Abstract
Although the role of the tumor microenvironment in the process of cancer progression has been extensively investigated, the contribution of different stromal components to tumor growth and/or evasion from immune surveillance is still only partially defined. In this study we analyzed fibroblasts derived from metastatic melanomas and provide evidence for their strong immunosuppressive activity. In coculture experiments, melanoma-derived fibroblasts sharply interfered with NK cell functions including cytotoxicity and cytokine production. Thus, both the IL-2-induced up-regulation of the surface expression of NKp44, NKp30, and DNAM-1 triggering receptors and the acquisition of cytolytic granules were inhibited in NK cells. This resulted in an impairment of the NK cell-mediated killing of melanoma target cells. Transwell cocultures and the use of specific inhibitors suggested that cell-to-cell contact was required for inducing DNAM-1 modulation. In contrast, modulation of NKp44 and NKp30 was due to PGE2 released by fibroblasts during coculture. Normal skin fibroblasts could also partially affect NK cell phenotype and function. However, the inhibitory effect of tumor-derived fibroblasts was far stronger and directly correlated with their ability to produce PGE2 either constitutively or upon induction by NK cells.
Keywords: activating receptors, cytotoxicity, tumor microenvironment
Over the past two decades, major advances have been made in the definition of NK cell function including their cytolytic activity against virus infected or tumor cells (1–3). The molecular mechanisms regulating NK cell functions have been defined thanks to the discovery of various activating and inhibitory receptors. These receptors primarily include inhibitory receptors specific for HLA class I molecules, such as killer-Ig-like receptors (KIRs) and NKG2A and activating receptors, such as NKp30, NKp46, NKp44 (collectively termed natural cytotoxicity receptors; NCRs), NKG2D, DNAM-1, NKp80, 2B4, NTBA, CRTAM. The activating receptors recognize various ligands expressed by transformed cells and/or by certain stressed or activated cells (4–8). Certain activating receptors including NKp44 and CD69 are induced (9, 10), while others are up-regulated (NKp30, NKG2D, and, partially, NKp46) at the NK cell surface by cytokines, such as IL-2, known to potentiate NK cell activity (11). Upon recognition of specific ligands, NK-receptors can focalize NK cell responses against potentially harmful target cells avoiding reactivity with autologous HLA-class I+ normal cells (4). Initially, this information could be achieved by the in vitro analysis of purified peripheral blood NK cell populations and clones expanded in the presence of IL-2 (10, 12). Those studies however, did not explain how NK cell functions could actually be modulated in tissues in vivo. Recently, several studies attempted to clarify this point. For example, it has been shown that NK cell function can be modified sharply by several cytokines or enzymes including IL-15, IL-18, IL-21, IL-23, TGF-β, or indoleamine 2,3-dioxigenase (IDO) (11, 13–18). In addition, different cell types including dendritic cells (DCs), plasmacytoid DC (pDC), monocytes, and mesenchymal stem cells (MSCs) have been demonstrated to interact with NK cells giving rise to different functional outcomes (19–22). Finally, in pathologic conditions including allergy, NK-type lymphoproliferative disease of granular lymphocytes, non-small cell lung cancer, and AML, NK cells have been shown to display unique functional and/or phenotypic alterations (23–26). These findings imply that effective NK cell responses established in vitro against transformed cells and/or pathogens may substantially differ in vivo as a consequence of NK cell interaction with different cell types in different inflammatory or tumor sites. In this context, it became increasingly evident that tumors can influence the nature and the efficacy of the immune responses of the host (27). For example, induction of regulatory T cells (Tregs) or myeloid suppressor cells and/or production of several immunosuppressive factors [including arginase-1, NOS-2, IDO, or TGF-β] by tumor cells or components of tumor microenvironment may result in functional subversion of tumor-infiltrating immunocompetent cells (27). In this context, tumor-associated fibroblasts are a relevant component of the tumor microenvironment (28). However, very limited information is available on their ability to suppress immune responses. In this study, we show that fibroblasts derived from melanomas profoundly affect NK cell functions. Thus, NK cells reaching the tumor site may be greatly impaired in their anti-tumor activity by the tumor stroma itself.
Results
Comparative Analysis of Fibroblasts Derived from Melanomas or Normal Skin.
Four primary fibroblast cell lines were established either from two melanoma metastases (lines TF1 and TF2) or from skin of two healthy individuals (lines HF1 and HF2). Their surface phenotype was analyzed by immunofluorescence and cytofluorimetric analysis. As shown in Table 1, all expressed prolyl-4-hydroxylase (P4H; an enzyme involved in the biosynthesis of collagen), but not the CD106 adhesion molecule (that is expressed by MSCs) (22, 29) nor the tumor marker GD2 ganglioside. Notably, fibroblasts derived from tumor or normal tissue displayed some phenotypic differences: TF1 and TF2 were CD56-negative and expressed high levels of fibroblast activation protein (FAP); while HF1 and HF2 were CD56+ and expressed lower levels of FAP. The expression of FAP has been reported as a distinctive feature of activated fibroblasts (30). Thus, the phenotypic profile suggests a different activation level on tumor-derived or normal fibroblasts, respectively. Regarding the known ligands for activating NK receptors, both tumor and normal fibroblasts expressed the DNAM-1 receptor ligands PVR and Nectin-2, whereas NKG2D ligands were not expressed at significant levels (4).
Table 1.
Phenotype of different fibroblast populations
| Fibroblast cell lines | FAP | P4H | MIC A* | ULBP1* | ULBP2* | ULBP3* | PVR† | Nectin-2† | CD56 | CD106 | GD2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HF1 | +‡ | + | − | − | ± | − | ++ | + | + | − | − |
| HF2 | + | + | − | − | − | − | + | ± | + | − | − |
| TF1 | +++ | + | − | − | ± | − | +++ | + | − | − | − |
| TF2 | ++ | + | ± | − | ± | − | ++ | + | − | − | − |
Data are expressed as negative control m.f.i. (mean fluorescence intensity). Fold Increase, 0 (−); 1–4 (± ); 4–9 (+); 9–16 (++); >16 (+++).
*, NKG2D ligands.
†, DNAM-1 ligands.
Tumor-Derived Fibroblasts Inhibit NK Cell Function.
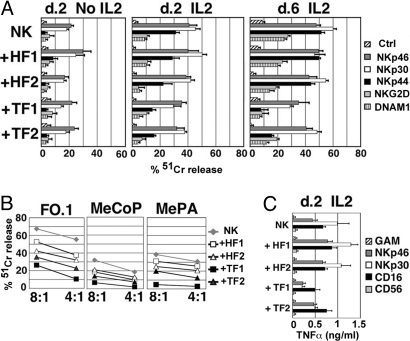
To investigate a possible effect of tumor-derived fibroblasts on NK cells, mixed cell cultures were set up. NK cells, freshly isolated from unrelated healthy donors were cocultured with different fibroblast cell lines for 2 or 6 days. The function of the major NK cell triggering receptors (including NKp46, NKp30, NKp44, NKG2D, and DNAM-1) was then analyzed by the use of specific mAbs in a redirected killing assay against the FcγR+ P815 target cell line. At day 2 (Fig. 1A, Left) and day 6 (Table S1) neither normal (HF1, HF2) nor tumor-derived fibroblasts (TF1, TF2) had any effect on the receptor-mediated triggering of the NK cell cytotoxicity. On the other hand, independent of their origin, fibroblasts appeared to preserve the NK cell viability (which is generally poor in the absence of cytokines). The same experimental approach was used to assess the effect of fibroblasts on the function of NK cells exposed to IL-2 (Fig. 1A, Center and Right). After 2 days of culture in the presence of IL-2, NK cells were partially triggered by mAb specific for NKp44 (a receptor expressed upon NK cell activation), while responses induced by anti-NKp46 and anti-NKp30 mAbs were substantially increased. At day 6, the increment of cytotoxicity was more evident and involved also NKG2D and DNAM-1 receptors. Addition of tumor-derived fibroblasts at the onset of the cultures resulted in partial inhibition of these functional changes. Although this effect was detectable at day 2 of coculture, it became more evident at day 6. In particular, in the presence of TF1 and TF2 the triggering capability of NKp44 and DNAM-1 was impaired. The NKp30 and NKG2D activation pathways were partially affected, while the NKp46 pathway was only marginally inhibited. At variance with tumor-derived fibroblasts, normal fibroblasts (lines HF1 and HF2) only minimally modified NK cell receptor functions.
Fig. 1.
Tumor-derived fibroblasts affect NK cells at functional level. NK cells were cultured either alone or with the indicated fibroblast cell lines and analyzed in functional assays. (A) At day 2 or 6 of culture (d. 2, d. 6), NK cells were tested in a redirected killing assay (E/T ratio: 2/1), either in the absence or in the presence of the mAbs to the indicated receptors. As indicated, NK cells were cultured either in the absence or in the presence of IL-2. Bars indicate the value means (± SD) from four independent experiments. (B) At day 6 of culture in IL-2, NK cells were also tested for their ability to kill the indicated melanoma cell lines. The E/T ratios are shown. The results are representative of three independent experiments. (C) At day 2 of culture in IL-2, NK cells were stimulated with mAbs to the indicated molecules and the supernatants were analyzed for TNF-α content. Bars indicate the value means (± SD) from three independent experiments.
We next assessed whether fibroblasts could also affect the ability of NK cells to kill tumors. As shown in Fig. 1B, NK cells that had been exposed to IL-2 for 6 days were able to kill (although with variable efficiency) both the established melanoma cell line FO-1 and the primary tumor cell lines derived from metastatic melanomas (MeCoP and MePA). However, cytolytic activity was substantially reduced in NK cells cocultured with tumor-derived fibroblasts. Also in this case, normal fibroblasts induced only a partial decrease of NK cell function. Cytofluorimetric analysis performed by the use of specific mAbs or soluble chimeric molecules (NKp44-Fc and NKp30-Fc) revealed that the melanoma cell lines analyzed expressed at their surface the ligands recognized by those NK receptors that were affected by coculture of NK cells and fibroblasts (Table 2).
Table 2.
Melanoma cell lines phenotype: analysis of the surface molecules involved in the NK cell/tumor cell interaction
| MIC A* | ULBP1* | ULBP2* | ULBP3* | PVR† | Nectin-2† | NKp30Fc | NKp44Fc | ICAM1 | |
|---|---|---|---|---|---|---|---|---|---|
| FO.1 | +‡ | − | + | ± | ++++ | ++ | − | ± | ++++ |
| MeCoP | ++ | − | ± | ± | ++++ | ++ | − | + | ± |
| MePA | − | − | ± | − | ++++ | ++ | − | + | ++++ |
Data are expressed as negative control m.f.i. (mean fluorescence intensity). Fold Increase, 0 (−); 1–4 (± ); 4–9 (+); 9–16 (++); 16–40 (+++); >40 (++++).
*NKG2D ligands.
†DNAM-1 ligands.
We further analyzed whether cytokine production induced by activating NK receptors engagement was affected as well. In particular, we analyzed NKp46, NKp30, and CD16 (i.e., those receptors that are known to induce cytokine release in short-term IL-2-cultured NK cells). After 2 days of culture either in the presence or in the absence of fibroblasts, NK cells were stimulated by receptor-specific mAbs, and the supernatants were assessed for the presence of TNF-α. As shown in Fig. 1C, upon coculture with tumor fibroblasts, NK cells displayed defective NKp30-induced TNF-α release, while the NKp46- or the CD16-mediated one was only minimally affected. A similar inhibitory effect was also detected on IFN-γ but not on GM-CSF production (Fig. S1).
Tumor-Derived Fibroblasts Inhibit the Expression of NK Receptors, Perforins, and Granzymes.
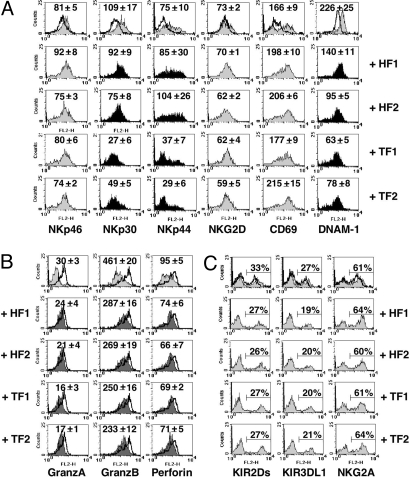
We next analyzed whether the functional defects documented in NK cells cocultured with tumor-derived fibroblasts would reflect an altered expression of certain NK cell surface receptors and/or of the molecules involved in NK cytolytic activity (i.e., perforins and granzymes). To this end, NK cells that had been cultured for 6 days in IL-2 either alone or in the presence of fibroblasts were analyzed by immunofluorescence and FACS analysis for the expression of activating or inhibitory NK receptors as well as for the presence of the major components of cytolytic granules. After 6 days of culture in the presence of IL-2, an increased surface density of NKp30, DNAM-1, and (partially) NKG2D activating receptors could be detected. In addition, NK cells de novo expressed NKp44 and CD69 (Fig. 2A). Remarkably, tumor-derived fibroblasts inhibited the cytokine-induced up-regulation of NKp44, NKp30, and DNAM-1 receptors, while only marginally affected the expression of NKp46 and NKG2D (Fig. 2A). In contrast, normal fibroblasts did not affect the expression of NKp44 (although they could partially inhibit the up-regulation of DNAM-1 and NKp30). Neither normal nor tumor-associated fibroblasts inhibited surface expression of CD69. The expression of cytolytic granules is generally up-regulated by IL-2. Fibroblasts (independent on their origin) slightly interfered with this process (Fig. 2B). Finally, analysis of HLA-specific receptors (KIR2DL1/S1, KIR3DL1, and NKG2A, shown in Fig. 2C) indicated that neither their surface density nor the percentage of receptor-positive cells was substantially modified by coculturing NK cells with fibroblasts.
Fig. 2.
Tumor-derived fibroblasts affect NK cells phenotype. NK cells were cultured in IL-2 either alone or with the indicated fibroblast cell lines, and analyzed by FACS. (A) The surface expression of the triggering NK receptors was analyzed at day 0 (i.e., freshly drawn NK cells; open profiles) or at day 6 (gray and black profiles). Black profiles indicate those markers whose expression significantly varied in the different culture conditions. (B) Intracytoplasmic analysis of the expression of granzymes and perforin. Gray profiles, day 0; open profiles, day 6 (NK cells cultured alone); deep gray profiles, day 6 (NK cells cocultured with fibroblasts). (C) Analysis of the HLA-specific NK-receptor surface expression. Open profiles, day 0; gray profiles, day 6 (NK cultured in IL-2 either alone or with the indicated fibroblast cell lines). The mean fluorescence intensities (m.f.i.) (indicated as m.f.i. mean ± SEM of four independent experiments) (A and B) or the percentage of receptor-positive cells (C) are referred to day 6 of culture. In NK cells derived from three additional donors (displaying different percent of KIR+ and NKG2A+ cells) the percentage of KIR2DL/S1+, KIR3DL/S1+, and NKG2A+ cells was not substantially modified by fibroblasts.
Both Cell-to-Cell Interaction and PGE2 Release Are Involved in the Inhibitory Effect of Tumor-Derived Fibroblasts on NK Cells.
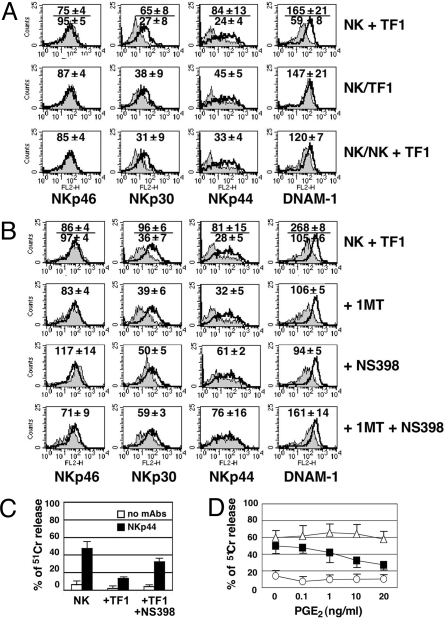
In an attempt to investigate the mechanism(s) responsible for the inhibitory effects of tumor-associated fibroblasts, coculture experiments were performed either in transwells or in the presence of inhibitors of soluble factors (produced by fibroblasts) known to regulate the NK cell function. As shown in Fig. 3A, under transwell conditions, the inhibitory effect of tumor-derived fibroblasts on the expression of NKp30 and NKp44 (although reduced) was still present, while no effect could be detected on DNAM-1. When the NK cell population was split and cultured in the same well, half in contact with fibroblasts and half in the upper transwell, the effect on NKp44 and NKp30 in NK cells cultured in the transwell (Fig. 3A, bottom line) was similar to that observed when NK cells were cultured in contact with fibroblasts (Fig. 3A, upper line). Also, under these experimental conditions, DNAM-1 expression was minimally affected (Fig. 3A, lower line). These results suggest that tumor-derived fibroblasts constitutively release soluble factor(s) capable of interfering with the NKp44 and NKp30 cytokine-induced up-regulation. Such release would be enhanced by the direct interaction between fibroblasts and NK cells. In contrast, inhibition of DNAM-1 expression appears to be dependent on cell-to-cell interaction. The main fibroblast-derived factors capable of immunoregulatory functions are PGE2, IDO/kynurenine, and TGF-β. As shown in Fig. 3B addition of the PGE2 inhibitor NS398 to cocultures restored the expression of NKp44 and NKp30. This also resulted in a significant recovery of NKp44 function (Fig. 3C). On the other hand, the IDO inhibitor 1methyl-tryptophan (1MT) had no effect (a slight effect was detected only when used in combination with NS398) (Fig. 3B). In no instances a blocking anti-TGF-β mAb could modify the NKp44 and NKp30 expression (Fig. S2). In line with the results shown in Fig. 3A the fibroblasts-induced inhibition of DNAM-1 was only marginally affected by the simultaneous blockade of PGE2 and IDO (Fig. 3B), or PGE2 and TGFβ (Fig. S2). Taken together, these experiments indicate that PGE2 can contribute to the inhibitory effect exerted by tumor-derived fibroblasts on NK cells. To assess whether PGE2 would act directly on NK cells, we analyzed its effect on NKp44-mediated cell activation. This was assessed in a redirected killing assay using NK cells that had been cultured for 6 days in the presence of IL-2 in combination with different doses of PGE2. As shown in Fig. 3D, NK cells cultured in the presence of PGE2 displayed a marked down-regulation of the NKp44-induced activation. Remarkably this occurred at concentration values detectable in our NK/fibroblasts cocultures (see Table 3). In line with these results, NK cells exposed to PGE2 also reduced the surface expression density of NKp44 (Fig. S3).
Fig. 3.
NK cells inhibition involves both cell-to-cell interactions and PGE2 release by tumor fibroblasts. (A–C) NK cells were cultured for 6 days in IL-2, either alone or with tumor-derived fibroblasts and were analyzed by FACS (open and gray profiles, respectively) or in a redirected killing assay. NK cells were cocultured with fibroblasts in the same well (NK + TF1), in Transwell devices (NK/TF1) or (in the same well) half with fibroblasts and half in Transwell (NK/NK + TF1) (A). NK cells were cocultured with fibroblasts either in the absence or in the presence of the indicated inhibitors and analyzed by FACS (B) or in cytolytic assay (C). NK cells cultured for 6 days in IL-2 plus the indicated PGE2 concentrations were analyzed in a redirected killing assay either in absence (white circle) or in presence of anti-NKp30 (white triangle) or anti-NKp44 (black square) mAbs (D). In A and B, the m.f.i. mean ± SEM (n = 3) are indicated. In C and D, the percentage of cytotoxicity mean ± SEM (n = 3) are indicated.
Table 3.
Analysis of the effect of fibroblasts on the expression of NKp44 and correlation with the ability to release PGE2
| Fibroblast cell lines | % NKp44 m.f.i. inhibition (Fibr/NS398)* | PGE2 (pg/ml) without NK | PGE2 (pg/ml) with NK | |
|---|---|---|---|---|
| Patient #1 | TF1 | 69/48 | 4,294.6 | 6,916 |
| Patient #2 | TF2 | 84/59 | 1,802 | 4,617 |
| Patient #3 | TF3 | 62/n.d. | 1,580 | 6,810 |
| Patient #4 | TF4 | 95/0 | 6,616 | 6,650 |
| Patient #5 | TF5 | 71/n.d. | n.d. | n.d. |
| Patient #6 | TF6 | 71/70 | 296 | 1,302 |
| Patient #7 | TF7 | 55/18 | 557.3 | 1,033.3 |
| Patient #8 | TF8 | 61/0 | 344 | 6,092 |
| Patient #9 | TF9 | 58/n.d. | 6,710 | 6,812 |
| Patient #10 | TF10 | 92/44 | 3,041.3 | 6,622 |
| Patient #11 | TF11 | 72/55 | 4,677 | 15,107 |
| Healthy #1 | HF1 | 0 | 25.4 | 41.4 |
| Healthy #2 | HF2 | 0 | 29.2 | 394.7 |
| Healthy #3 | CRL-2106 | 9/2 | 37.7 | 88.7 |
| Healthy #4 | CRL-2201 | 41/38 | 212.2 | 231.2 |
*values indicate the % of NKp44 m.f.i. inhibition induced by fibroblasts either in the absence or in the presence of PGE2 inhibitor NS398.
Correlation Between PGE2 Release and Inhibitory Functions in Tumor Fibroblasts Derived from 11 Patients.
To further define the role of PGE2 in the inhibitory function of tumor-derived fibroblasts, primary fibroblast cell lines were derived from melanoma lesions of nine additional patients and analyzed (together with TF1 and TF2) for their ability to release PGE2 and to inhibit NKp44 expression in fibroblasts/NK cells cocultures. In the same experiment HF1, HF2, and two additional primary fibroblast cell lines (CRL-2106 and CRL-2201) derived from the skin of healthy subjects were also analyzed. Fibroblasts and NK cells were cultured in IL-2 for 6 days (either alone or in combination). Culture supernatants were harvested and analyzed for the presence of PGE2 by ELISA, while NK cells were assessed by FACS for the expression of NKp44. As shown in Table 3, all tumor-derived fibroblast cell lines, inhibited NKp44 expression in NK cells. Inhibition was completely blocked or reduced by the addition of NS398 at the onset of the cocultures (Table 3). Supernatant of tumor fibroblasts cultured alone contained PGE2, thus implying its spontaneous release. More importantly, PGE2 release was greatly enhanced in the presence of NK cells. Among normal fibroblast cell lines, only CRL-2201 displayed a slight inhibitory effect on NKp44 expression in NK cells and was found to spontaneously release low amounts of PGE2 (which was not increased in coculture experiments).
Discussion
In the present study, we provide evidence that fibroblasts isolated from metastatic melanoma specimens sharply interfere with the induction of NK cell effector functions. This inhibition profoundly impacts on the ability of NK cells to kill melanoma cells. We show that tumor-associated fibroblasts inhibit the IL-2-driven up-regulation of triggering receptors that are involved in the NK-mediated recognition and killing of tumor cells. This suppressive function, which is largely dependent on the PGE2 release, seems to be specifically induced on fibroblasts by the tumor microenvironment, because skin fibroblasts from healthy subjects only minimally affect NK cells and, also, do not release PGE2.
It is well known that the development and survival of tumors are largely dependent on the ability to create a microenvironment that would favor tumor growth and invasion and subvert defense mechanisms (27, 28, 30). In this context, cells of mesenchymal origin, including fibroblasts, may play a pivotal role. Different soluble factors released by tumor cells (such as bFGF, TGF-β, and PDGF) activate local fibroblasts, which, in turn, may substantially modify their phenotypic and functional features (28, 31, 32). In particular, when exposed to these stimuli, fibroblasts express proteases (such as matrix metalloproteases and FAP), which can favor remodeling of ECM and tumor invasion. In addition, they release growth factors and cytokines [including insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1)], which can favor tumor cell proliferation and angiogenesis and may also affect immune responses (28, 31, 32). In our study, we comparatively analyzed fibroblasts that were derived from either skin or lymph node (L.N.) metastases with normal skin fibroblasts (see Materials and Methods). Thus, at least in the case of skin metastases, the functional phenotype of fibroblasts appears to be related to the presence of the tumor. It should be noted, however, that, although displaying differences in the spontaneous PGE2 release capability, nodal and skin tumor-derived fibroblasts showed similar capability of modulating NK cells. These data suggest a general role for the melanoma microenvironment in the induction of the suppressive phenotype of tumor-associated fibroblasts. So far only few studies have investigated the capability of tumor-associated fibroblasts to directly modulate the function of cells involved in the local immune response against tumors (33, 34). Nazareth and colleagues (34) recently reported that fibroblasts from lung tumors could modulate CD3-induced T cells activation. Conflicting data have also been obtained on the capability of mesenchymal cells isolated from normal tissues of modulating T or NK cell functions. For example, fibroblasts isolated from different tissues could inhibit T cell functions (35, 36), while in other studies, bone marrow-derived mesenchymal stromal cells could activate NK cell functions (37). Under our experimental conditions, the capability of inhibiting NK cell function was essentially confined to tumor-derived fibroblasts. Accordingly, it has recently been shown that mesenchymal tumor cells share common molecular and functional features with MSCs (38), a cell type whose immunosuppressive capabilities have been widely demonstrated (22, 39–41).
With regard to the mechanism of inhibition, we show that normal skin fibroblasts can inhibit only DNAM-1 and, partially, cytolytic granule expression. Moreover, this effect only occurs in a cell-to-cell contact-dependent way. In contrast, tumor-derived fibroblasts, also inhibit sharply the expression of NKp44 and NKp30 activating receptors and this effect mainly reflects the production of PGE2, although PGE2 may not represent the sole agent involved. Indeed in some cases (TF6 and TF7; Table 3), the PGE2 released in cocultures reached levels that are poorly effective in functional assays (Fig. 3D and Fig. S3).
Killing melanoma cells by NK cells exposed to normal or tumor-derived fibroblasts, appears to correlate with the degree of down-regulation of critical activating NK-receptors. Indeed, the ligands of both NKp44 and DNAM-1 were expressed by melanoma target cells analyzed (Table 2). This is in line with a recent report indicating the prominent role of NCR and DNAM-1 in the NK-mediated recognition and killing of melanoma cells (3). Interestingly, modulation of NK cell function can occur (although at variable extent) at different NK/fibroblast ratios (see Table S2), thus suggesting that a similar inhibitory effect may occur also in the case of relevant NK cell infiltration in vivo. Notably, the slight inhibitory activity elicited by normal fibroblasts may not reflect their actual function in vivo, since, in culture, they may undergo artifactual activation (as also suggested by their faint but detectable, FAP expression; Table 1). Although this experimental bias may also involve tumor-derived fibroblasts, the sharp functional difference displayed by the two cell types in vitro (Table 3), together with the fact that fibroblasts populating tumor stroma are constitutively activated (28, 30), suggests that the ability to modulate NK cell function may be a property of tumor-associated fibroblasts in vivo.
Another, remarkable finding regards the expression of CD56 that is confined to normal fibroblasts (HF1 and HF2; Table 1). Importantly, these data are in agreement with the concept that the down-regulation of N-CAM mediated homophilic interactions may be one of the mechanisms by which tumor cells reduce adhesion to the tumor stroma and acquire metastatic properties (42).
Up-regulated expression of COX-2 and PGE2 production has been documented in different tumors including melanomas (27, 43, 44). However, the debate on which cells, within the melanoma lesion, actually express COX-2 is still open. Different studies report the COX-2 expression either on melanoma cells (44) or fibroblasts or keratinocytes or inflammatory cells (43). These findings suggest that, besides fibroblasts, other cells of the tumor microenvironment should be considered in studies aimed at assessing the actual NK cell activity at the tumor site.
That tumor-induced fibroblasts could have a dramatic effect on NK cell activity has not yet been documented. Moreover the concept that, at the tumor site, NK cells could trigger a paradoxical suppressive loop by enhancing the PGE2 release by tumor associated fibroblasts (Table 3), opens an interesting area of investigation in the field of the immunosubversion strategies developed by tumors. Indeed, this observation suggests that tumor-associated fibroblasts not only suppress NK cells, but may also interact with them eliciting a reciprocal active cross-talk.
Remarkably, the effect of tumor-associated fibroblasts on NK cells, did not involve a general blockade of the process of NK cell activation. Indeed the surface expression of CD69 and NKp46 that are either up-regulated (NKp46) or induced ex-novo (CD69) after exposure to IL-2 (9, 11) was not inhibited. In this context, the expression of the different IL-2 receptor subunits (CD122, CD132, and CD25) was not significantly modified by the interaction with fibroblasts (Table S3). Finally, the analysis of KIRs, NKG2A (Fig. 2) or CD56 and CD16 (Fig. S4) expression indicate that neither tumor-derived nor normal fibroblasts were able to favor or to inhibit proliferation of known defined NK cell types (such as KIR+, NKG2A+, or CD56brightCD16+/− NK cell subsets) (45).
Both normal and tumor-derived fibroblasts exerted a protective effect on resting NK cells since, in the absence of cytokines, NK cell viability was higher in coculture conditions as compared to fibroblast-free cultures (Table S4). In this context, it has recently been proposed that fibroblasts could favor the differentiation of CD56bright NK cells into CD56dim mature effector cells (46). Thus, once NK cells have reached tissues, thanks to the function exerted by stromal fibroblasts, they may be induced to mature and may also be maintained viable until they would be fully activated by appropriate stimuli. The tumor microenvironment, however, may interfere with this mechanism by inducing fibroblasts to limit NK cell killing capabilities. That such fibroblast/NK cell interaction could actually occur at the tumor site is indicated by our immunohistochemistry data (Fig. S5). Indeed, these experiments clearly show that NK cells are detectable in close vicinity to fibroblasts surrounding the metastatic lesion (at least in L.N. metastases).
The fact that convergent strategies, including the presently described inhibitory activity induced in tumor-associated fibroblasts, the down-regulation of NKG2D-ligands from the cell surface, or their release as soluble molecules (47), are adopted by melanomas to escape NK cells, witnesses the selective pressure exerted by these cells on tumor progression in melanomas. Further studies should be performed to extend our knowledge on this matter also in view of the recent attempts to exploit NK cell-based cellular therapies against melanoma (47).
Materials and Methods
Monoclonal Antibodies.
The following mAbs, produced in our laboratory, were used in this study: c127 (IgG1, anti-CD16), c218 (IgG1, anti-CD56), AZ20 (IgG1, anti-NKp30), BAB281 (IgG1, anti-NKp46), Z231 (IgG1, anti-NKp44), BAT221 (IgG1, anti-NKG2D), c227 (IgG1, anti-CD69), GN18 (IgG3, anti-DNAM-1), GL183 (IgG1, anti-KIR2DL2/L3/S2), Z27 (IgG1, anti-KIR3DL1), Z199 (IgG2b, anti-NKG2A), M7E22 (IgG1, anti-ICAM-1), M5A10 (IgG1, anti-PVR). 170818, 165903, and 166510 mAbs (IgG2a, anti-ULBP-1, anti-ULBP2, and anti-ULBP3, respectively; R& D System Europe); 14.G2a mAb (anti-GD2; BD PharMingen); 5B5 mAb (anti-4PH; Dako Italia); F19 mAb (anti-FAP, IgG1; ATCC); PE-anti-perforin mAb and anti-CD106 (Ancell); PE-anti-granzyme A (BD PharMingen); PE-anti-granzyme B (clone GB12; CALTAG) were commercially available. BAM195 (IgG1, anti-MICA); L14 (IgG2a, anti-Nectin-2) were provided by D. Pende (Genova, Italy).
Isolation of NK Cells.
To obtain purified NK cells, the RosetteSep NK Cell Enrichment kit (StemCell Technologies) was used as described in ref. 11. Only those populations displaying >95% of CD56+ CD3− CD14− NK cells were selected.
Fibroblasts and Melanoma Cells.
A panel of 12 pathology-confirmed metastatic melanoma tumor resections was obtained in accordance with consent procedures approved by the Institutional Review Board for these studies. Mechanically dissociated tissues underwent enzymatic digestion in 500 units/mL collagenase type IA and 300 units/mL hyaluronidase (Sigma-Aldrich). Cell suspensions derived from tumor specimens were seeded in 6-well tissue plates and cultured in RPMI 1640 medium plus 10% FCS. Wells giving rise to cell populations homogeneously displaying fibroblast or melanoma cell morphology were selected. All fibroblast and melanoma cell cultures were phenotypically characterized and assessed for purity by the analysis of informative markers including P4H, FAP, CD146 (Mel-CAM), and GD2. Fibroblasts cell lines (TF1–11) and melanoma cell lines (MeCoP and MePa) were then used within 15 passages. TF1, 4, 5, 9, 10, 11, and TF2, 3, 6, 7, 8 were derived from L.N. and skin metastases, respectively. TF2 and MeCop cell lines were from the same tumor specimen. Normal skin fibroblasts were obtained in our laboratory (HF1 and HF2) or purchased from ATCC (CRL-2201 and CRL-2106).
NK Cell/Fibroblasts Coculture.
NK cells were cultured in RPMI-1640 10% FCS and (when indicated) 100 IU/mL rhIL-2 (proleukin; Chiron), in 96-well flat bottom microtiter plates (5 × 104 cells per well) either in the absence or in the presence of fibroblasts (NK/fibroblasts ratio: 2.5/1). At the indicated time intervals NK cells were harvested, counted, and analyzed. When indicated, 1 mM 1MT (Sigma–Aldrich) and/or 5 μM NS398 (Cayman Chemicals) were added at the onset of cocultures.
Preparation of Soluble Chimeric Receptors.
The constructs for the production of NKp44Fc and NKp30Fc molecules were prepared as described in ref. 48.
Flow Cytofluorimetric Analysis and Cytolytic Activity.
For cytofluorimetric analysis (FACSCalibur; Becton Dickinson) cells were stained with the appropriate unlabeled mAb or soluble chimeric receptors produced in our laboratory (NKp30Fc or NKp44Fc) or purchased from R&D Systems Europe (NKp30Fc). Staining was followed by PE-conjugated isotype specific goat anti-mouse or goat anti-human second reagent (Southern Biotechnology Associated). NK cells were tested for cytolytic activity in a 4-h 51Cr-release assay. The concentration of the mAbs added in the assays was 1 μg/mL.
Cytokine Secretion Assays.
NK cells were stimulated as follows. NK cells (5 × 104 per well) were cultured overnight in 96-well microtiter plates precoated with goat anti-mouse (GAM; ICN) in the presence of the mAb supernatants indicated. The culture supernatants were then collected and analyzed for the presence of TNFα by specific ELISA (BioSource International).
Supplementary Material
Acknowledgments.
We thank S. Lonardi and C. Rossini (University of Brescia) for technical support, A. Poggi (Istituto Nazionale per la Ricerca sul Cancro di Genova) for fruitful discussion on the study, F. Cafiero and N. Solari (Istituto Nazionale per la Ricerca sul Cancro di Genova) for providing metastatic melanoma biopsies. This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro; Ministero della Salute: Ricerca Oncologica Project of integrated program 2006–20008, and Ricerca Finalizzata (2005 and 2006); Istituto Superiore di Sanità; Ministero dell'Istruzione dell'Università e della Ricerca (Programmi di Ricerca di rilevante Interesse Nazionale) 2006 and 2007.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906481106/DCSupplemental.
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 3.Lakshmikanth T, et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119:1251–1263. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moretta L, et al. Different checkpoints in human NK-cell activation. Trends Immunol. 2004;25:670–676. doi: 10.1016/j.it.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 6.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 7.Boles KS, Barchet W, Diacovo T, Cella M, Colonna M. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood. 2005;106:779–786. doi: 10.1182/blood-2005-02-0817. [DOI] [PubMed] [Google Scholar]
- 8.Pogge von Strandmann E, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Moretta A, et al. CD69-mediated pathway of lymphocyte activation: Anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J Exp Med. 1991;174:1393–1398. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitale M, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells is involved in non-MHC restricted tumor cell lysis. J Exp Med. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Della Chiesa M, et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood. 2006;108:4118–4125. doi: 10.1182/blood-2006-03-006700. [DOI] [PubMed] [Google Scholar]
- 12.Ferrini S, Miescher S, Zocchi MR, von Fliedner V, Moretta A. Phenotypic and functional characterization of recombinant interleukin 2 (rIL 2)-induced activated killer cells: Analysis at the population and clonal levels. J Immunol. 1987;138:1297–1302. [PubMed] [Google Scholar]
- 13.Fehniger TA, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: Implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 14.Parrish-Novak J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 15.van de Wetering D, de Paus RA, van Dissel JT, van de Vosse E. IL-23 modulates CD56+/CD3- NK cell and CD56+/CD3+ NK-like T cell function differentially from IL-12. Int Immunol. 2009;21:145–153. doi: 10.1093/intimm/dxn132. [DOI] [PubMed] [Google Scholar]
- 16.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mailliard RB, et al. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–953. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castriconi R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez NC, et al. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 20.Moretta A, et al. Early liaisons between cells of the innate immune system in inflamed peripheral tissues. Trends Immunol. 2005;26:668–675. doi: 10.1016/j.it.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Welte S, Kuttruff S, Waldhauer I, Steinle A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 22.Spaggiari GM, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 23.Scordamaglia F, et al. Perturbations of natural killer cell regulatory functions in respiratory allergic diseases. J Allergy Clin Immunol. 2008;121:479–485. doi: 10.1016/j.jaci.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 24.Zambello R, et al. Expression and function of KIR and natural cytotoxicity receptors in NK-type lymphoproliferative diseases of granular lymphocytes (LDGL) Blood. 2003;102:1797–1805. doi: 10.1182/blood-2002-12-3898. [DOI] [PubMed] [Google Scholar]
- 25.Carrega P, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–875. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 26.Fauriat C, Moretta A, Olive D, Costello RT. Defective killing of dendritic cells by autologous natural killer cells from acute myeloid leukemia patients. Blood. 2005;106:2186–2188. doi: 10.1182/blood-2005-03-1270. [DOI] [PubMed] [Google Scholar]
- 27.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: Immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 28.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 29.Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair—Current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 30.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 31.Li G, et al. Function and regulation of melanoma-stromal fibroblast interactions: When seeds meet soil. Oncogene. 2003;22:3162–3171. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- 32.Silzle T, Randolph GJ, Kreutz M, Kunz-Schughart LA. The fibroblast: Sentinel cell and local immune modulator in tumor tissue. Int J Cancer. 2004;108:173–180. doi: 10.1002/ijc.11542. [DOI] [PubMed] [Google Scholar]
- 33.Silzle T, et al. Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur J Immunol. 2003;33:1311–1320. doi: 10.1002/eji.200323057. [DOI] [PubMed] [Google Scholar]
- 34.Nazareth MR, et al. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol. 2007;178:5552–5562. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 35.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 36.Haniffa MA, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 37.Poggi A, et al. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: Role of NKp30 and NKG2D receptors. J Immunol. 2005;175:6352–6360. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 38.Galiè M, et al. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene. 2008;27:2542–2551. doi: 10.1038/sj.onc.1210920. [DOI] [PubMed] [Google Scholar]
- 39.Di Nicola M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 41.Corcione A, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 42.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 43.Goulet AC, et al. Analysis of cyclooxygenase 2 (COX-2) expression during malignant melanoma progression. Cancer Biol Ther. 2003;2:713–718. [PubMed] [Google Scholar]
- 44.Becker MR, Siegelin MD, Rompel R, Enk AH, Gaiser T. COX-2 expression in malignant melanoma: A novel prognostic marker? Melanoma Res. 2009;19:8–16. doi: 10.1097/CMR.0b013e32831d7f52. [DOI] [PubMed] [Google Scholar]
- 45.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 46.Chan A, et al. CD56bright human NK cells differentiate into CD56dim cells: Role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 47.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: Moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 48.Vacca P, et al. Regulatory role of NKp44, NKp46, DNAM-1 and NKG2D receptors in the interaction between NK cells and trophoblast cells. Evidence for divergent functional profiles of decidual versus peripheral NK cells. Int Immunol. 2008;20:1395–1405. doi: 10.1093/intimm/dxn105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.