Abstract
During injury to the nervous system, innate immune cells mediate phagocytosis of debris, cytokine production, and axon regeneration. In the neuro-degenerative disease amyotrophic lateral sclerosis (ALS), innate immune cells in the CNS are activated. However, the role of innate immunity in the peripheral nervous system (PNS) has not been well defined. In this study, we characterized robust activation of CD169/CD68/Iba1+ macrophages throughout the PNS in mutant SOD1G93A and SOD1G37R transgenic mouse models of ALS. Macrophage activation occurred pre-symptomatically, and expanded from focal arrays within nerve bundles to a tissue-wide distribution following symptom onset. We found a striking dichotomy for immune cells within the spinal cord and PNS. Flow cytometry and GFP bone marrow chimeras showed that spinal cord microglia were mainly tissue resident derived, dendritic-like cells, whereas in peripheral nerves, the majority of activated macrophages infiltrated from the circulation. Humoral antibodies and complement localized to PNS tissue in tandem with macrophage recruitment, and deficiency in complement C4 led to decreased macrophage activation. Therefore, cross-talk between nervous and immune systems occurs throughout the PNS during ALS disease progression. These data reveal a progressive innate and humoral immune response in peripheral nerves that is separate and distinct from spinal cord immune activation in ALS transgenic mice.
Keywords: innate immunity, macrophage, peripheral nervous system, neuroimmunology, amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disorder characterized by muscular weakness and paralysis; mortality usually results within 2 to 5 years. Disease progression leads to selective death of motor neurons in the CNS and denervation of neuromuscular synapses in the peripheral nervous system (PNS). Although the majority of cases are sporadic (90%), the most common form of familial ALS is linked to mutations in the Cu/Zn superoxide dismutase 1 (SOD1) gene (1). In mice, transgenic (Tg) overexpression of human SOD1 mutant proteins induces motor neuron disease resembling ALS (2, 3).
In patients and mouse models of ALS, inflammatory responses accompany motor neuron degeneration (4). In the CNS, microglia and astrocytes are activated during disease progression (5, 6), whereas peripheral T and natural killer cells infiltrate the spinal cord (6, 7). Recent studies have shown that these non-neuronal cells play an active role in motor neuron death. Selective ablation of mutant SOD1 within astrocytes and microglial cells by conditional deletion and neonatal bone marrow (BM) transplantation resulted in increased motor neuron survival and lifespan (8, 9). Deficiency in T cells, in contrast, led to accelerated disease progression in mutant SOD1 Tg mice (7, 10). These recent studies of neuro-inflammation in ALS have mainly focused on the CNS.
In contrast, the role of immune activation in the PNS has not been well analyzed. Degeneration of motor axons in the periphery is an early and significant pathological feature in ALS patients and mutant SOD1 mice (11, 12). Mutant SOD1 also induces defects in peripheral axon transport, which may be a primary determinant of motor neuron death (13). In acute models of PNS injury, myeloid cells have been shown to mediate the processes of myelin clearance and subsequent axon regeneration (14). Whether and how the immune system participates in motor axon loss during ALS disease progression remains unexplored.
In this study, we examined immune activation in the PNS of mSOD1G93A and mSOD1G37R mice. Specific and progressive accumulation of monocytes/macrophages was observed along the length of degenerating nerve fibers in ventral roots, sciatic nerves, and muscles. Concurrently, antibodies and complement are deposited in PNS tissue. Moreover, flow cytometry and BM chimera studies demonstrated distinct origins for PNS macrophages compared with spinal cord microglia.
Results
Macrophage Activation Occurs Throughout the Peripheral Nervous System of Mutant SOD1 Mice.
Glial cell activation is closely associated with motor neuron degeneration in the spinal cord of patients and mouse models of ALS (4–6). Similarly, in our study, activated CD68+ microglia and GFAP+ astrocytes were observed along the rostral-caudal axis of mSOD1G93A mouse spinal cord, but not spinal cord of non-Tg litter-mates [supporting information (SI) Fig. S1]. In addition to spinal cord, we observed that CD68, a lysosomal marker for activated microglia/macrophages, was significantly expressed in ventral nerve roots of mSOD1G93A mice (Fig. 1 and Fig. S1).
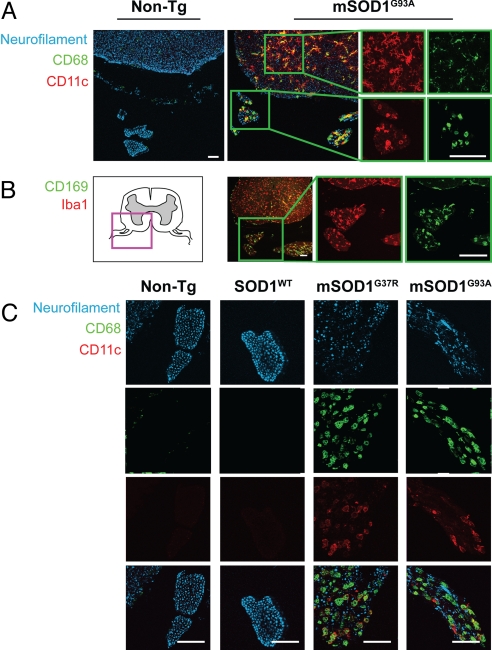
Fig. 1.
Morphologically activated macrophages expressing CD68, Iba1, CD11c, and CD169 accumulate between degenerating axons in ventral nerve roots of mutant SOD1 mice. (A) Both microglia in spinal cord and macrophages in ventral nerve roots of mSOD1G93A mice show significant expression of the myeloid activation marker, CD68 (green), and dendritic cell receptor, CD11c (red). Axons were labeled with anti-neurofilament (blue). Innate immune activation was absent in non-Tg sections. Magnified views (Insets) show images of representative microglia (Top) and nerve root macrophages (Bottom). (B) Anatomical schematic depicting spinal cord with ventral roots (Left). Magnification of mSOD1G93A section (Inset) shows nerve root macrophages expressing sialoadhesin, CD169 (green), and calcium adaptor, Iba1 (red). (C) Ventral roots in non-Tg, SOD1WT, and end-stage mSOD1G37R, mSOD1G93A mice stained for neurofilament (blue) and macrophage markers (CD11c, red; CD68, green). In mutant SOD1 mice, activated macrophages accumulate in spaces between degenerating axons. (Scale bars: 100 μm.)
We used a panel of antibodies to further characterize the immunological phenotype of these CD68+ cells. These cells were found to co-express the microglia/macrophage cytoplasmic calcium adaptor Iba1, dendritic cell marker CD11c, sialic acid binding receptor CD169 (Fig. 1 A and B), and myeloid cell integrin CD11b (Fig. S2). Based on presence of these innate immune markers, and their rounded morphology (compared with spinal cord microglia, Fig. 1A), we conclude that these cells are activated macrophages accumulating within peripheral nerve roots.
Macrophages localized to spaces adjacent to axons (stained with anti-neurofilament) in ventral nerve roots of mSOD1G93A and mSOD1G37R mice (Fig. 1C). In contrast, non-Tg and SODWT roots showed intact axon bundles with a few un-activated, resident macrophages (Fig. 1C). We next analyzed PNS sites downstream of spinal cord and proximal to the muscle.
Whole-mount, longitudinal, and transverse sections of sciatic nerves were stained for markers of innate immune activation (Fig. 2). Concurrent with axon loss in mutant SOD1 mice, reflected by decreased neurofilament staining, sciatic nerves were filled with activated macrophages (Fig. 2A). CD68/Iba1+ macrophages were present throughout mSOD1G93A nerves by end stage of disease and absent in non-Tg litter-mates (Fig. 2B). These macrophages also expressed the myeloid cell markers CD169, F4/80, and CD11c at various levels (Fig. S2). Toluidine blue staining showed accumulation of phagocytic cells ingesting myelinic debris in mSOD1G93A peripheral nerves (Fig. S3).
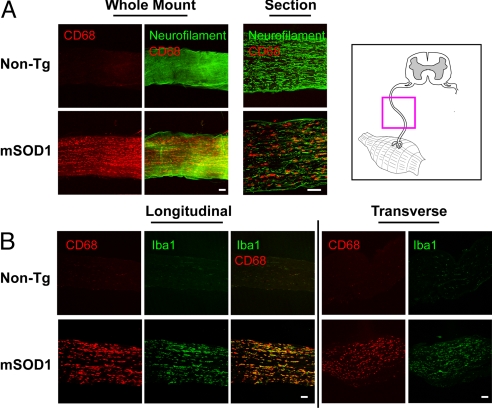
Fig. 2.
Sciatic nerves in mutant SOD1 mice but not WT mice show intra-axonal activation of macrophages. (A) Whole-mount segments and longitudinal sections of distal sciatic nerve from end-stage mSOD1G93A and non-Tg litter-mates were stained for macrophages (CD68) and axons (neurofilament). For whole-mount stains, confocal microscopy images 100 μm into the nerve are shown as a composite. (B) Longitudinal and transverse sciatic nerve sections were stained for macrophage markers CD68 (red) and Iba1 (green). mSOD1G93A nerves at end stage show an abundance of activated, CD68/Iba1+ macrophages compared with non-Tg litter-mates. An anatomical schematic of peripheral nerves is shown (Upper Right). (Scale bars: 100 μm.)
Further downstream in the PNS, we analyzed immune activation in muscle tissues (Fig. 3). CD68+ macrophages accumulated within degenerating nerve bundles in mSOD1G93A muscle but not non-Tg muscle (Fig. 3A). To better visualize innervating connections, mSOD1G93A mice were bred with mice expressing Thy1-YFP, which labels motor neurons and peripheral axons (15). We found that more severely affected muscles, such as the Tibialis, showed greater infiltration of macrophages compared with diaphragm (Fig. 3B). Although a few macrophages were observed in the vicinity of end-plate neuromuscular synapses, the majority of activated macrophages accumulated within fascicles of innervating axon tracts (Fig. 3C).
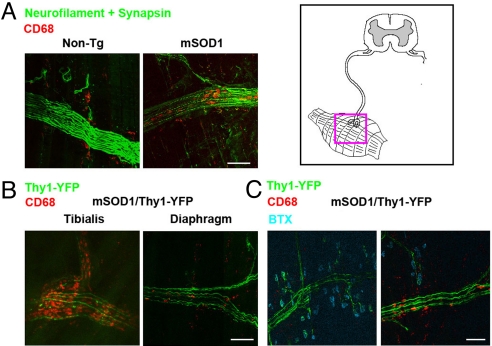
Fig. 3.
Whole-mount muscle staining reveals macrophage infiltration within innervating axon fascicles. (A) Diaphragms from end-stage mSOD1G93A and non-Tg mice were stained for macrophages (CD68, red), axons (neurofilament, green), and synapses (synapsin, green). Intra-nerve macrophages were found in mSOD1G93A but not non-Tg muscles. (B) Muscles from end-stage mSOD1G93A/Thy1-YFP animals (YFP, green) were stained for macrophages (CD68, red). Activation of macrophages and degeneration of YFP axons was more extensive in the tibialis compared with diaphragm. (C) Triple staining for macrophages (CD68, red), axons (YFP, green), and motor endplates [bungarotoxin (BTX), blue]. Activated macrophages were found within mutant SOD1 axonal fascicles but not at neuromuscular synapses. (Scale bars: 100 μm.)
Early, Focal Immune Activation in the Mutant Nervous System Becomes Pervasive Following Onset of Symptoms.
We examined the dynamics of immune activation in the PNS and CNS relative to disease progression (Fig. 4 and Figs. S5 and S6). By monitoring, the earliest sign of symptom onset in the B6/SJL mSOD1G93A mice was initiation of weight loss, which occurred at 12 weeks of age [mean, 85.8 d ± 4.1 (SEM), n = 11, Fig. S5a]. Visible signs of muscle weakness were observed soon afterward (90.64 d ± 1.83, n = 11; Fig. S5b). Sciatic nerves and spinal cords of mSOD1G93A, non-Tg litter-mates, and SOD1WT mice were analyzed in parallel at several disease time points.
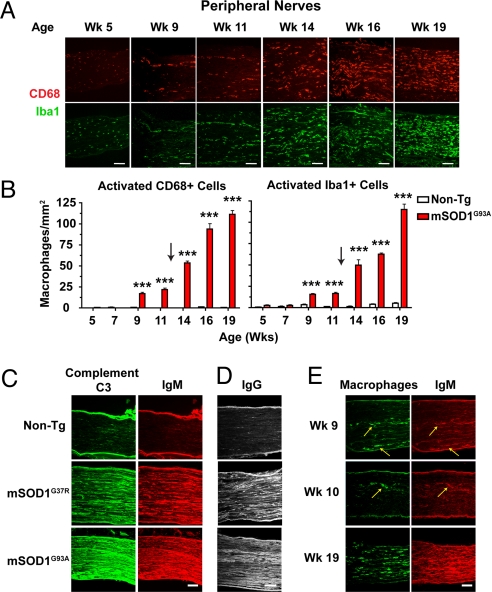
Fig. 4.
Age-dependent progression in PNS macrophage activation is accompanied by significant deposition of antibodies and complement. (A) Representative sciatic nerves from mSOD1G93A mice were stained for CD68/Iba1+ macrophages. At week 5, endoneural macrophages are morphologically un-activated. At weeks 9 and 11, macrophages accumulate in nerves, becoming activated morphologically, often assembling in rows. At weeks 14 to 19, activated macrophages spread throughout nerve parenchyma. (Scale bars: 100 μm.) (B) Quantification of progressive macrophage activation in sciatic nerves of mSOD1G93A and non-Tg mice (20× fields of non-consecutive 14 μm sections analyzed; mean ± SEM, ***, P < 0.001). (C) Week 19 mSOD1G93A, week 19 non-Tg, and 6-month-old mSOD1G37R sciatic nerve sections were stained for complement C3 (green) and antibody IgM (red). Strong deposition of complement and antibodies was observed in ALS Tg nerves. (D) Separate week-19 sections show anti-IgG reactivity in mSOD1G93A but not non-Tg nerves. (E) At presymptomatic weeks 9 and 10, antibody IgM (red) co-localizes in PNS tissue with strands of activated macrophages (CD68, green).
In the PNS of mutant SOD1 mice, macrophage activation proceeded in two distinct phases (Fig. 4A). From weeks 5 through 7, no evidence of innate activation was found in mSOD1G93A peripheral nerves. These nerves were populated by resting, endoneural macrophages (<250 μm2). From weeks 9 through 11, which are presymptomatic time points, enlarged CD68/Iba1+ macrophages (>250 μm2) accumulated in cellular strands along the peripheral nerve. At weeks 14, 16, and 19, which are post-symptomatic time points, macrophage activation in the PNS became generalized, distributing throughout the tissue parenchyma. Image quantification analyses confirmed these 2 phases of activation, with symptom onset acting as a transition point (n = 4 nerves/time point; Fig. 4B).
In addition to immunostaining for macrophage activation, we also analyzed mRNA levels of cytokines and chemokines in sciatic nerves by real-time PCR. The chemokine MCP-1, which is involved in monocyte recruitment during Wallerian degeneration (16), was progressively increased in mSOD1G93A compared with non-Tg and SODWT nerves (Fig. S4). In contrast, classic pro-inflammatory cytokines TNF-α and IL-6 did not show significant changes.
In the spinal cord, we found that the time course of immune activation progressed concurrently with PNS macrophage activation. Two parameters were used to assess this: surface/morphological activation of microglia and infiltration of CD4+ and CD8+ T cells (Figs. S5 and S6). By immunostaining, spinal cord sections from presymptomatic mSOD1G93A mice (week 9) showed focal activation of microglia and astrocytes in ventral horns (Fig. S5b). Following symptom onset (week 12), hypertrophic microglia was observed throughout the gray and white matter, peaking in activation at end stage (week 19). Flow cytometry demonstrated a similar time course, with mSOD1G93A microglia showing population-wide shifts in surface activation markers CD11c and CD86 after symptomatic onset (Fig. S6 b and d). Microglial activation was not observed in spinal cords of SODWT and non-Tg mice. Strikingly, infiltration of CD4+ and CD8+ T cells also occurred only following onset of symptoms (Fig. S6c).
These observations, in conjunction with previous studies (5–7), indicate that immune activation in the PNS and spinal cord of mutant SOD1 mice progresses in parallel. At presymptomatic time points (i.e., weeks 9–12), focal activation occurs for PNS macrophages and spinal cord microglia. Following clinical onset (i.e., weeks 12–19), activation of innate immunity becomes widespread in both tissues as indicated by FACS and histological analysis.
A Role for Humoral Immunity in PNS Macrophage Activation.
Humoral system components, including circulating antibodies and complement, are known to deposit in the ALS spinal cord, and may play significant roles in damaging motor neurons (6, 17). In acute PNS injury, complement mediates the opsonization of myelin debris and recruitment of myeloid cells (18, 19). Therefore, we analyzed the role of the humoral immune system in the PNS of ALS Tg mice.
We detected intense deposits of IgM, complement C3, and IgG throughout the parenchyma of mSOD1G93A and mSOD1G37R sciatic nerves at end stage, but not in non-Tg age-matched mice (Fig. 4 C and D). This humoral deposition was specific, as isotype control and secondary antibodies alone did not stain mutant SOD1 nerves. Interestingly, at pre-symptomatic time points weeks 9 and 10, IgM deposited in the same areas of nerve that also recruited strands of activated macrophages (Fig. 4E). Co-localization of humoral components and macrophages was observed at every time point analyzed.
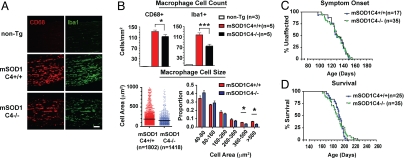
These data led us to hypothesize that antibody deposition may lead to downstream activation of the classical complement cascade and subsequent recruitment of macrophages (classical pathway, IgG and IgM → C1q → C4 → C3 → immune cells). To test this hypothesis, we bred mSOD1G93A mice onto a background deficient in complement C4, which is necessary for activation of both the classical and lectin complement pathways (20). Ablation of C4 was confirmed by PCR and serum ELISA analysis (Fig. S7 a and b). At disease end stage, significantly fewer activated macrophages were found in sciatic nerves of mSOD1G93AC4-/- compared with mSOD1G93AC4+/+ mice (Fig. 5A). Based on image quantification, both activated CD68+ (P = 0.0477, t test) and Iba1+ (P = 0.0003) macrophages showed significant decreases in mSOD1G93AC4−/− mice at end stage (Fig. 5B; n = 5). In particular, larger-sized macrophages (>360 μm2) were fewer in number in the absence of C4 (Fig. 5B, histogram). Nonetheless, not all macrophages were eliminated in mSOD1G93AC4−/− nerves. To ascertain the effect of complement C4 deficiency on disease progression, we analyzed development of motor symptoms and mortality. Onset of symptoms was unaffected in mSOD1G93AC4−/− mice compared with mSOD1G93AC4+/+ mice (Fig. 5C; P = 0.769 by log-rank test). Furthermore, weight loss and motor score analysis demonstrated similar downward trajectories in mSOD1G93AC4+/+, mSOD1G93AC4±, and mSOD1G93AC4−/− animals (Fig. S7 c and d). Finally, Kaplan–Meier survival curves showed that lifespan was unaltered in mSOD1G93AC4−/− compared with mSOD1G93AC4+/+ animals (Fig. 5D; 191.1 d ± 2.52 and 192.4 d ± 1.88; P = 0.592 by log-rank test). Therefore, complement C4 plays a partial role in PNS macrophage activation, but does not significantly affect survival in mSOD1G93A mice.
Fig. 5.
Complement C4 partially mediates macrophage activation during motor neuron degeneration. To ascertain the role of humoral immunity in ALS, mSOD1G93A mice were bred onto a complement C4-deficient background. (A) Representative images of Iba1/CD68+ macrophages in end-stage mSOD1C4+/+ and mSOD1C4−/− sciatic nerves, with age-matched non-Tg nerves. (Scale bars: 100 μm.) (B) Quantification analysis of CD68+ and Iba1+ macrophages in end-stage mSOD1C4+/+ (n = 5), mSOD1C4−/− sciatic nerves (n = 5). Individual macrophage size data (CD68+) are shown in vertical scatter plot and histogram analysis (mean ± SEM, *, P < 0.05 by t test). (C and D) Kaplan Meier curves of symptom onset and survival in mSOD1C4+/+ (n = 25; male, n = 14; female, n = 11) and mSOD1C4−/− mice (n = 35; male, n = 18; female, n = 17).
Phenotypic Dichotomy of Myeloid Cell Activation in the ALS Peripheral Nervous System Versus Spinal Cord.
The phenotype of myeloid cells is significantly influenced by the surrounding tissue and cytokine microenvironment (21). In spinal cord, mutant SOD1 microglia are induced to express insulin-like growth factor and osteopontin during disease progression (7). Activated PNS macrophages may express a distinct set of factors in response to motor axon degeneration. Furthermore, myeloid cells in each compartment may originate from tissue resident progenitors or differentiate from infiltrating, blood-derived monocytes (22, 23).
To characterize the traits of innate immune activation in the PNS and CNS, we compared macrophages isolated from mSOD1G93A sciatic nerves with spinal cord microglia from the same mice. By flow cytometry, surface receptor profiles of activated PNS macrophages differed distinctly from microglia (Fig. 6A). Whereas mSOD1G93A microglia expressed dendritic cell markers CD11c, CD86 (B7–2), and CD54 (ICAM-1), PNS macrophages showed high surface levels of MHC class II. Microglia and macrophages expressed similar levels of CD11b and CD45.
Fig. 6.
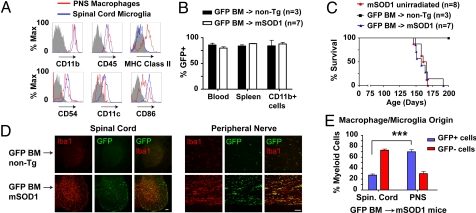
Flow cytometry and GFP chimeras demonstrate unique nature and origin for ALS PNS macrophages compared with spinal cord microglia. (A) Sciatic nerve macrophages and spinal cord microglia from end-stage mSOD1G93A mice were compared by FACS for MHC class II, CD54 (ICAM-1), CD11c, and CD86. Histograms show surface expression of PNS macrophages (red) and spinal cord microglia (blue) relative to isotype controls for PNS macrophages (gray). (B-E) mSOD1G93A (n = 8) or non-Tg (n = 3) mice were transplanted at 7 weeks with BM from EGFP mice (GFP BM). Un-irradiated mSOD1G93A litter-mates (n = 7) were analyzed in parallel. (B) FACS analysis of GFP chimerism for blood leukocytes and total and CD11b+ splenocytes in transplanted mice at end-stage. (C) Kaplan–Meier survival analysis of BM-transplanted and un-irradiated mice. (D) In spinal cord, Iba1+ microglia are mainly negative for GFP, indicating tissue resident origin. In contrast, sciatic nerve sections imaged for Iba1+ macrophages show significant co-expression of GFP, indicating a BM origin. (Scale bars: 100 μm.) (E) Quantification of BM-derived spinal cord microglia and PNS macrophages in mutant SOD1/GFP mice (***, P < 0.001; n = 4).
To determine the origin of PNS and CNS myeloid cell types during motor neuron degeneration, GFP+ BM was transplanted into irradiated mSOD1G93A mice and non-Tg litter-mates at 7 weeks of age. Splenocytes and blood leukocytes, including CD11b+ myeloid cells, showed >75% GFP chimerism in transplanted mice (Fig. 6B). Replacement of endogenous BM with GFP+ cells did not significantly affect disease progression (Fig. 6C). We next examined spinal cord and peripheral nerves of GFP transplanted mice (Fig. 6D). Using Iba1 as a general marker for microglia/macrophages, it was evident that the majority of activated spinal cord microglia originated from within the CNS; the converse was true for macrophages in sciatic nerves and ventral roots (Fig. 6 D and E and Fig. S9). Only 27.3% ± 2.2% of spinal cord microglia were BM-derived, whereas 69.9% ± 4.14% of PNS macrophages were GFP+ and originated from the peripheral circulation (Fig. 6E, mean ± SEM, n = 4).
GFP transplantation also revealed specific innate immune cell subsets (Fig. S10 a and b). In spinal cord, GFP tissue-resident microglia were mainly CD11c+, whereas GFP+ BM-derived microglia were mainly CD169+. In the PNS, most macrophages were CD169+GFP+ BM-derived cells. Spinal cord microglia also showed more heterogeneity in cell size than peripheral macrophages (Fig. S10c). Thus, based on the results of FACS analysis and BM transplantation, PNS macrophage activation in mutant SOD1 mice is distinct in nature and origin compared with spinal cord microglia.
Discussion
Motor neuron death is associated with a robust cellular response by CNS microglia and astrocytes (4, 5). In this study, we characterize a system-wide infiltration of macrophages in the PNS of mutant SOD1 mice that accompanies axon degeneration in ventral roots, sciatic nerves, and muscle tissues. Concurrently, increased levels of antibodies and complement are detected in the affected nerves. These findings broaden the dimensions of neuro-immunological pathology in ALS, and have ramifications for immune modulation of motor neuron survival.
Our results show that 2 separate immune cell compartments undergo activation in ALS Tg mice. Degeneration of different anatomical regions of the motor neuron likely elicits distinct functional responses by the immune system. In the spinal cord, loss of motor neuron cell bodies induces the activation of resident microglia and infiltration of T cells (5, 7, 10). Here we show that in the PNS, denervation and degeneration of motor axons leads to significant peripheral macrophage activation. Although an earlier microscopy study suggested the presence of macrophages in peripheral nerves of mutant SOD1 mice (24), here we prove their existence and characterize them in detail during disease progression. We find that the origin and nature of CNS myeloid cells are distinct from PNS myeloid cells in ALS. We find that microglia in mutant SOD1 mice are primarily tissue resident cells, in agreement with earlier studies using parabiotic and BM chimeric mice (22, 23). By contrast, PNS macrophages in ALS Tg mice are mainly derived from the circulation. Diverse signals may regulate immune recruitment to each tissue: one set of chemokines may attract T cells to the ALS spinal cord, whereas other signals mediate macrophage recruitment to the PNS. For example, we find that MCP-1, a monocyte chemoattractant that functions during acute peripheral nerve injury (16), is up-regulated in sciatic nerves of mutant SOD1 mice. Furthermore, myeloid deficiency in CCR2, the receptor for MCP-1, leads to disease acceleration in these mice (10). Therefore, MCP-1 and CCR2 may significantly affect PNS macrophage recruitment in ALS.
CNS and PNS immunity may play distinct functional roles. Although spinal cord microglia acquired dendritic cell surface receptors during disease progression, PNS macrophages became phagocytic. In immune responses, dendritic cells are antigen-presenting cells that activate T cells; ALS microglia may use similar cell pathways to interact with T cells infiltrating the spinal cord. Conversely, the primary role of macrophages in peripheral nerves may be the phagocytic removal of debris following axonal degeneration.
Despite these functional differences, we find that immune activation in the CNS and PNS follow similar kinetics. In the PNS, we observed activated macrophages forming cellular strands along the nerve at pre-symptomatic time points; following symptom onset, macrophage activation occupies a majority of the parenchyma. In the spinal cord, microglia are discretely activated in ventral horns at presymptomatic time points; following onset, glial activation becomes widespread and T cells infiltrate from the periphery. What is the significance of the symptom onset time point? Neuropathologically, neuromuscular degeneration and motor neuron loss proceeds in a stepwise fashion. In the PNS, denervation at the neuromuscular synapse occurs as early as day 40 in the mutant SOD1 mouse (11). By onset, decreases in cholinergic motor neuron count (25), ventral root motor axons (60% loss), and neuromuscular junctions become significant (11). Nevertheless, a large subset of motor neurons and axons remain intact at symptom onset (11, 25). The role of the immune system may be to impact the survival of this remaining neuronal subset. Evidence for a post-symptomatic role of immunity is reflected in several phenotypic studies. When the mutant SOD1 gene is selectively deleted from microglia/macrophages, only the symptomatic phase of disease is affected, nonetheless leading to significant extensions in lifespan (8, 9). In contrast, selective removal of mutant SOD1 within motor neurons affects both pre- and post-symptomatic phases of disease (9). Recently, it was shown that blocking adaptive immunity in ALS mice does not alter disease onset, but leads to acceleration of post-symptomatic progression (7, 10). For most patients with ALS, the window for therapeutic modulation necessitates targeting post-symptomatic disease mechanisms; therefore, understanding immune activation processes holds relevance to clinical treatment.
We find that antibodies and complement may play a significant role in PNS neuro-degeneration. Natural antibodies are endogenous, circulating antibodies of IgG and IgM isotypes that are poly-reactive (26). Natural antibodies efficiently activate complement, acting to clear apoptotic debris and as a first line of defense against pathogens (26). In situations of sterile inflammation such as ischemia/reperfusion injury, natural IgM and complement can bind self-antigens and initiate pathologic damage (27). We detected IgG, IgM, and complement deposition in mutant SOD1 sciatic nerves concomitant with macrophage accumulation. It is not known how natural antibodies enter the PNS and whether they recognize specific peripheral nerve antigens. In acute nerve injury, depletion of complement or the membrane attack complex decreases macrophage activation (18, 19). Similarly, in our study, mSOD1C4−/− mice showed significantly decreased macrophage levels and activation. Although C4 deficiency did not affect overall survival or motor decline, other molecular pathways may play compensatory roles in immune activation and macrophage recruitment.
Is peripheral immune activation a secondary response to motor axon death or a primary result of the ALS disease process? Wallerian degeneration of the PNS following acute injury is mediated by both innate and humoral immune pathways (28). However, it is not known whether the same cascade of events occur in ALS; our experiments and other studies suggest significant similarities. In Wallerian degeneration, animals deficient in Toll-like receptors and the downstream signaling molecule MyD88 show impaired macrophage-mediated debris clearance and axon regeneration (29). Kang et al. showed that transplantation of MyD88−/− BM cells into ALS mice led to significant acceleration of motor neuron disease (30). Therefore, Toll-like receptors and MyD88 in macrophages may play analogous roles in Wallerian degeneration and ALS. Conversely, although the WldS protein slows axon loss in Wallerian degeneration, it induces minimal effects on motor axon survival and neuronal loss in ALS Tg mice (31). Depletion of mutant SOD1 from myeloid cells leads to a significant lifespan extension in ALS Tg mice (8, 9), suggesting that macrophages harboring mutant SOD1 protein may not function equivalently as WT macrophages in Wallerian degeneration.
Although tissue inflammation is generally thought to be detrimental, there is mounting evidence that controlled immune activation can be beneficial for regenerative processes (14, 21). In ALS, we and others found that T cells polarize a neuroprotective response in spinal cord microglia (7, 10). In the PNS, significant re-innervation of motor end-plates occurs during disease progression, indicating that ALS motor axons may actively regenerate (25, 32). Recently, Barrette et al. demonstrated that macrophage depletion significantly slowed myelin clearance, axon regeneration, and functional recovery following sciatic nerve injury (14). Despite robust macrophage activation in the nerves of mutant SOD1 mice, we did not detect significant expression of the pro-inflammatory cytokines TNF or IL-6, suggesting that immune activation in the PNS may not be actively detrimental.
Therapeutic targeting in ALS has been traditionally difficult because of the necessity for treatments to cross the blood-brain barrier. The finding that significant immunopathology occurs in the PNS offers a more accessible target for modulation. Our results show that large proteins, including IgG and IgM, infiltrate peripheral nerves during disease progression. This suggests that the ALS blood-nerve barrier would be relatively permeable to chemical therapeutic agents and antibodies. Furthermore, signaling pathways for peripheral macrophages have been well defined, and can be readily targeted in ALS Tg mice.
During neurodegeneration in ALS, dysfunction occurs at both the motor neuron cell body and peripheral motor axon. In this study, we found progressively increased innate and humoral activation in the PNS of mutant SOD1 mice, from spinal ventral roots distal to innervating axons of affected muscles. Therefore, activation of the immune system is intimately connected with the process of motor neuron degeneration. Future analysis of neuroimmune communication will hopefully lead to targeted treatments to extend motor neuron survival in ALS.
Materials and Methods
Mice.
B6/SJL mSOD1G93A, non-Tg, SOD1WT, B6 congenic mSOD1G93A, mSOD1G37R, Thy1-YFP, Actin-EGFP (Jackson Laboratories), and B6.C4−/− mice (generated previously in the laboratory) were bred and maintained in a full-barrier, specific pathogen-free facility. For visualization of axons, B6.mSOD1G93A mice were bred with Thy1-YFP mice. To analyze the role of complement, B6.mSOD1G93A mice (low copy strain) were bred with C4−/− mice; C4 ablation was confirmed by PCR genotyping and serum ELISA. To analyze myeloid cell origins, BM from Actin-EGFP mice was transplanted into irradiated 7-week-old B6.mSOD1G93A mice and non-Tg litter-mates. Survival and motor symptom progression was analyzed. Experiments were conducted according to institutional animal care guidelines. A summary of animals used in this study is in Table S1. For details on breeding, motor symptomatic analysis, BM transplantation, measurement of GFP chimerism, and C4 ELISA, see SI Methods.
Flow Cytometry.
Mice were perfused with PBS solution, and immune cells were directly isolated from spinal cord and nerves, stained, and analyzed by FACS. Flow cytometry was conducted on a FACScalibur machine (BD Biosciences). For details on cell isolation, surface labeling, and flow cytometry, see SI Methods.
Tissue Processing.
For immunostaining, mice at different time points were intracardially perfused with 4% paraformaldehyde/PBS solution. Sciatic nerve, muscle, and spinal cord were subsequently dissected, embedded, and cryo-sectioned. For quantitative PCR, freshly isolated sciatic nerves were homogenized in TRIzol (Invitrogen) for RNA extraction and cDNA synthesis. For details on tissue processing, real-time PCR, and toluidine blue staining, see SI Methods.
Immunofluorescence and Image Analysis.
Longitudinal and transverse cryo-sections of spinal cord, sciatic nerves, as well as whole-mount muscle and sciatic nerve tissues were immunostained for cellular and immunological antigens. Primary antibodies were as follows: rat anti-CD68 (1:1,000; Serotec), rabbit anti-GFAP (1:1,000; Sigma), hamster anti-CD11c (1:50, BD Biosciences), rabbit anti-neurofilament 200 (1:1,000; Sigma), rat anti-CD169 (1:200; Serotec), rabbit anti-Iba1 (1:500; Wako), rat anti-CD11b (1:50; BD Biosciences), rat anti-F4/80 (1:100; Serotec), goat anti-IgM (1:100; Southern Biotech), rat anti-C3 (1:100; Abcam), and Cy3 goat anti-mouse IgG (1:500; Jackson Immunoresearch). Secondary antibodies were as follows: Alexa 488, Alexa 568, or Alexa 633 goat anti-rat, Alexa 488 rabbit anti-rat, Alexa 568 rabbit anti-goat, Alexa 488 or Alexa 633 goat anti-rabbit (Invitrogen), and Cy3 goat anti-Armenian hamster IgG (Jackson Immunoresearch). Motor end-plates were labeled with Alexa 633 bungarotoxin (1:1,000, Invitrogen). For details on immunostaining, microscopy, and image analysis, see SI Methods.
Supplementary Material
Acknowledgments.
We thank Lisa Pitcher, Bela Kosaras, Hilary Bowden, and Ryan Conway for technical help. We thank Robert Brown, Jr, Vijay Kuchroo, Beth Stevens, Jack Strominger, and Ben Barres for helpful advice. This work was supported by the National Institutes of Health and the ALS Association.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911405106/DCSupplemental.
References
- 1.Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 2.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 3.Bruijn LI, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 4.Mcgeer P, Mcgeer E. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- 5.Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- 7.Chiu IM, et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci USA. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beers DR, et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boillée S, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 10.Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer L, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal A. Detection of preclinical motor neurone loss in SOD1 mutation carriers using motor unit number estimation. J Neurol Neurosurg Psychiatry. 2002;73:199–201. doi: 10.1136/jnnp.73.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ström AL, et al. Retrograde axonal transport and motor neuron disease. Journal of neurochemistry. 2008;106:495–505. doi: 10.1111/j.1471-4159.2008.05393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrette B, et al. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 16.Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff TM, et al. The complement factor C5a contributes to pathology in a rat model of amyotrophic lateral sclerosis. J Immunol. 2008;181:8727–8734. doi: 10.4049/jimmunol.181.12.8727. [DOI] [PubMed] [Google Scholar]
- 18.Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci. 1998;18:6713–6722. doi: 10.1523/JNEUROSCI.18-17-06713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramaglia V, et al. The membrane attack complex of the complement system is essential for rapid Wallerian degeneration. J Neurosci. 2007;27:7663–7672. doi: 10.1523/JNEUROSCI.5623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll MC, Fischer MB. Complement and the immune response. Curr Opin Immunol. 1997;9:64–69. doi: 10.1016/s0952-7915(97)80160-4. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 22.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 23.Solomon JN, et al. Origin and distribution of bone marrow-derived cells in the central nervous system in a mouse model of amyotrophic lateral sclerosis. Glia. 2006;53:744–753. doi: 10.1002/glia.20331. [DOI] [PubMed] [Google Scholar]
- 24.Dal Canto MC, Gurney ME. Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am J Pathol. 1994;145:1271–1279. [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu AY, et al. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6:349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- 26.Fleming SD, Tsokos GC. Complement, natural antibodies, autoantibodies and tissue injury. Autoimmun Rev. 2006;5:89–92. doi: 10.1016/j.autrev.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- 29.Boivin A, et al. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007;27:12565–12576. doi: 10.1523/JNEUROSCI.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang J, Rivest S. MyD88-deficient bone marrow cells accelerate onset and reduce survival in a mouse model of amyotrophic lateral sclerosis. J Cell Biol. 2007;179:1219–1230. doi: 10.1083/jcb.200705046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer L, et al. The gene WLDs modestly prolongs survival in the SOD1 fALS mouse. Neurobiol Dis. 2005;19:293–300. doi: 10.1016/j.nbd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer A, Sanes J, Lichtman J. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.