Abstract
The ubiquitin-conjugating enzyme Ubc13 mediates lysine-63-specific protein ubiquitination involved in signal transduction by immune receptors; however, the in vivo physiological functions of Ubc13 remain incompletely understood. Using Ubc13 conditional knockout mice, we show that somatic deletion of the Ubc13 gene causes severe loss of multi lineages of immune cells, which is associated with profound atrophy of the thymus and bone marrow, as well as lethality of the mice. Ubc13 has a cell-intrinsic function in mediating hematopoiesis and is essential for the survival and accumulation of hematopoietic stem cells in the bone marrow. Interestingly, loss of Ubc13 results in accumulation of β-catenin and hyperexpression of Wnt target genes, a condition known to cause impaired hematopoiesis. These results establish Ubc13 as a crucial regulator of hematopoiesis and suggest a role for Ubc13 in the control of Wnt signaling in hematopoietic stem cells.
Keywords: inducible knockout, ubiquitination, Wnt signaling
Mature blood cells have a relatively short life span and thus require continuous replenishment for the maintenance of their steady levels in an adult animal (1). Blood cell regeneration is mediated by hematopoiesis, an orchestrated process that produces all lineages of blood cells from the pluripotent hematopoietic stem cells (HSCs) (1). In adult mammals, HSCs reside in the bone marrow, where they undergo proliferative renewal and differentiation to produce lineage-specific progenitor cells (2), including common lymphoid progenitors (CLPs), common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte-erythroid progenitors (MEPs) (3). The lineage commitment of the HSCs and the subsequent lineage-specific expansion are subject to tight regulations, since deregulation of hematopoiesis can cause severe diseases, such as anemia and blood malignancies.
A large number of transcription factors have been implicated in the regulation of different stages of hematopoiesis, although the signaling mechanism that controls the hematopoietic transcription network is poorly understood. Nevertheless, genetic evidence suggests an important role for the Wnt signaling pathway in the regulation of early stages of hematopoiesis (3, 4). A central step of the canonical Wnt signaling pathway is stabilization of β-catenin (5). In the absence of Wnt signaling, β-catenin is constantly phosphorylated by a kinase, GSK-3β, and targeted for degradation by the proteasome. Binding of Wnt proteins to their receptors causes inactivation of GSK-3β and stabilization of β-catenin. The β-catenin then forms a complex with T cell factor (TCF) family of transcription factors, TCF-1 and Lef-1, thereby inducing the transcription of a set of Wnt-target genes (5). Whereas, the Wnt signaling is important for specific steps of hematopoiesis and thymocyte development, this pathway is subject to tight control, since its deregulation impairs hematopoiesis at the stage of HSCs (6, 7). Although the mechanism of Wnt regulation remains largely unclear, a recent study suggests the involvement of lysine (K) 63 type of protein ubiquitination in the negative control of Wnt signaling (8).
Protein ubiquitination is an emerging mechanism that regulates signal transduction in different biological processes (9, 10). Although traditionally viewed as a mechanism that targets proteins for degradation in the proteasome, ubiquitination is now known to also mediate various nondegradative functions, including signal transduction (9–11). Polyubiquitin chains are formed via linkage of the C-terminal glycine residue of one ubiquitin with an internal K residue of another ubiquitin, and the ubiquitin chains formed with different internal K residues may mediate distinct functions. In particular, K63-linked ubiquitin chains play an important role in mediating signal transduction (9). A ubiquitin-conjugating enzyme (E2), Ubc13, specifically catalyzes K63-linked ubiquitin chains when forming a dimeric complex with a noncanonical E2, Uve1A (MMS2 in yeast) (12, 13). RNA interference (RNAi) assays suggests a critical role of Ubc13 in the regulation of signal transduction mediating activation of NF-κB (14–16), a transcription factor involved in the activation of lymphocytes and innate immune cells (17, 18). NF-κB is also involved in the development of specific lineages of blood cells, although it appears to be dispensable for the early stage of hematopoiesis involving HSCs (19–22).
Recent studies demonstrate that conditional knockout of Ubc13 in lymphocytes attenuates NF-κB activation by antigen receptors (23, 24). Ubc13 appears to also regulate activation of NF-κB and MAP kinase signaling pathway in innate immune cells by the Toll-like receptors (23, 25). On the other hand, Ubc13 is largely dispensable for the development of T cells and the follicular B cells, although it regulates the formation of marginal zone B cells (23, 24). However, due to the embryonic lethality of the Ubc13 conventional knockout mice (23), the role of Ubc13 in early stages of immune cell development and hematopoiesis has not been investigated. In the present study, we investigated the function of Ubc13 using a conditional knockout mouse model that allows inducible knockout of Ubc13 in adult mice. We show that Ubc13 has a pivotal role in regulating hematopoiesis. The Ubc13 deficiency causes drastic loss of multi lineages of blood cells, as well as atrophy of the thymus and bone marrow. Interestingly, the Ubc13 deficiency results in accumulation of β-catenin and aberrant expression of several Wnt-target genes. These findings not only establish Ubc13 as a pivotal regulator of hematopoiesis but also suggest a signaling role for Ubc13 in the negative regulation of Wnt pathway.
Results
Conditional Knockout of Ubc13 in Adult Mice Leads To Blood Cell Deficiency and Lethality.
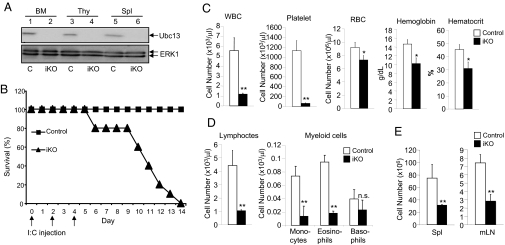
Since Ubc13 knockout mice are embryonic lethal, we used a conditional knockout strategy to study the role of Ubc13 in early stages of hematopoiesis. We crossed the Ubc13 floxed (Ubc13fl/fl) mice with Mx1-Cre mice, in which the Cre gene is under the control of the type I IFN (IFN)-inducible Mx promoter. To induce Cre-mediated Ubc13 somatic knockout, we injected the Ubc13f/f MxCre+ or control Ubc13+/fMx1-Cre+ mice with an IFN inducer, polyinosinic-polycytidylic acid [poly(I:C)]. The poly(I:C)-injected Ubc13f/f Mx1-Cre+ (hereafter called Ubc13 inducible KO, or iKO) mice, but not poly(I:C)-induced Ubc13+/flMx1-Cre+ (hereafter called control) mice had nearly complete loss of Ubc13 in the bone marrow cells and thymocytes (Fig. 1A). As expected from many other studies, the control mice were tolerant to the poly(I:C) injection and did not display any visible abnormalities (Fig. 1B). In contrast, the Ubc13 iKO mice all became moribund or died within 2 weeks of the initial poly(I:C) injection (Fig. 1B).
Fig. 1.
Mx1-Cre-induced Ubc13 deletion in mice results in blood cell deficiency and lethality. Control and Ubc13 iKO mice were prepared by poly(I:C) injection, as described in Materials and Methods. Unless specified, mice were used for experiments 9 days after the initial poly(I:C) injection in this and all of the subsequent figures. (A) Immunoblotting (IB) assays to detect the Ubc13 protein expression in the bone marrow (BM) cells, thymocytes (Thy), and splenocytes (Spl) of control (C) and iKO mice. (B) Survival curves of control (n = 10) and iKO (n = 10) mice with the three poly(I:C) injection dates indicated. (C) Whole-blood counts of control and iKO mice (n = 3) showing numbers of white blood cell (WBC), platelet, and red blood cell (RBC), concentrations of hemoglobin, and percentage of hematocrit. (D) Blood samples from C were subjected to blood count analysis to determine the number of lymphocytes and the indicated subsets of myeloid cells. (E) Total cell numbers in spleen (Spl) and mesenteric lymph node (mLN) from control and iKO mice (n = 3 each). Data in C–E are presented as mean ± SD. *, P < 0.05; **, P < 0.01; n.s. is nonsignificant.
In searching for potential causes of the lethality associated with Ubc13 knockout, we noticed that peripheral blood of the Ubc13 iKO mice had greatly reduced cellularity. Cytological analyses revealed a striking reduction in the number of white blood cells (WBC) and platelets in the Ubc13 conditional knockout mice (Fig. 1C). The Ubc13 deficiency also caused a partial loss of red blood cells (RBC), which was associated with reduced hemoglobin concentration and hematocrit (Fig. 1C). We next examined the effect of Ubc13 iKO on different WBC populations. Both lymphocytes and the myeloid cell subsets were severely affected by this genetic deficiency (Fig. 1D). The number of WBCs in secondary lymphoid organs, including spleen and mesenteric lymph nodes (mLN), was also drastically reduced (Fig. 1E). Thus, loss of Ubc13 in adult mice causes hematopoietic failure and lethality.
Ubc13 Deficiency Causes Severe Thymic Atrophy and an Arrest of Thymocyte Development at the Most Immature Stage.
To further understand the mechanism by which Ubc13 regulates the production of mature blood cells, we examined the effect of inducible Ubc13 KO on the development of immune cells in primary lymphoid organs. Compared with the control mice, the Ubc13 iKO mice had a strikingly smaller thymus (Fig. S1A), the primary lymphoid organ that produces T lymphocytes. Histological analysis revealed greatly reduced density of thymocytes in the H & E stained thymic sections of the iKO mice (Fig. S1B). Indeed, the total thymocyte number of the iKO mice was markedly lower than the control mice (Fig. S1C).
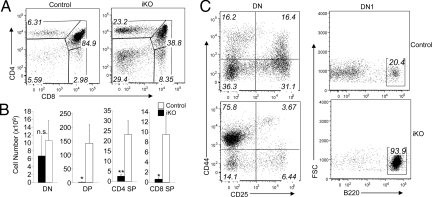
Thymocyte development initiates from bone marrow-derived T cell progenitors and progress through different stages, which can be defined based on surface expression of the T cell coreceptors, CD4 and CD8 (26). The initial stage includes CD4−CD8− double-negative (DN) thymocytes, which undergo TCR gene rearrangement and become CD4+CD8+ double positive (DP) thymocytes. The DP cells then undergo positive and negative selections and eventually become the mature thymocytes that express either CD4 (CD4+ single positive, or SP, cells) or CD8 (CD8+ SP cells). Despite their severe thymic atrophy, the Ubc13 iKO mice were competent in generating the mature SP thymocytes, as well as the immature DN and DP thymocytes (Fig. 2A). This result was consistent with our prior study using Lck-Cre-mediated T cell conditional Ubc13 KO mice (24). However, the loss of Ubc13 appeared to attenuate thymocyte development at an early stage, since the DN population was relatively accumulated in the Ubc13 iKO mice (Fig. 2B). The DN cells are further divided into four different developmental stages, DN1–DN4, defined based on their surface expression of CD44 and CD25 markers (27). The Ubc13 iKO mice had a great reduction in the DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) thymocytes with concurrent increase in the most immature DN1 (CD44+CD25−) thymocytes (Fig. 2C). These results thus suggest an arrest of the thymocyte development at the DN1 stage in Ubc13 iKO mice.
Fig. 2.
Thymic atrophy and DN1 arrest in Ubc13 iKO mice. Control and Ubc13 iKO mice were prepared as described in Materials and Methods. (A) Flow cytometry of thymocytes from control and iKO mice to determine the percentage of CD4+ and CD8+ SP cells, CD4+CD8+ DP cells, and CD4−CD8− DN cells within the total thymocyte population. (B) Absolute numbers of the indicated subpopulations of thymocytes were calculated based on flow cytometry and total thymocyte number counts and presented as mean ± SD (n = 3 each). (C) Flow cytometry to determine the percentage of different stages of DN cells, DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) (left). The DN1 population was further analyzed based on expression of IgM and B220 (gated on IgM negative DN1 cells) (right).
DN1 thymocytes form a heterogeneous population of cells that contains progenitors with both T- and nonT-lineage potentials (28, 29). Although the signaling mechanism that governs T-lineage commitment in the thymus remains incompletely understood, previous work suggests the requirement of specific transcription factors. For example, genetic deficiency of the transcription factor Notch1 abrogates T cell development and promotes the development of B cells in the thymus (30). We noted that whereas the control DN1 cells contained both CD44-high and CD44-intermediate cell populations, the Ubc13 iKO DN1 cells were almost exclusively CD44-intermediate (Fig. 2C, left panels). To examine the nature of the DN1 cells accumulated in the Ubc13 iKO mice, we examined their surface markers by flow cytometry. Interestingly, more than 90% of the Ubc13 iKO DN1 cells displayed the B-cell marker B220, whereas only about 20% of the control DN1 cells were B220 positive (Fig. 2C, right panels). These results suggest that Ubc13 has a role in the control of an initial step of T cell development, which may partially contribute to the thymic atrophy of the Ubc13 iKO mice.
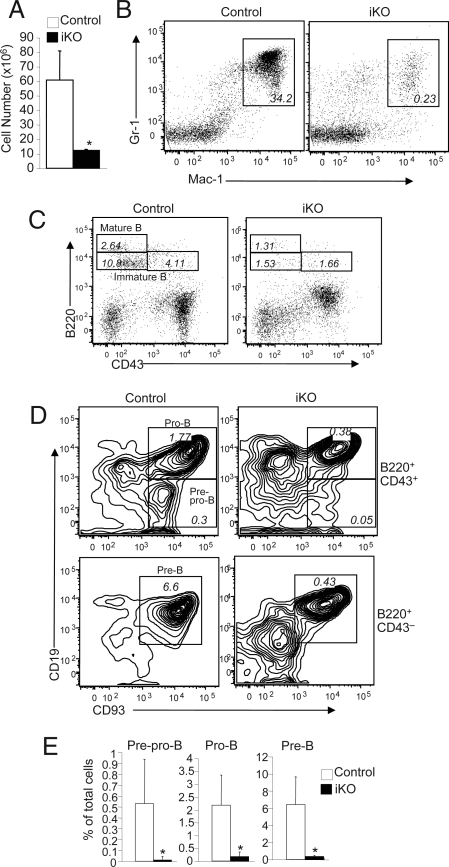
Ubc13 iKO Mice Display Bone Marrow Atrophy and Deficiency in Myeloid and B Cells.
Bone marrow is the primary lymphoid organ that produces multiple lineages of blood cells. Because of the severe deficiency of Ubc13 iKO mice in mature blood cells and the thymic atrophy, we examined the effect of Ubc13 deficiency on bone marrow function. Compared with the control femurs, the Ubc13 iKO femurs were considerably darker, indicating loss of bone marrow cells (Fig. S2A). Indeed, the bone marrow of Ubc13 iKO mice had a drastic reduction in the number of nucleated cells (Fig. 3A). The loss of bone marrow cells in Ubc13 iKO mice was further demonstrated by histological analysis of bone sections (Fig. S2B). Flow cytometry analysis revealed a remarkable reduction in the frequency of myeloid cells with Gr-1+Mac-1+ surface markers (Fig. 3B). The frequency of mature B cells (B220hiCD43−), as well as early stages of immature B cells (B220+CD43− and B220+CD43+) was also reduced (Fig. 3C). More detailed flow cytometry analysis demonstrated severe reduction in the frequency of several immature B-cell populations, including prepro-B, pro-B, and pre-B cells (Fig. 3D). These results were striking, considering that the total number of bone marrow cells was also greatly reduced in the iKO mice (Fig. 3A). Indeed, when calculated into absolute numbers, all of the developing stages of B cells displayed marked deficiency in the bone marrow of iKO mice (Fig. 3E). Thus, although loss of Ubc13 does not block the bone marrow development of B cells (23), it causes an overall reduction of B-lineage cells, as well as severe loss of myeloid cells. These findings suggested the possible involvement of Ubc13 in early stages of hematopoiesis.
Fig. 3.
Bone marrow atrophy and loss of myeloid cells and B cells in Ubc13 iKO mice. Control and Ubc13 iKO mice were prepared as described in Materials and Methods. (A) Total bone marrow cell counts from age-matched control and Ubc13 iKO mice [9 days after poly(I:C) injection]. Data are mean ± SD (right) of three mice. (B) Flow cytometry analysis of Gr-1 and Mac-1 expression on bone marrow cells. The percentages of Gr-1+Mac-1+ myeloid cells are indicated. (C) Flow cytometry analysis of B220 and CD43 expression on total bone marrow cells showing the frequency of mature B cells and two populations of immature B cells. (D) The gated CD43− and CD43+ populations of immature B cells were further analyzed based on their expression of CD19 and CD93. Numbers indicate the percentage of prepro-B (B220+CD43+CD93+CD19−), pro-B (B220+CD43+CD93+CD19+), and pre-B (B220+CD43−CD93+CD19+) in total bone marrow cells of the control and iKO mice. (E) Summary of the flow cytometry results from E, showing average percentages (mean ± SD) of the indicated subsets of B cells from three control and three Ubc13 iKO mice. *, P < 0.05.
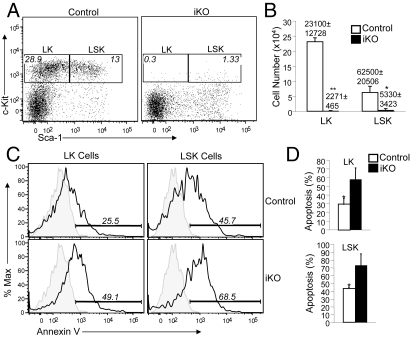
Severe Loss of Bone Marrow Progenitor Cells in Ubc13 iKO Mice.
The results discussed above suggest defects in early steps of hematopoiesis. Since all lineages of blood cells are derived from bone marrow progenitor cells in adult mice, we examined whether loss of Ubc13 affected the bone marrow progenitors. Two major populations of progenitor cells, LSK and LK, could be detected by flow cytometry based on their expression of IL-7 receptor alpha chain (IL-7Rα), lineage markers (Lin), stem cell antigen-1 (Sca-1), and the receptor tyrosine kinase c-Kit (31). The LSK population (IL-7Rα−Lin−Sca-1+c-Kit+) contains the multipotent HSCs that can further produce lineage-specific progenitors, whereas the LK population (IL-7Rα−Lin−Sca-1−c-Kit+) contains common myeloid progenitors (CMP). Compared to the control mice, the Ubc13 iKO mice had drastically reduced frequency of both LSK and LK cells (Fig. 4A). The reduction in the absolute number of these progenitor cell populations was even more striking in the iKO mice (Fig. 4B) because of their great reduction in total number of bone marrow cells (Fig. 3A).
Fig. 4.
Ablation of bone marrow progenitor cells by inducible deletion of Ubc13. Control and Ubc13 iKO mice were prepared as described in Materials and Methods. (A) Flow cytometry analysis of c-Kit and Sca-1 expression on gated Lin−IL7Ra− bone marrow cells. Numbers indicate percentage of LK (Lin−Sca-1−c-Kit+) and LSK (Lin−Sca-1+c-Kit+) cells within total bone marrow cells. Data are representative of three control and three iKO mice. (B) Absolute numbers of LK and LSK cells from the bone (femurs and tibia) of control and Ubc13 iKO mice. Data are mean ± SD of three mice of each genotypes. (C) Bone marrow cells of control and iKO mice were stained with annexin V as well as the progenitor-detection antibodies used in A. Apoptosis (annexin V staining) was analyzed in gated LK and LSK cells. Shaded area indicates background staining. Percentages of annexin V positive cells are indicated. (D) Summary of data from C. Data are mean ± SD of three control and three iKO mice. *, P < 0.05; **, P < 0.01.
To understand the mechanism by which Ubc13 regulates the homeostasis of bone marrow progenitor cells, we analyzed the apoptosis of LK and LSK cells derived from control and Ubc13 iKO mice. Although apoptotic cells were detected from both the control and iKO progenitors, the frequency of the iKO apoptotic cells was markedly higher (Fig. 4 C and D). Considering the rapid clearance of apoptotic cells in vivo, this result was remarkable. Thus, Ubc13 is important for the survival of bone marrow progenitor cells.
Cell Intrinsic Function of Ubc13 in Hematopoiesis.
Hematopoiesis requires both hematopoietic cells and bone marrow microenvironment (32). To examine whether the function of Ubc13 in hematopoiesis is intrinsic to hematopoietic cells, we performed mixed bone marrow adoptive transfer studies. Briefly, bone marrow cells from Ubc13+/+ (with CD45.1 congenital marker) and Mx1-Cre+Ubc13fl/fl (with CD45.2 congenital marker) mice were mixed at 1:1 ratio and adoptively transferred into lethally irradiated Ubc13+/+ recipient mice (CD45.1+CD45.2+). In contrast to the Ubc13 iKO mice, the poly(I:C)-treated bone marrow chimeras did not show reduction in the frequency of Gr-1+Mac-1+ myeloid cells (Fig. S3A, left panels). However, analysis of the CD45 congenital markers revealed a nearly complete loss of the CD45.2+ (iKO) cells within the myeloid cell population of the poly(I:C)-treated chimeric mice, which was associated with the concurrent increase in the frequency of CD45.1 (Ubc13+/+) cells (Fig. S3A, right panels). Since the Ubc13+/+ control cells and the Mx1-Cre+Ubc13fl/fl cells were treated in the same recipient mice, this result suggested a cell-intrinsic role for Ubc13 in regulating the generation of myeloid lineage of cells.
Using the same approach, we analyzed the production of B cells and progenitor cells. As seen with the myeloid cells, poly(I:C) treatment of the bone marrow chimeric mice led to selective loss of the CD45.2+ (iKO) cells and the concurrent increase in the CD45.1+ (control) cells within the B220+ cell population (Fig. S3B). A similar result was obtained in the analysis of the LK and LSK progenitor cells (Fig. S3C). Although poly(I:C) treatment did not interfere with the overall production of LK and LSK cells (Fig. S3C, left panels), these progenitors contained exclusively the CD45.1 control cells and no CD45.2 Ubc13 iKO cells (Fig. S3C, right two panels). These data further demonstrated a cell-intrinsic role for Ubc13 in the regulation of hematopoiesis.
To examine whether Ubc13 also plays a role in stromal function in hematopoiesis and thymocyte development, we performed reciprocal bone marrow adoptive transfer assays. As expected from the mixed bone marrow transfer experiment, transfer of iKO bone marrow to control recipients led to severe defect in both thymocyte development (Fig. S4A, left panel) and generation of bone marrow progenitors (Fig. S4B, left panel). On the other hand, transfer of control bone marrow to iKO recipients resulted in competent development of thymocytes and bone marrow progenitor cells (Fig. S4, right panels). Compared to the regular control mice (Fig. 2A), the iKO recipients of control bone marrow cells had a slight reduction in the frequency of CD4 SP thymocytes (Fig. S4A, right panel). Whether this represents variations between nontransferred and transferred mice or a stromal function of Ubc13 requires additional studies. Nevertheless, these results suggest that the function of Ubc13 in hematopoiesis and early stages of thymocyte development is predominantly cell-intrinsic.
Deregulated Wnt Signaling in Ubc13 iKO Cells.
To investigate the mechanism by which Ubc13 regulates hematopoiesis, we examined the effect of Ubc13 deficiency on Wnt signaling, which is known to be crucial for early steps of hematopoiesis although controversies exist regarding the underlying mechanism (3, 6, 7). Remarkably, gene expression analyses revealed that the Ubc13 iKO bone marrow cells had elevated expression of several Wnt target genes, including Axin2, Ccnd1, Lef1, and Tcf1 (Fig. 5A). Consistently, the steady level of β-catenin was strongly enhanced in the iKO bone marrow cells, as well as in thymocytes and splenocytes (Fig. 5B). To further confirm this finding, we performed flow cytometry assays to measure the level of β-catenin in different populations of bone marrow cells of control and iKO mice. Consistent with the IB assays, flow cytometry revealed increased levels of β-catenin in the total bone marrow cells (Fig. 5C). Moreover, the increased β-catenin expression was also detected in the Lin+ and Lin− cell populations as well as in the LK and LSK progenitor cells. On the other hand, the loss of Ubc13 only had a moderate effect on β-catentin expression in B- and myeloid-lineage of cells. Consistently, the elevated expression of Wnt target genes was drastic in the progenitor cells and moderate in the B- and myeloid-lineage cells (Fig. S5). Parallel studies revealed that poly(I:C) did not alter the expression level of β-catenin in control mice (Fig. S6), thus suggesting a specific role for Ubc13 in regulating the steady level of β-catenin and Wnt gene expression. These results thus uncovered an unexpected function of the Ubc13 in the negative regulation of Wnt signaling. Given that aberrant Wnt signaling causes hematopoietic defect (6, 7), these findings provide important insight into the mechanism by which Ubc13 regulates hematopoiesis.
Fig. 5.
Elevated expression of Wnt target genes and β-catenin protein in Ubc13 iKO bone marrow cells. Control and Ubc13 iKO mice were prepared as described in Materials and Methods. (A) RNA was isolated from total bone marrow cells of control and Ubc13 iKO mice (three of each) and subjected to real-time RT-PCR analysis to determine the relative mRNA level of the indicated Wnt target genes. Data are presented as fold relative to the control samples (arbitrarily set to 1). (B) Whole-cell lysates were from prepared from bone marrow cells (BM), thymocytes (Thy), or splenocytes (Spl) and subjected to IB to detect the protein level of β-catenin or loading control ERK1. (C) Flow cytometry of intracellular staining for β-catenin in bone marrow cells. β-catenin levels in total, lineage positive (Lin+), lineage negative (Lin−), B220 positive (B220+), LK (Lin−Sca-1−c-Kit+), LSK (Lin−Sca-1+c-Kit+), and myeloid (Gr-1+Mac-1+) cells are shown.
Discussion
K63 type of protein ubiquitination has been implicated in the regulation of signal transduction by immune receptors, but the in vivo biological function of this nondegradative type of ubiquitination is still quite unclear. Since Ubc13 is a K63-specific ubiquitin-conjugating enzyme, the Ubc13 KO mice provide a powerful tool for the study of K63 ubiquitination. In the present study, we have used an inducible knockout mouse model that allows Ubc13 knockout in adult mice. We crossed the Ubc13fl/fl mice with Mx1-Cre mice and generated Ubc13 inducible knockout (iKO) mice by inducing the cre-mediated Ubc13 gene disruption by poly(I:C) injection. Using this approach, we have discovered a crucial role for Ubc13 in regulating hematopoiesis. The Ubc13 iKO mice have a drastic reduction in multi lineages of blood cells, which is associated with severe loss of bone marrow progenitor populations. Our bone marrow adoptive transfer experiments suggest that the role of Ubc13 in hematopoiesis is cell intrinsic. Thus, these findings suggest that K63 ubiquitination has an indispensable role in the early steps of hematopoiesis.
Our previous study using T cell conditional (Lck-cre) Ubc13 KO mice suggests that Ubc13 is dispensable for the development of thymocytes (24). Consistently, the Ubc13 iKO mice are competent in producing mature thymocytes, as well as the DN and DP immature thymotye populations. However, the Ubc13 iKO mice display a defect in the most immature stage (DN1) that is apparently bypassed by the Lck-cre mediated Ubc13 gene inactivation, since productive Lck-cre expression does not occur until DN3 stage of thymocyte development (33). The DN1 population is accumulated in the Ubc13 iKO mice, which is associated with reduced frequency of DP thymocytes. Moreover, the Ubc13 iKO mice have severe thymic atrophy, affecting mostly the DP and SP cells. It is likely that early-stage defect of thymocyte development, together with the severe loss of bone marrow progenitor cells, contributes to the thymic atrophy of the Ubc13 iKO mice.
The DN1 stage of thymocytes is known to be heterogeneous and has the ability to produce not only T cells but also other lineages of cells, including B cells, within the thymus (28). Under normal situations, the CLPs present in DN1 cells predominantly develop into T cells. The molecular mechanism regulating the T cell fate specification has not been well defined, but the Notch signaling pathway is known to play a critical role. Inducible knockout of Notch1 using the Mx1-Cre strategy causes a defect in T cell fate specification, and the Notch1-deficient T cell precursors adopt a default cell fate and develop into B cells (30, 34). Like the Ubc13 iKO mice, the Notch1 iKO mice have severe thymic atrophy. Interestingly, the DN1 cells of Ubc13 iKO mice also contain mostly B220+ B cells, suggesting the involvement of K63 ubiquitination in the control of T cell fate specification. Future studies will examine whether Ubc13 has a role in Notch signaling.
Whereas the early-stage defect of thymocyte development at least partially contributes to the T cell deficiency in Ubc13 iKO mice, the hematopoiesis failure of these mutant animals is clearly responsible for the severe loss of multi lineages of blood cells. The bone marrow of Ubc13 iKO mice has a drastic reduction in the number of B- and myeloid-lineage of cells, which is apparently due to the loss of progenitor cells. Precisely how Ubc13 regulates hematopoiesis needs to be further investigated. Ubc13 has been implicated in the regulation of NF-κB activation in mature T and B cells. However, since the Ubc13 iKO mice contain extremely low numbers of bone marrow progenitor cells, it is technically difficult to analyze NF-κB activation in these cells. The development of a flow cytometric method of IKK/NF-κB detection is essential for such studies. Nevertheless, it is unlikely that the hematopoiesis failure of Ubc13 iKO mice is mainly caused by NF-κB deficiency, since loss of the prototypical NF-κB member RelA or combined knockout of RelA and c-Rel, does not lead to defect in pluripotent HSCs or global loss of different lineages of blood cells (19, 20). In fact, the RelA/c-Rel combined deficiency causes a dramatic expansion of granulocytes (granulocytosis) in both the bone marrow and spleen (20). Thus, although NF-κB activation may contribute to the function of Ubc13 in regulating hematopoiesis, Ubc13 likely has additional targets in bone marrow progenitor cells.
Our data suggest that Ubc13 has a role in negatively regulating Wnt signaling. The Ubc13 deficiency causes a striking increase in the expression of several Wnt target genes, which is associated with upregulated steady level of the key Wnt signaling component β-catenin. The elevated expression of β-catenin is seen in both total bone marrow cells and bone marrow progenitor cells. Notably, deregulated Wnt signaling is known to cause hematopoiesis deficiency, although the precise underlying mechanism is incompletely understood (6, 7). It is currently unclear how Ubc13 regulates Wnt signaling. Nevertheless, K63-type of protein ubiquitination has recently been shown to negatively regulate Wnt signaling (8). A K63-specific deubiquitinase, Trabid, binds to and deubiquitinates APC (adenomatous polyposis coli), a tumor suppressor that mediates β-catenin degradation and functional inactivation (35). Future studies will examine whether the K63-linked ubiquitination of APC requires Ubc13.
Materials and Methods
Ubc13 Conditional Knockout Mice.
Ubc13fl/fl mice were generated using the loxP targeting system as previously described (23). The Ubc13fl/fl mice were crossed with Mx1-Cre transgenic mice (Jackson Laboratory) to obtain Ubc13+/flMx1-Cre+ and Ubc13fl/flMx1-Cre+ mice. To induce the deletion of floxed Ubc13 gene allele, 4-week-old mice were injected i.p. with poly(I:C) (GE Healthcare; 250 μg/mouse in PBS) for three consecutive times at 2-day intervals. At day 9 following the initial injection, the mice were used for hematopoiesis studies. At this time, Ubc13 expression is undetectable in bone marrow cells of the poly(I:C)-injected Ubc13fl/flMx1-Cre+ mice, which are designated as Ubc13 inducible knockout (iKO) mice. In contrast, the poly(I:C)-injected Ubc13+/flMx1-Cre+ mice, designated as control mice, express abundant Ubc13 in the bone marrow cells. In some experiments, Ubc13fl/flMx1-Cre− mice were used as control, which gave similar phenotypes to the Ubc13+/flMx1-Cre+ mice. All animal experiments were in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
Complete Blood Count (CBC) Test.
CBC test was performed with the help of the Department of Veterinary Medicine of MD Anderson Cancer Center. Whole blood samples from control and Ubc13 iKO mice were subjected to the CBC test using an Advia 120 hematology analyzer (Siemens Diagnostic), and the reading was confirmed by blood smear review using microscope.
Histology.
Freshly isolated thymi and legs (femurs and tibias) from killed mice were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned for H&E staining in the Department of Immunology Histology Core of MD Anderson Cancer Center. Slides were analyzed under a microscope (Olympus BX 41), and pictures were taken from typical sections.
Bone Marrow Adoptive Transfer.
Ubc13+/+ mice (CD45.1+) were bred to B6.SJL mice (CD45.2+) for one generation to obtain Ubc13+/+ recipient mice (CD45.1+CD45.2+), which were lethally irradiated (1,100 rads) with a field flattener in a Nordion International GammaCell 220s irradiator (MDS Nordion). Twelve hours later, irradiated mice were injected intravenously with a mixture containing equal proportions (2 million cells each) of Ubc13+/+ (CD45.1+CD45.2−) and Ubc13fl/flMx1-Cre+ (CD45.1−CD45.2+) bone marrow cells. After 6 weeks, reconstitution efficiency was analyzed by flow cytometry based on the expression of CD45.1 and CD45.2 congenital markers on nucleated cells purified from peripheral blood. The recipient mice were subsequently subjected to poly(I:C) injection to induce Ubc13 gene deletion as described above.
Real-Time Quantitative RT-PCR.
Total RNA was isolated from bone marrow cells using TRI reagent (Molecular Research Center, Inc.) and subjected to cDNA synthesis using MMLV reverse transcriptase (Invitrogen) and oligo (dT) primers. Real-time quantitative PCR was performed using iCycler Sequence Detection System (Bio-Rad) and iQTM SYBR Green Supermix (Bio-Rad). Expression of individual genes was calculated by a standard curve method and normalized to the expression of GAPDH. Sequences of the GAPDH primers were: 5′-CTC ATG ACC ACA GTC CAT GCC ATC-3′ and 5′-CTG CTT CAC CAC CTT CTT GAT GTC-3′, and the other gene-specific primer sets were as described (6).
Statistical Analyses.
Two-tailed unpaired t-test statistical analysis was performed using the Microsoft Excel software. P value <0.05 and <0.01 means significant and very significant, respectively.
See SI Materials and Methods for information about antibodies, reagents, plasmids, flow cytometry, and cell sorting.
Supplementary Material
Acknowledgments.
We thank Drs. Huiyuan Zhang and Stephanie Watowich for valuable suggestions and personnel from the Department of Immunology Histology Core Facility (Yihong Wang and Pam Grant), Department of Veterinary Medicine, and the flow cytometry core facility (Karen Martinez, David He, and Amy Cortez) of MD Anderson Cancer Center for technical assistance. This study was supported by National Institutes of Health Grants AI064639 and GM084459 (to S.-C.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906547106/DCSupplemental.
References
- 1.Orkin SH. Diversification of haematopoietic stem cells to specific lineages. Nat Rev Genet. 2000;1:57–64. doi: 10.1038/35049577. [DOI] [PubMed] [Google Scholar]
- 2.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 3.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 4.Staal FJ, Clevers HC. WNT signalling and haematopoiesis: A WNT-WNT situation. Nat Rev Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 5.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 6.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 7.Scheller M, et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 8.Tran H, Hamada F, Schwarz-Romond T, Bienz M. Trabid, a new positive regulator of Wnt-induced transcription with preference for binding and cleaving K63-linked ubiquitin chains. Genes Dev. 2008;22:528–542. doi: 10.1101/gad.463208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 10.Liu YC, Penninger J, Karin M. Immunity by ubiquitylation: A reversible process of modification. Nat Rev Immunol. 2005;5:941–952. doi: 10.1038/nri1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell. 1999;96:645–653. doi: 10.1016/s0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- 13.Deng L, et al. Activation of the IkB kinase complex by TRAF6 requires a dimeric ubiqutitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Deng L, Ea C-K, Xia Z-P, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, et al. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 16.Andersen PL, et al. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. J Cell Biol. 2005;170:745–755. doi: 10.1083/jcb.200502113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 19.Alcamo E, et al. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-kappa B in leukocyte recruitment. J Immunol. 2001;167:1592–1600. doi: 10.4049/jimmunol.167.3.1592. [DOI] [PubMed] [Google Scholar]
- 20.Grossmann M, et al. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc Natl Acad Sci USA. 1999;96:11848–11853. doi: 10.1073/pnas.96.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottero V, Withoff S, Verma IM. NF-kappaB and the regulation of hematopoiesis. Cell Death Differ. 2006;13:785–797. doi: 10.1038/sj.cdd.4401888. [DOI] [PubMed] [Google Scholar]
- 22.Guo F, Tänzer S, Busslinger M, Weih F. Lack of nuclear factor-kappa B2/p100 causes a RelB-dependent block in early B lymphopoiesis. Blood. 2008;112:551–559. doi: 10.1182/blood-2007-11-125930. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, et al. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat Immunol. 2006;7:962–970. doi: 10.1038/ni1367. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M, et al. Cutting edge: Pivotal function of Ubc13 in thymocyte TCR signaling. J Immunol. 2006;177:7520–7524. doi: 10.4049/jimmunol.177.11.7520. [DOI] [PubMed] [Google Scholar]
- 25.Fukushima T, et al. Ubiquitin-conjugating enzyme Ubc13 is a critical component of TNF receptor-associated factor (TRAF)-mediated inflammatory responses. Proc Natl Acad Sci USA. 2007;104:6371–6376. doi: 10.1073/pnas.0700548104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germain RN. T cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 27.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 28.Porritt HE, et al. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Zediak VP, Maillard I, Bhandoola A. Closer to the source: Notch and the nature of thymus-settling cells. Immunity. 2005;23:245–248. doi: 10.1016/j.immuni.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Radtke F, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki H, Akashi K. Hematopoietic developmental pathways: On cellular basis. Oncogene. 2007;26:6687–6696. doi: 10.1038/sj.onc.1210754. [DOI] [PubMed] [Google Scholar]
- 32.Ramakrishnan A, Deeg HJ. A novel role for the marrow microenvironment in initiating and sustaining hematopoietic disease. Expert Opin BIol Ther. 2009;9:21–28. doi: 10.1517/14712590802603093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantrell DA. Transgenic analysis of thymocyte signal transduction. Nat Rev Immunol. 2002;2:20–27. doi: 10.1038/nri703. [DOI] [PubMed] [Google Scholar]
- 34.Wilson A, MacDonald HR, Radtke F. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J Exp Med. 2001;194:1003–1012. doi: 10.1084/jem.194.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoki K, Taketo MM. Adenomatous polyposis coli (APC): A multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- 36.Reiley WW, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.