53:OL930–942. [PubMed] [Google Scholar]
- 32.Shilatifard A. Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr Opin Cell Biol. 2008;20:341–348. doi: 10.1016/j.ceb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: Intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 37.Collins A, et al. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS One. 2009;4:e4330. doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson MB, et al. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graff J, Mansuy IM. Epigenetic codes in cognition and behavior. Behav Brain Res. 2008;192:70–87. doi: 10.1016/j.bbr.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 41.Chandramohan Y, Droste SK, Arthur JS, Reul JM. The forced swimming-induced behavioral immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signaling pathway. Eur J Neurosci. 2008;27:2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- 42.Cleck JN, Ecke LE, Blendy JA. Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice. Psychopharmacology. 2008;201:15–28. doi: 10.1007/s00213-008-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volkel P, Angrand PO. The control of histone lysine methylation in epigenetic regulation. Biochimie. 2007;89:1–20. doi: 10.1016/j.biochi.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8:307–318. doi: 10.1038/nrm2143. [DOI] [PubMed] [Google Scholar]
- 45.Kubicek S, Jenuwein T. A crack in histone lysine methylation. Cell. 2004;119:903–906. doi: 10.1016/j.cell.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Irwin LN. Gene expression in the hippocampus of behaviorally stimulated rats: Analysis by DNA microarray. Brain Res Mol Brain Res. 2001;96:163–169. doi: 10.1016/s0169-328x(01)00269-8. [DOI] [PubMed] [Google Scholar]
- 47.Tsolakidou A, et al. Acute stress regulation of neuroplasticity genes in mouse hippocampus CA3 area–possible novel signaling pathways. Mol Cell Neurosci. 2008;38:444–452. doi: 10.1016/j.mcn.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Ejchel-Cohen TF, et al. Chronic restraint stress decreases the expression of glutathione S-transferase pi2 in the mouse hippocampus. Brain Res. 2006;1090:156–162. doi: 10.1016/j.brainres.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 49.Feldker DE, et al. The effect of chronic exposure to highly aggressive mice on hippocampal gene expression of non-aggressive subordinates. Brain Res. 2006;1089:10–20. doi: 10.1016/j.brainres.2006.02.110. [DOI] [PubMed] [Google Scholar]
- 50.Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Lachner M, Jenuwein T. The many faces of histone lysine methylation. Curr Opin Cell Biol. 2002;14:286–298. doi: 10.1016/s0955-0674(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 52.Grewal SI, Jia S. Heterochromatin revisited. Nat Rev Genet. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- 53.Jakobsson J, et al. KAP1-mediated epigenetic repression in the forebrain modulates behavioral vulnerability to stress. Neuron. 2008;60:818–831. doi: 10.1016/j.neuron.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 54.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 55.Luo L, Tan RX. Fluoxetine inhibits dendrite atrophy of hippocampal neurons by decreasing nitric oxide synthase expression in rat depression model. Acta Pharmacol Sin. 2001;22:865–870. [PubMed] [Google Scholar]
- 56.Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 57.Bessa JM, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2008;14:764–773. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 58.Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 59.Anisman H, Zacharko RM. Behavioral and neurochemical consequences associated with stressors. Ann N Y Acad Sci. 1986;467:205–225. doi: 10.1111/j.1749-6632.1986.tb14630.x. [DOI] [PubMed] [Google Scholar]
- 60.Romeo RD, et al. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.