Abstract
Unlike other neuronal counterparts, primary synaptic proteins are not known to be involved in vascular physiology. Here, we demonstrate that neurexins and neuroligins, which constitute large and complex families of fundamental players in synaptic activity, are produced and processed by endothelial and vascular smooth muscle cells throughout the vasculature. Moreover, they are dynamically regulated during vessel remodeling and form endogenous complexes in large vessels as well as in the brain. We used the chicken chorioallantoic membrane as a system to pursue functional studies and demonstrate that a monoclonal recombinant antibody against β-neurexin inhibits angiogenesis, whereas exogenous neuroligin has a role in promoting angiogenesis. Finally, as an insight into the mechanism of action of β-neurexin, we show that the anti-β-neurexin antibody influences vessel tone in isolated chicken arteries. Our finding strongly supports the idea that even the most complex and plastic events taking place in the nervous system (i.e., synaptic activity) share molecular cues with the vascular system.
Keywords: angiogenesis, vessel tone, cell-to-cell adhesion, nervous–vascular parallels, synapses
Neurexins and neuroligins, which are transmembrane synaptic proteins of the central nervous system, are codified in humans by families of 3 and 5 genes, respectively (1, 2). Neurexins, produced in long (α) and short (β) forms, have been widely studied because of their extended alternative splicing (1, 3) and have been localized indirectly at the presynaptic membrane (4). Neuroligins are localized at the postsynaptic membrane (5) and interact with neurexins from the opposite side (in trans) of the synaptic cleft in a calcium-dependent manner (6). Both proteins display a strong and selective synapse formation-promoting activity in vitro (7, 8). Nonetheless, knockdown of the expression of the 3 α forms of neurexins (9) or neuroligins 1–3 (10) in the mouse demonstrates that these proteins have a more fundamental role in the modulation of synaptic transmission than in the early adhesive steps of synapse formation. The same studies revealed a redundancy of function between isoforms of the same gene family. Neurexins and neuroligins are part of a large set of synaptic cell adhesion molecules whose elimination in mice surprisingly results in an overall maintenance of synaptic structures (11).
The vasculature, much like the nervous system, consists of a hierarchical organization of vessels that form a distributed network to reach all regions of the body. Blood vessels form through 2 main mechanisms: vasculogenesis, which is typical of embryonic development and is based on the differentiation of endothelial cells (ECs) from mesodermal precursors, and angiogenesis, which is the creation/remodeling of blood vessels from preexisting ones (12). Angiogenesis is carried out by different overlapping cellular processes, including, among others, the proliferation and migration/adhesion of ECs and the recruitment of pericytes and vascular smooth muscle cells (VSMCs) during the final steps of maturation (13). The larger blood vessels are formed by a luminal coating of ECs, called the “intima,” and a vessel wall, which in turn is divided into the media, consisting of VSMCs, and the adventitia, the outer layer of the vessel built by connective tissue and fibroblasts. These 3 compartments, with different functions, are involved in a multitude of physiological and pathological events, including control of vessel tone and atherosclerosis (14).
Unlike many other neuronal cues (15), none of the known key synaptic proteins had been shown to participate in blood vessel function. We have explored this possible involvement and demonstrated that various isoforms of neurexins and neuroligins are produced by vascular cells in embryo and adult animals. They are alternatively spliced, are involved in blood vessel remodeling, and form endogenous complexes analogous to their behavior in the brain. We next used the chicken chorioallantoic membrane (CAM) as a system to investigate the role of these proteins in angiogenesis. We show that a monoclonal humanized recombinant antibody against β-neurexin (anti-βΝRXN) inhibits vascular remodeling/angiogenesis, whereas the overexpression of neuroligin 1 by ECs inserted in a tumor environment induces CAM vessel growth. Finally, we demonstrate that the anti-βΝRXN antibody modulates the vascular tone of isolated arteries, providing important insights into the molecular activity of β-neurexins.
Results
Expression of Neurexin and Neuroligin in the Vascular System.
The original aim of this study was to investigate the expression of neurexin and neuroligin in an in vivo setting. Preliminary immunohistochemical/immunofluorescence screening was performed with a monoclonal anti-neuroligin antibody (monoclonal anti-NLGN, ref. 5) and a home-made polyclonal anti-pan-neurexin antibody (polyclonal anti-NRXN) that recognizes both α- and β-neurexins (see Figs. S1, S2, and S3 for validation and controls of specificity). The analysis revealed that neurexin and neuroligin are expressed in the blood vessel walls of the chicken and mouse and in human tissues. Furthermore, the analysis demonstrated that smooth muscle cells (SMCs) of nonvascular origin (lung bronchiolar SMCs) also can express neurexin (Fig. S3). Prompted by these findings, we next focused on large blood vessels. Fig. 1 displays the peculiar expression pattern of neurexin and neuroligin that was revealed. To illustrate the pervasiveness of neurexin and neuroligin expression in the vascular system, we performed a thorough immunohistochemical analysis on E5 chicken embryos (Fig. 2A), E18 chicken embryo organs (liver, lung, and spleen; Fig. S3), and brains from E18 chicken embryos and adult mice (SI Text R2 and Figs. S4 and S5). From this analysis we obtained a general quantification of blood vessel labeling by neurexin and neuroligin antibodies (Table 1 and Figs. S4 and S5). These results indicate that both the arterial and venous compartments of the vasculature produce neurexin and neuroligin. Moreover, in the vast majority of vessels that we analyzed, both proteins are expressed throughout the vessel wall in a pattern similar to anti-α-smooth muscle actin (α-SMA, Figs. 2A and S3). This is particularly clear in the immature vessels of the E5 chicken embryo. Only in the well-structured and muscularized arteries (Figs. 1 and S5) is neurexin expression limited to a subset of SMCs. Another distinction that can be made is that the expression of neuroligin in the large arteries of the mouse brain (Fig. S5C) appears to cover both the endothelial and mural layers, whereas it is restricted to the endothelium in adult chicken brain arteries (Fig. 1, P and R). Whether this difference is related to the different amount of coverage by SMCs in the 2 types of vessel or depends on the subtype of vessel or function will need further analysis. In Fig. S5 we also provide a quantification of the number of vessels in which neurexin expression is independent of neuroligin expression in the vessel wall.
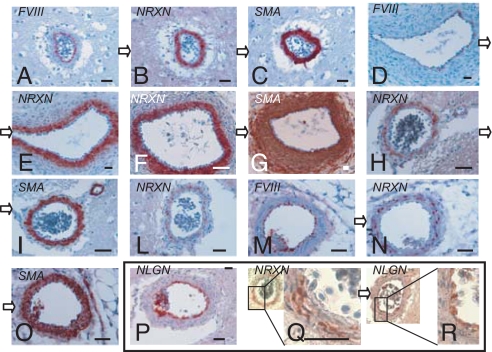
Fig. 1.
Neurexin and neuroligin expression in large blood vessels. Pictures show immunostaining of blood vessels from adult chicken brain (A–C, H, I, L, P–R), E11 chicken embryos (D–G), and adult mouse kidneys (M–O). Sections were stained with polyclonal anti-NRXN (B, E, F, H, L, N, Q), anti-NLGN 4F9 (P, R), anti-αSMA (C, G, I, O), or anti-FVIII (A, D, M) antibodies. Sections linked by open arrows (A–C; D and E; F and G; H and I; M–O; Q and R) are consecutive. (Scale bar, 50 μm) See SI Text R1 for a further description of these results.
Fig. 2.
Expression and co-localization of neurexin and neuroligin in early (E5) chicken embryo. (A) Immunostaining of E5 chicken embryo sagittal sections showing comparison of endothelial markers, neurexin, neuroligin, and α-SMA. Class 3 vessels (see Table 1) are shown in low-magnification (Top) and high-magnification (Middle) images of a cephalic region proximal to the developing optic lobe. Sections of blood vessels of various wall thicknesses located peripherally to the optic lobe as well as vessels entering the nerve tissues are stained by the neurexin, neuroligin, α-SMA, and FVIII antibodies. (Bottom) Class 4 vessels: aortic arches expressing neurexin, neuroligin, α-SMA, and VEGFR-2. (B) Immunofluorescence and confocal analysis of E5 chicken embryo sagittal sections showing that neurexin (red) is expressed by αSMA-positive cells (green) in 3 examples of developing vessels: a portion of the vessel wall of the developing aorta (Top), an aortic arch section (Middle), and a small vessel of the head vascular plexus (Bottom). (C) Co-localization between neurexin (red) and neuroligin (green) in 2 different vessels: a section of a developing aortic vessel wall (Upper) and a portion of the neck vascular plexus (Lower). DAPI (blue) is nuclear staining. (Scale bar, 50 μm.)
Table 1.
Quantification of neurexin and neuroligin expression in the E5 chicken embryo
| Class | Range of wall thickness (μm), by SMA staining | Number of vessels in the class | Range of vessel length (μm) | Range of vessel diameter (μm) | α-SMA staining intensity | NRXN staining intensity | NLGN staining intensity | Representative vessels of the class |
|---|---|---|---|---|---|---|---|---|
| 1 | 0–4 | 17 | 20–250 | 4.5–135 | 0/+ | + | + | Arteries and veins of the primary cephalic plexus |
| 2 | 4–6 | 13 | 21–270 | 5–250 | + | + | + | Intersomitic vessels |
| 3* | 6–10* | 17* | 34–1650* | 13–115* | ++ | ++ | ++ | Dorsal aorta, subcardinal vein, primary cephalic plexus |
| 4* | >10* | 10* | 52–1130* | 33–116* | +++ | ++ | ++ | Aortic arches |
Medial sagittal sections from 6 entire E5 chicken embryos were immunohistochemically stained and blood vessels throughout the sections were evaluated for their expression of α-SMA, neurexin, and neuroligin. Next, various morphological parameters were measured, and the vessels were separated into 4 classes based on the thickness of the SMA staining in their walls. Arbitrary values of expression were reported for each marker: +++, strong; ++, good; +, weak. Data show that neurexin and neuroligin expression almost perfectly matches that of α-SMA.
*Examples of blood vessels included in classes 3 and 4 are presented in Fig. 2A.
To confirm the intriguing concept of a co-localization of neurexin and neuroligin in the wall of immature vessels, we performed immunofluorescence detection followed by a confocal analysis on E5 chicken embryos. Fig. 2 shows that anti-NRXN staining overlaps that of the anti-αSMA (Fig. 2B) and the anti-NLGN (Fig. 2C) antibodies up to the intimal cell layer in a portion of the developing aorta, aortic arch, and a small vessel of the head plexus. Hence, during blood vessel assembly, neurexin and neuroligin are expressed in a pattern that allows their interaction.
Neurexin and Neuroligin Exist in Endogenous Complexes in Chicken Embryo Arteries.
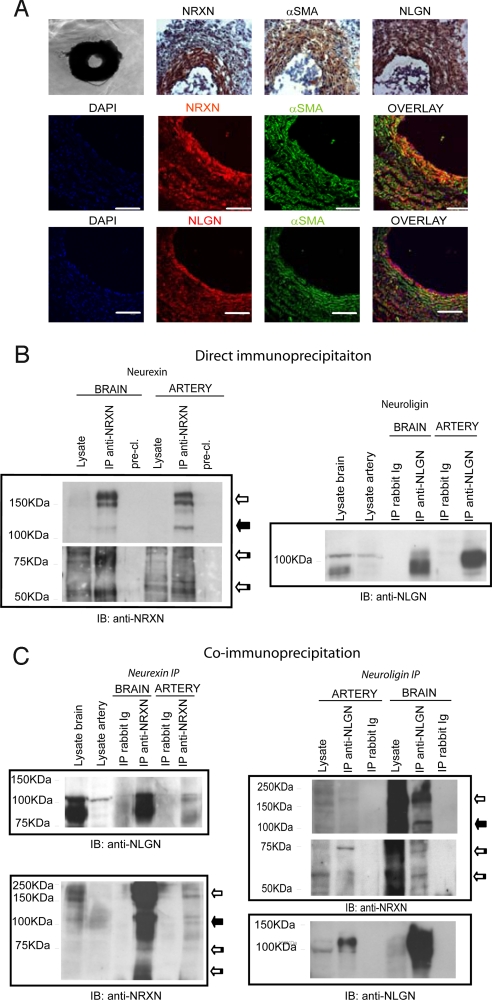
To continue our studies, we used surgically removed arteries from E18 chicken embryos. In these vessels, which we characterized to ensure that no nerve tissue was present in the preparation (Fig. S6 A), neurexin and neuroligin are produced by overlapping luminal layers of cells (Fig. 3A). Immunoprecipitation of neurexin (Fig. 3B, Left) with the polyclonal anti-NRXN antibody followed by immunoblotting with the monoclonal antibody indicated that neurexin proteins with molecular weights in the range of 150–180 kDa (compatible with the α isoforms) and 70–85 kDa (compatible with known β isoforms; ref 16 and Fig. S2) were expressed in both the brain and arteries. A 115-kDa protein band (possibly a highly glycosylated β-neurexin isoform) appeared clearly in the arteries and in a weak form in the brain.
Fig. 3.
Neurexin and neuroligin expression pattern, protein production, and co-precipitation in the E18 chicken embryo arteries. (A) (Top) Phase-contrast and immunohistochemical staining of artery sections. (Middle and Bottom) Immunofluorescence and confocal analyses reveal the co-localization of neurexin and neuroligin with αSMA-expressing cells. (Middle) Polyclonal anti-NRXN (red) and anti-αSMA (green) staining. (Bottom) Monoclonal anti-NLGN (red) and anti-αSMA (green) staining. (B and C) E18 chicken embryo arteries were lysed using immunoprecipitation or co-immunoprecipitation buffers (see Materials and Methods) (B) (Left) α and β isoforms of neurexin were immunoprecipitated from both E18 chicken embryo arteries and brain with the polyclonal anti-NRXN. Immunoblotting was performed with monoclonal anti-NRXN. (Right) Neuroligins were immunoprecipitated from E18 chicken embryo arteries and brain with the polyclonal anti-NLGN antibody. Immunoblotting was performed with monoclonal anti-NLGN. (C) Co-immunoprecipitation of neurexin and neuroligin in E18 chicken embryo arteries and brain. (Left) Similar neuroligin isoforms were immunoprecipitated with neurexin in the arteries and brain. (Right) β-Neurexin isoforms preferentially precipitate with neuroligin in chicken embryo arteries, whereas both α- and β-neurexin were enriched by neuroligin in the brain. Immunoblots shown are representative of 3 experiments. White arrow, α-neurexin; black arrow, 115-KDa neurexin; striped arrow, β-neurexin.
The immunoblotting bands relative to the β isoforms appeared as the sum of many thinner bands in both organs, a pattern probably caused by a complex splicing and glycosylation process. Neuroligin immunoprecipitation and immunoblotting (Fig. 3B, Right) demonstrated the expression of neuroligin proteins of similar molecular weights (about 118 kDa) in the brain and arteries.
Because most known biological activities of neurexins and neuroligins are related to their interaction (17), we dedicated our next set of experiments to identifying complexes of these 2 proteins in the chicken arteries and brain. For this purpose, we immunoprecipitated each of the 2 proteins in particular cation and detergent conditions (see Materials and Methods) and immunoblotted the resulting precipitate with the antibody against its respective partner. Fig. 3C shows that neurexin and neuroligin can be co-immunoprecipitated reciprocally in arteries as well as in brain. Notably, although all neurexin isoforms are produced by arteries, only β-neurexin bands (in a single discrete form, not as a stack of bands) co-precipitate with neuroligin, indicating a selective interaction of the 2 proteins in this tissue.
Role of Neurexin and Neuroligin in Angiogenesis.
At this stage, we set up an adaptation of the aortic ring assay (18) using E18 chicken embryo arteries embedded in Matrigel. The subsequent immunohistochemical analysis on the rings revealed that the original histological structure of the section was considerably altered (Fig. S6C, Upper, middle row). In particular, in these conditions cells expressing neurexin, neuroligin, and αSMA were no longer found in single continuous layers of cells but were scattered throughout the ring and among sprouted cells within the Matrigel matrix. Of particular interest was the complete overlap between cells expressing neuroligin and cells expressing VEGF receptor 2 (VEGFR2) in the sprouted region (Fig. S6C, Bottom).
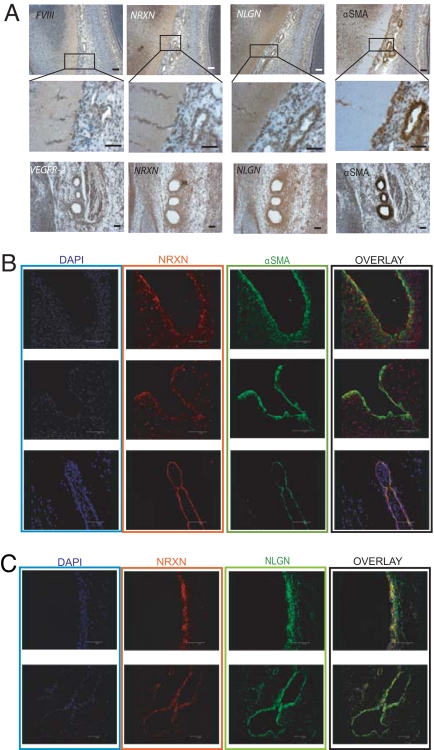
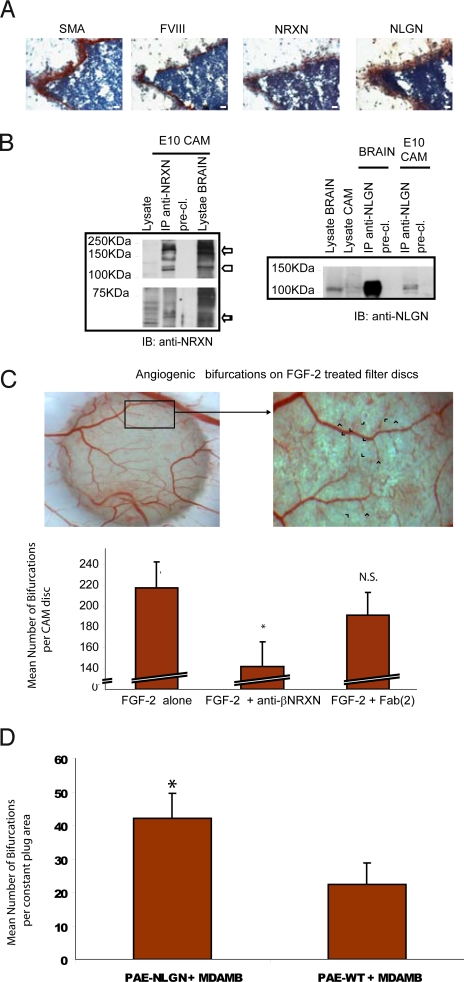
Because the chicken embryo aortic ring is not a classical angiogenesis assay (SI Text R5 and Fig. S6), we chose the CAM model to pursue functional studies on angiogenesis and targeted neurexin and neuroligin separately. For the former protein, we selected a specific isoform, β-neurexin, based on the following considerations: (i) It is the most studied isoform in terms of synaptogenic activity and neuroligin binding; (ii) unlike α-neurexins, no data are available on β-neurexin–null mice; and (iii) our co-precipitation experiments revealed a selective co-precipitation of β-neurexin with neuroligin in blood vessels (Fig. 3C). β-Neurexin was targeted with a recombinant antibody that specifically recognizes the native protein (Fig. S7). Fig. 4 shows that, in the 10-day-old CAM in which the assay was performed, the blood vessel wall produces neurexin and neuroligin (Fig. 4A) and that α- and β-neurexin (Fig. 4B, Left) as well as neuroligin proteins (Fig. 4B, Right) can be immunoprecipitated from this tissue. Isoform analysis by RT-PCR revealed the expression of both neurexin 1 and 3 (α and β) and of all neuroligin isoforms (Fig. S6B, Table).
Fig. 4.
Neurexin and neuroligin expression in the CAM and their role in angiogenesis. (A) Immunohistochemical expression analysis of neurexin and neuroligin in CAM blood vessels. CAMs were sectioned and stained with anti-αSMA, anti-FVIII, polyclonal anti-NRXN, and monoclonal anti-NLGN antibodies. Images show that neurexin and neuroligin are expressed in developing vessels of the E10 chicken CAM. (Scale bar, 50 μm) (B) Biochemical analysis of neurexin and neuroligin expression on E10 CAM: different isoforms of neurexin were immunoprecipitated from the CAM tissue with the polyclonal anti-NRXN antibody (Left). Neuroligins were immunoprecipitated from E10 chicken CAM as in the brain with the polyclonal anti-NLGN antibody (Right). (C) Effect of the recombinant anti-βNRXN antibody on FGF-2–induced angiogenesis. The CAM angiogenesis assay was used to evaluate the effects of the recombinant anti-βNRXN antibody on sprouting angiogenesis. The antibody decreases the number of vessel bifurcations compared with treatment with human IgG Fab(2) or with FGF-2 alone. (Top) A snapshot of the CAM disc. Blue arrowheads indicate the bifurcation used for scoring the result of the graph. (Bottom) Bars represent the number of vessel bifurcations per disc counted after the different treatments. n = 35 for FGF-2 alone, n = 31 for FGF-2 + anti-βNRXN antibody; n = 31 for FGF-2 + human IgG Fab(2). Error bars indicate 95% confidence intervals (CI). ANOVA gave F = 11.081. *, P < 0.01 for anti-βNRXN antibody vs. FGF-2 alone and FGF-2 + human IgG Fab(2) by Student Newman-Keuls test. N.S., P = 0.109 for FGF-2 alone vs. FGF-2 + human IgG Fab(2) by Student Newman-Keuls test. The mean angiogenic level in the untreated CAM discs (not presented in the graph) was 26.5 ± 12% (95% CI) or 26.5 ± 6% (SEM) higher than in the samples treated with anti-β-neurexin (n = 29). (P < 0.01 for anti-βNRXN antibody vs. untreated CAM discs. P = 0.91 for IgG Fab(2) vs. untreated CAM) (D) Pro-angiogenic effect of neuroligin 1 overexpression in ECs. Cultrex plugs (see Materials and Methods) of either nontransfected or neuroligin 1-overexpressing porcine aortic ECs (PAE cells) mixed with the tumor cell line MDA-MB-435 were incubated on CAM for 2 days, and vessel bifurcation was counted as in C. Bars represent the mean count of bifurcations in a fixed area. Error bars indicate 95% CI. n = 24 for PAE NLGN+MDA-MB-435; n = 16 for PAE-WT + MDA-MB-435. *, P < 0.01. Identical results were obtained with 2 different clones of PAE-NLGN.
The addition of the anti-βNRXN reduced the FGF-2–induced formation of capillary bifurcations (a sign of angiogenesis) by 35%, whereas a non-immune antibody in the same format (human IgG Fab(2)) did not have any significant effect (Fig. 4C).
To prove a role for neuroligin in angiogenesis, we set up an assay in which ECs, which constantly express this protein (see also SI Text R7 and Fig. S9), were mixed with the tumor cell line MDA-MB-435 (19) and laid on the CAM. It is known that reciprocal signaling between ECs of the growing vasculature and target cells in the surrounding organ, including tumors, is mediated by a variety of soluble and membrane-bound molecules (20). This phenomenon in turn modulates tumoral angiogenesis and metastasization (21). Our assay showed that, in a tumorigenic environment, a much stronger angiogenic response occurs with ECs overexpressing neuroligin 1 than with the WT ECs (Fig. 4D).
Anti-βNRXN Antibody Modulates Blood Vessel Tone.
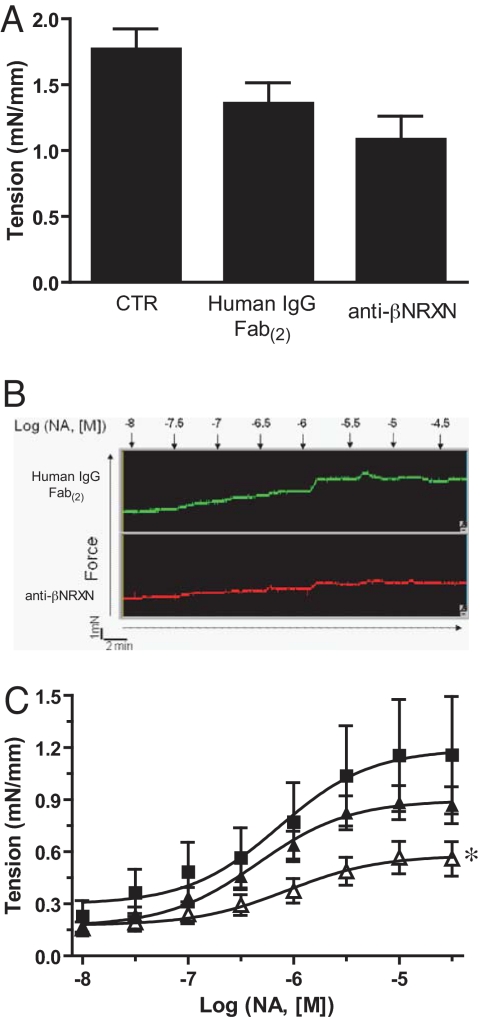
We next sought to gain insight into the cellular activities underlying the anti-angiogenic properties of the anti-βNRXN antibody. When we performed in vitro assays to analyze the influence of this reagent on the adhesive, proliferative, migratory, or survival responses of vascular mural cells, none of these responses altered significantly. The antibody also did not affect the neurexin- or neuroligin-mediated cell aggregation measured through a specific assay (SI Text R6 and Fig. S8). We then decided to investigate whether the reagent affected another important property of SMCs, i.e., their contractile activity. Indeed, there is a tight link between vascular tone, hemodynamics, and vascular remodeling, in both the embryo and adult organism (22, 23). To this aim, we tested the effect of the anti-βNRXN antibody on the tone of whole arteries stimulated either by membrane depolarization or by a soluble agonist. Although the tension induced by potassium depolarization remained unaffected by all treatments (Fig. 5A), the anti-βNRXN treatment (Fig. 5C) significantly reduced the development of vessel tension throughout the stimulation with noradrenalin (NA). The maximal response value (Emax) also was reduced significantly by the anti-βNRXN antibody (untreated = 1.19 ± 0.15 mN/mm (SEM); human IgG Fab(2) = 0.90 ± 0.05; anti-βNRXN 0.58 ± 0.06; P = 0.017 vs. human IgG Fab(2), P = 0.0022 vs. untreated).
Fig. 5.
Anti-βNRXN antibody inhibited NA-induced contraction on isolated E18 chicken embryo arteries. (A) Anti-βNRXN did not affect K+-induced contraction. Mesenteric arteries were incubated overnight with medium alone (n = 4), 20 μg/mL of human IgG Fab(2) (n = 7), or 20 μg/mL of anti-βNRXN antibody (n = 7). Bars represent mean tension recorded expressed as mN/mm ± SEM. (B) Original representative traces showing the dose–response curve to NA (0.01–100 μM) of 1 artery incubated with 20 μg/mL of human IgG Fab(2) (green) or 20 μg/mL of anti-βNRXN antibody (red). (C) Effect of recombinant anti-βNRXN on the NA dose–response curve. Mesenteric arteries were incubated overnight with (■) medium alone (n = 4), (▴) 20 μg/mL of human IgG Fab(2) (n = 7), or (▵) 20 μg/mL of anti-βNRXN antibody (n = 7). Anti-βNRXN antibody decreased the mean tension value of mesenteric arteries compared with medium alone or human IgG Fab(2). Data are expressed as mean ± SEM. ANOVA gave F = 10.653. *, P < 0.01 for anti-βNRXN antibody vs. medium alone and P = 0.018 vs. human IgG Fab(2) by Bonferroni posttest.
Discussion
Here we provide evidence that various isoforms of neurexins and neuroligins, exquisitely synaptic proteins, are expressed in the blood vessel wall where they exist in preformed complexes, as they do in the central nervous system. These data are based on transcription analysis as well as on biochemical and immunohistochemical studies performed using different affinity reagents, i.e., 2 different antibodies for both neurexin and neuroligin.
Using different approaches, we have shown that neurexin and neuroligin are involved in angiogenesis. We produced a specific reagent against β-neurexin that reduced angiogenesis in the CAM. The neuronal activity of this protein, i.e., organization of synaptic contacts, is not directly related to the best-studied cellular events of angiogenesis (proliferation, adhesion, or migration) and, in fact, the antibody treatment did not affect any of these activities. However, this reagent affects blood vessel tone, and this effect provides an interesting insight into the possible mechanism of action of β-neurexin. Indeed, SMCs are excitable cells, like neurons, and the other main neurexin isoform (α) is functionally coupled to voltage-gated calcium channels (9). Moreover, in addition to the links between hemodynamics and vascular remodeling, the contractile properties of supporting/mural cells appear to have a direct role in transcapillary pillar formation during intussusceptive angiogenesis, a fundamental process of blood vessel remodeling in various embryo organs including CAM (24).
Neuroligin is invariably produced in ECs. When ECs overexpressing neuroligin were introduced in a tumoral pro-angiogenic setting, their presence further promoted angiogenesis in the host CAM vessels. An interesting possibility is that neuroligin might mediate the secretion by ECs of direct or indirect inducers of angiogenesis (20, 25), analogous to its role in insulin release from pancreatic β cells (26). Although no vascular defects have been described for mice carrying null mutations within the 3 neuroligin genes (10), the experimental settings that we used involve the overexpression of the protein within an environment that notoriously induces an abnormal vascular response (27).
Our findings may be important from 2 perspectives. The first is the potential role of neurexin and neuroligin during the growth and remodeling of the vascular system. The complexity of these protein families greatly enlarges the number of their possible activities. We believe that the following general working hypotheses can be introduced. First, in analogy with the synaptic environment (11), a redundant collection of adhesive proteins that includes neurexins and neuroligins could finely regulate the plethora of blood vessel functions. Second, the most likely function of neurexins and neuroligins, which are physically associated within the developing vascular system, may be in regulating the EC–mural cell interactions, an important step during blood vessel disassembly and maturation. However, the functions of these 2 proteins can be analyzed both in relation to each other and separately. For example, neuroligin co-precipitates specifically with β-neurexins and not with α-neurexins, which can remain “orphans.” Third, neurexins and neuroligins could exploit some known or unknown vascular counterparts of their neuronal protein partners, such as calcium channels (9), as suggested by our data on vessel tone control, or others, such as dystroglycan (28) or intracellular PDZ domain-rich proteins, to carry out their biological activity. Alternatively, as many neuronal cues (29), they could produce their effect by interacting with the molecular machinery of key regulators of angiogenesis.
The second perspective concerns the possible existence of physical cross-talk between neurons and blood vessels mediated by neurexins and neuroligins. We mainly discuss the possibility that these proteins can mediate cross-talk during embryonic development or within plastic events involving both the nervous and vascular systems in the adult. In the developing embryo, blood vessels are innervated by neuronal axons of various origins that navigate through a maze of signals until they reach their target. Neurexins and neuroligins are not guidance molecules, but it is known that cell-to-cell adhesion mediated by the alpha 4 integrin expressed on axons and the VCAM1 adhesion receptor expressed on cardiac myocytes is important for the sympathetic innervation of the heart (30). Another intriguing possibility is that neurexin and neuroligin mediate cross-talk between the nervous and vascular systems during angiogenesis/synaptogenesis/neurogenesis events that take place in the cerebellum and hippocampus upon repetitive physical activities and/or motor skill learning (31, 32). It is particularly interesting that spatial motor learning in the rat results in the induction of neuroligin 1 in the hippocampus (33). Moreover, it is well known that neuroligin and neurexin produced by non-neural cells can induce pre- and post-synaptic specialization, respectively, in neurons (7, 34). Hence, in the right environment, these proteins presented by vascular cells could stimulate synaptic plasticity.
Materials and Methods
Detailed methods can be found in the SI Text.
Production of Antibodies.
The anti-β-neurexin humanized recombinant antibody (anti-βNRXN, clone AbyD02101) was produced by AbD Serotec MorphoSys AG, using the HuCALGOLD library (35). The β-neurexin–specific peptide HFHGSSKHHSVPIAIYRSPASLRG was used in the screening. This antibody is subject to restrictions for in vivo use in mammalian models and requires a license from MorphoSys AG.
The rabbit polyclonal anti-NRXN and anti-NLGN antibodies were raised against the peptide AKSANKNKKNKDKEYYV and PHPHPHSHSTTRV, respectively, and then were purified on the antigenic peptides.
Protein Immunoprecipitation and Co-Immunoprecipitation from Tissues.
Frozen tissues were disgregated with a tissue potter and lysed for 40 min on ice with lysis buffer (10 mM Tris HCl, pH 7.5; 150 mM NaCl; 5 mM EDTA, pH 8; 10% glycerol; 1% Triton X-100; and 1% 3-[(3-Cholamidopropyl)dimethylammonio]1-propanesulfonate (CHAPS)) and protease and phosphatase inhibitors (50 μg/mL pepstatin, 50 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mM PMSF, 100 μM ZnCl2, 1 mM Na orthovanadate, and 10 mM NaF). Samples then were pre-cleared with protein A-Sepharose (Amersham Biosciences) and incubated for 1 h with rabbit anti-NRXN anibody (1.5 μg/mg) or rabbit polyclonal anti-NLGN antibody (2.5 μg/mg). Immune complexes were analyzed by immunoblot. To detect neurexin, the membrane was incubated with the mouse monoclonal anti-NRXN antibody (Becton-Dickinson); to detect neuroligin, the membrane was incubated with mouse monoclonal anti-NLGN antibody (4F9; Synaptic Systems). For the co-immunoprecipitation experiments, some modifications were applied, as detailed in the SI Text, to preserve the neurexin–neuroligin interaction.
Aortic Ring Assay.
Mesenteric arteries were removed surgically from E18 chicken embryos, transferred to a petri dish, and rinsed in PBS. Next, they were cleaned of fibroadipose tissue, and rings about 1 mm in length were cut and incubated in Growth Factor Reduced Matrigel (Becton-Dickinson) for 4 days before immunohistochemical analysis.
CAM Angiogenesis Assays.
Cortisone acetate-treated filter disks (5 mm) saturated with 5 μL of 100 ng/mL FGF-2 (R&D Systems) and 9 μg of anti-βNRXN antibody or human IgG Fab(2) (Jackson ImmunoResearch) were laid on the CAMs of 10-day-old embryos. For the neuroligin angiogenesis studies, 1 × 106 MDA-MB-435 + PAE-NLGN or 1 × 106 MDA-MB-435 + PAE-WT were embedded in Cultrex Basement Membrane Extract (R&D Systems) and laid on the CAM. After 2 to 3 days of incubation, the angiogenic bifurcations were counted.
Contractile Response in Embryonic Chicken Mesenteric Artery.
Isometric tension exerted by the vessels was recorded using the wire-myograph technique (Danish Myo Technologies) according to Mulvany and Halpern (36). The preparations were stimulated with isotonic depolarizing KCl solutions. After washout, cumulative dose–response curves to NA (0.01–100 μM) (Sigma-Aldrich) were performed.
Supplementary Material
Acknowledgments.
We thank G. Serini and A. Gualandris for critically reading the manuscript, Prof. L. Capussotti of the Institute for Cancer Research and Treatment (IRCC) for providing the surgical samples, Prof M. Trovati and Prof. B. Bussolati (University of Torino) for providing the VSMC derived from Zucker lean rat aortas, HSMC, and human microvascular ECs, Prof. T. C Sudhof (Dallas, Texas) for providing the neurexin 1 α and β cDNAs, E. Vigna (IRCC) for providing the third-generation lentiviral vectors, and Famarco S.p.A. and Susa Trasporti S.p.A. for providing white Leghorn chicken eggs. This study was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Sixth Framework Program of European Union Contract LSHM-CT-2003-503254, Regione Piemonte [Ricerca Finalizzata 2006, Ricerca Scientifica Applicata 2004, (D10, A150, A17); Ricerca Industriale e Competitiva 2006, Grant PRESTO; Ricerca Tecnologie Convergenti 2007, Grant PHOENICS; Piattaforme Tecnologiche per le Biotecnologie, Grant Druidi], Fondazione CRT-Torino, and Ministero della Salute (Programma Ricerca Oncologica 2006, Ricerca Finalizzata 2006).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition footnote: The following Gallus gallus coding sequences were deposited in a NCBI Gene Bank: neurexin 1β precursor (EU702427), neurexin 3α (EU702428), neurexin 3β precursor (EU702430), and neurexin 3β arterial isoform (EU702429).
This article contains supporting information online at www.pnas.org/cgi/content/full/0809510106/DCSupplemental.
References
- 1.Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: Insight into the mechanism of alternative splicing. Genomics. 2002;79(6):849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- 2.Bolliger MF, Frei K, Winterhalter KH, Gloor SM. Identification of a novel neuroligin in humans which binds to PSD-95 and has a widespread expression. Biochem J. 2001;356(Pt 2):581–588. doi: 10.1042/0264-6021:3560581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rissone A, et al. Comparative genome analysis of the neurexin gene family in Danio rerio: Insights into their functions and evolution. Mol Biol Evol. 2007;24(1):236–252. doi: 10.1093/molbev/msl147. [DOI] [PubMed] [Google Scholar]
- 4.Sudhof TC. α-Latrotoxin and its receptors: Neurexins and CIRL/låtrophilins. Annu Rev Neurosci. 2001;24:933–962. doi: 10.1146/annurev.neuro.24.1.933. [DOI] [PubMed] [Google Scholar]
- 5.Song JY, Ichtchenko K, Sudhof TC, Brose N. neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA. 1999;96(3):1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem. 1997;272(41):26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- 7.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101(6):657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 8.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119(7):1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Missler M, et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423(6943):939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 10.Varoqueaux F, et al. neuroligins determine synapse maturation and function. Neuron. 2006;51(6):741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Piechotta K, Dudanova I, Missler M. The resilient synapse: Insights from genetic interference of synaptic cell adhesion molecules. Cell Tissue Res. 2006;326(2):617–642. doi: 10.1007/s00441-006-0267-4. [DOI] [PubMed] [Google Scholar]
- 12.Drake CJ, Hungerford JE, Little CD. Morphogenesis of the first blood vessels. Ann N Y Acad Sci. 1998;857:155–179. doi: 10.1111/j.1749-6632.1998.tb10115.x. [DOI] [PubMed] [Google Scholar]
- 13.Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997;22(7):251–256. doi: 10.1016/s0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- 14.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(5):812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmeliet P. Blood vessels and nerves: Common signals, pathways and diseases. Nature Reviews Genetics. 2003;4(9):710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- 16.Ushkaryov YA, et al. Conserved domain structure of beta-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. J Biol Chem. 1994;269(16):11987–11992. [PubMed] [Google Scholar]
- 17.Craig AM, Kang Y. neurexin–neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17(1):43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63(1):115–122. [PubMed] [Google Scholar]
- 19.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50(3):717–721. [PubMed] [Google Scholar]
- 20.Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9(6):661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 21.Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190(3):310–329. doi: 10.1002/(SICI)1096-9896(200002)190:3<310::AID-PATH525>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Girard H. Arterial pressure in the chick embryo. Am J Physiol. 1973;224(2):454–460. doi: 10.1152/ajplegacy.1973.224.2.454. [DOI] [PubMed] [Google Scholar]
- 23.Langille BL. Remodeling of developing and mature arteries: Endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol. 1993;21(Suppl 1):S11–S17. doi: 10.1097/00005344-199321001-00003. [DOI] [PubMed] [Google Scholar]
- 24.Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: Its emergence, its characteristics, and its significance. Dev Dyn. 2004;231(3):474–488. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130(4):691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suckow AT, et al. Expression of neurexin, neuroligin, and their cytoplasmic binding partners in the pancreatic beta-cells and the involvement of neuroligin in insulin secretion. Endocrinology. 2008;149(12):6006–6017. doi: 10.1210/en.2008-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 28.Sugita S, et al. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154(2):435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neufeld G, Kessler O, Herzog Y. The interaction of neuropilin-1 and neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol. 2002;515:81–90. doi: 10.1007/978-1-4615-0119-0_7. [DOI] [PubMed] [Google Scholar]
- 30.Wingerd KL, et al. Alpha 4 integrins and vascular cell adhesion molecule-1 play a role in sympathetic innervation of the heart. J Neurosci. 2002;22(24):10772–10780. doi: 10.1523/JNEUROSCI.22-24-10772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: Angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 32.Van der Borght K, et al. Physical exercise leads to rapid adaptations in hippocampal vasculature: Temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19(10):928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- 33.Haberman RP, Lee HJ, Colantuoni C, Koh MT, Gallagher M. Rapid encoding of new information alters the profile of plasticity-related mRNA transcripts in the hippocampal CA3 region. Proc Natl Acad Sci USA. 2008;105(30):10601–10606. doi: 10.1073/pnas.0804292105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam CI, Chen L. Postsynaptic assembly induced by neurexin–neuroligin interaction and neurotransmitter. Proc Natl Acad Sci USA. 2005;102(17):6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knappik A, et al. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol. 2000;296(1):57–86. doi: 10.1006/jmbi.1999.3444. [DOI] [PubMed] [Google Scholar]
- 36.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.