Abstract
Accumulating evidence suggests that hyperproliferating intestinal stem cells (SCs) and progenitors drive cancer initiation, maintenance, and metastasis. In addition, chronic inflammation and infection have been increasingly recognized for their roles in cancer. Nevertheless, the mechanisms by which bacterial infections can initiate SC-mediated tumorigenesis remain elusive. Using a Drosophila model of gut pathogenesis, we show that intestinal infection with Pseudomonas aeruginosa, a human opportunistic bacterial pathogen, activates the c-Jun N-terminal kinase (JNK) pathway, a hallmark of the host stress response. This, in turn, causes apoptosis of enterocytes, the largest class of differentiated intestinal cells, and promotes a dramatic proliferation of SCs and progenitors that serves as a homeostatic compensatory mechanism to replenish the apoptotic enterocytes. However, we find that this homeostatic mechanism can lead to massive over-proliferation of intestinal cells when infection occurs in animals with a latent oncogenic form of the Ras1 oncogene. The affected intestines develop excess layers of cells with altered apicobasal polarity reminiscent of dysplasia, suggesting that infection can directly synergize with the genetic background in predisposed individuals to initiate SC-mediated tumorigenesis. Our results provide a framework for the study of intestinal bacterial infections and their effects on undifferentiated and mature enteric epithelial cells in the initial stages of intestinal cancer. Assessment of progenitor cell responses to pathogenic intestinal bacteria could provide a measure of predisposition for apoptotic enterocyte-assisted intestinal dysplasias in humans.
Keywords: cancer, cell polarity, cytokines, tumor, virulence factors
The intestine is one of the fastest-renewing tissues of metazoa and its rapid cell turnover is a necessary response to enterocyte apoptosis or exfoliation caused by the passage, digestion, and absorption of food and various xenobiotics (1). Concurrently, the intestine must tolerate an extensive load of microbiota and is susceptible to various types of acute and chronic infection that can elicit intestinal inflammation. Not surprisingly, alterations in human microbiota, as well as bacterial infections, mainly due to Helicobacter species, have been inextricably linked to gastrointestinal disease and cancer (2–4). Yet while bacterial infection has been associated with blood cell infiltration and the induction of immune responses, its role in carcinogenesis has not been demonstrated conclusively (3–5). In addition, while the roles of the Notch and K-Ras signaling pathways in human colorectal cancer have been unequivocally demonstrated (6, 7), the contribution of these pathways in stem cells (SCs) and progenitors in intestinal tumor initiation remains elusive.
The evidence that hyperproliferating intestinal SCs and progenitors drive cancer initiation, maintenance, and metastasis (8–10), and that chronic inflammation has a role in cancer (4, 5, 11), indicates that under some conditions, hyperproliferation diverts from homeostasis to tumorigenesis. Among the various oncogenes, K-Ras is a commonly identified factor that drives gastrointestinal tumors (6). We hypothesized that cytologically undetectable activation of K-Ras and other oncogenes or reduction in expression of tumor suppressors might lead to perturbations in intestinal progenitors during infection. We therefore sought to model tumor initiation upon infection in the genetically-tractable model organism Drosophila melanogaster, a species that shares striking similarities with mammals in terms of overall physiology, cell biology, and signal transduction pathway components (12).
We analyzed the intestinal cell changes that accompany infection with P. aeruginosa, a prevalent human opportunistic pathogen (13), especially in immunocompromised and neutropenic cancer patients undergoing radiation, chemotherapy or bone marrow transplant (14, 15) in D. melanogaster. We found that virulent P. aeruginosa induces apoptosis, c-Jun N-terminal kinase (JNK) pathway activation and the proliferation of intestinal SCs and progenitors upon infection. Importantly, infection of flies bearing a latent oncogenic form of the Ras1 proto-oncogene, produces a profound increase in SCs and progenitors, epithelial multilayering and changes in the apicobasal polarity marker Armadillo.
Results and Discussion
P. aeruginosa Infection Increases the Number of esg-Positive SCs or Progenitors
In this study, flies were infected with P. aeruginosa strains via feeding and the highly virulent PA14 strain (16) killed almost all flies within 20 days, while the avirulent CF5 strain (16) failed to establish fatal infections (Fig. S1). Subsequent analysis of the expression of the SC and progenitor marker gene escargot (esg) using either the esg-lacZ or esg-GAL4 UAS-GFP reporter (17) revealed that intestinal infection of flies with the highly-virulent P. aeruginosa strain PA14 caused a striking increase in esg-positive (esg+) cells following infection, predominantly in the posterior part of the midgut (Fig. 1 A and B and Fig. S2 A and C). In contrast, the avirulent P. aeruginosa strain CF5 did not promote such a prominent effect (Fig. 1C). PA14 infection resulted in tissue hyperplasia, manifested by a significant increase in the width of the posterior midgut compared to that of uninfected flies (Fig. S3A). Interestingly, the phenotype of hyperplasia (Fig. S3A) and the concomitant increase in the number of esg+ cells (Fig. S4 A and C) was reversible upon bacteria clearance, suggesting that the presence of virulent bacteria is a prerequisite for this phenotype.
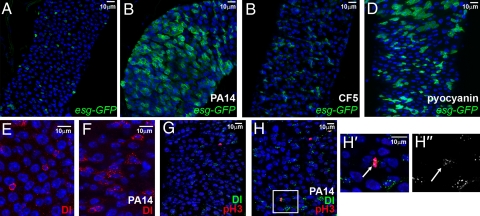
Fig. 1.
P. aeruginosa infection induces intestinal progenitor expansion. (A–D) Posterior midgut cells of uninfected (A), strain PA14 infected (B), strain CF5 infected (C) and pyocyanin-fed (D) flies. SCs and progenitors of esg-GAL4 UAS-srcGFP flies fed for 5 days are shown in green. (E and F) Posterior midgut SCs marked with α-Delta antibody (red) of uninfected (E) and PA14-fed (F) flies. (G, H) Posterior midgut SCs marked with α-Delta (green) and phospho-histone-H3 (pH3; red) antibody, of uninfected (G) and PA14-fed (H) flies. (H′ and H″) Magnification of rectangular region in (H), showing colocalization of pH3 (H′; arrow) and Delta (H′ and H″; arrow) staining. Nuclei in all panels are marked with DAPI (blue).
In control experiments, flies infected with heat-killed PA14 or subjected to PA14 clearance postinfection were not killed (no mortality over the course of 20 days) and did not have an altered SC compartment (Fig. S4). In addition, fly feeding on media containing purified pyocyanin, a secreted virulence factor of P. aeruginosa that is not produced by the CF5 strain, as assessed by comparing the absorbance at OD520 nm of the fully pyocyanin producer strain PA14 to that of CF5, increased the percentage of esg+ cells (P = 0.03, n = 5 intestines) (Fig. 1D). This finding indicates that pyocyanin secretion by virulent P. aeruginosa is one of the factors that stimulate proliferation of SCs and progenitors during infection.
Hyperplasia in P. aeruginosa Infected Fly-Gut Is Associated Predominantly with SC Divisions
To distinguish whether the observed increase in esg+ cells was due to proliferation of SCs or progenitors, or to some other mechanism, such as dedifferentiation, we looked at two indicators to determine if SCs divide during infection: the SC restricted expression of Delta (Dl) (18) and the presence of phosphorylated histone-H3 (pH3), a marker for mitosis. We found a 2.5-fold increase in Delta-positive (Dl+) cells upon infection (P = 0.007, n = 6 intestines, >1,000 cells) (Fig. 1 E and F), indicating that the increase occurs predominantly in SCs. Likewise, we found a 3-fold increase in mitosis (P = 0.0005, n = 6 intestines, >1,000 cells) (Fig. 1 G and H), indicating that the effect of infection is not due to dedifferentiation of cells, but rather to the expansion of cells. Importantly, as in uninfected (18), the pH3 positive (pH3+) cells of infected flies were restricted to the SCs (Fig. 1 H′ and H″), as marked by Delta, indicating that the infection caused an overproliferation of SCs. It is worth noting that transient-amplifying cells that arise from SCs, and that are necessary for the amplification of the mammalian intestine progenitor population, have not been previously described in Drosophila. Nevertheless, they might arise from Drosophila SCs upon infection and the increase in mitosis of Dl+ cells upon infection may reflect the divisions of such transient-amplifying cells rather than SCs per se.
Infection Does Not Inhibit Intestinal Cell Differentiation Rate
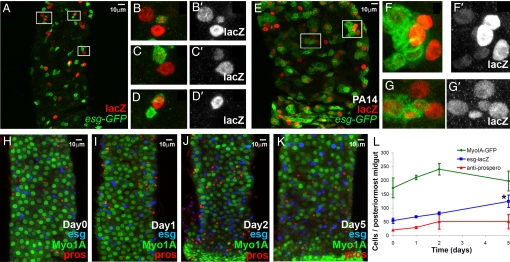
To assess if infection affects other aspects of gut homeostasis, such as the differentiation of SCs into mature cells, we used the flip-out lineage tracing technique (19) to mark lacZ clones of cells arising from esg+ cells. Fig. 2 shows that clones with differentiated cells were present in both noninfected and infected flies. At day 5 post-PA14 infection, 64 ± 18% of the clones contained mature cells, as evidenced by the lack of esg expression (esg−) and the big nucleus size. Similarly, in wild-type (wt) animals, 67 ± 11% of clones contained mature (esg−) cells, indicating that infection did not alter the frequency at which SCs gave rise to differentiated cells (n = 96 clones) (Fig. 2 E–G). Thus, the infection appeared to alter SC and progenitor numbers, without restricting subsequent differentiation steps. To assess if the production of differentiated cells during PA14 infection results in mature cell homeostasis, we performed a time-course analysis measuring the numbers of intestinal cells expressing myosin1A, prospero and esg, which correspond to the mature enterocytes, the enteroendocrine cells, and the undifferentiated cells, respectively. We observed no overlap in the expression of these markers under any condition tested (n = >10 intestines), indicating that, these markers can be used to label distinct cell populations in infected flies. We did not find a significant increase in the numbers of mature cells, although the numbers of esg+ cells gradually increased approximately 2.5-fold in the course of infection (Fig. 2 H–L). Thus, it can be deduced that infection increases progenitor population leading to the production of mature cells, establishing cellular homeostasis.
Fig. 2.
Mature midgut cells are produced and cellular homeostasis is sustained during infection. (A–K) Lineage tracing of midgut clones induced by esg expressing cells in the absence (A–D) or presence (E–G) of PA14 infection. Green represents GFP driven by esg-GAL4 and red represents nuclear lacZ, the lineage-tracing marker. esg+ cells produce mature enterocytes in both cases (B–D, F, and G), although in the case of PA14 infection, the clone size increases (E–G). (H–L) Time-course of PA14 infection. Myo1A-Gal4 UAS-GFP flies were fed on PA14-containing food for 0 (H), 1 (I), 2 (J), and 5 days (K) and the different cell types [Myo1A+, mature enterocytes (green), esg-lacZ+, SCs and progenitors (blue) and prospero+, enteroendocrine cells (red)] comprising their posterior-most midguts were counted. (L) Only esg+ cell numbers changed significantly during the course of infection (≈2.5-fold increase at day 5, *, P = 0.05; n = 3 intestines).
Infection Induces Apoptosis of Mature Cells
The observation that there was an increase in progenitor population without a continuous increase in enterocytes and enteroendocrine cells, also suggests that the mature cells may have been dying at a faster rate due to the infection. SCs may multiply to compensate for the loss of dying cells. This possibility is consistent with the observation in mammals that proliferating SCs preserve the integrity of the gut (1). To assess this, we used antibodies against the activated cleaved form of caspase-3, which marks apoptotic cells. We also used acridine orange, another indicator of apoptosis, and observed increased staining in patches of cells in infected flies (n = 5 intestines). Importantly, esg+ cells did not show significant overlap with apoptotic cells (Fig. 3 B, E, and F), indicating that mature cells are more prone to apoptosis.
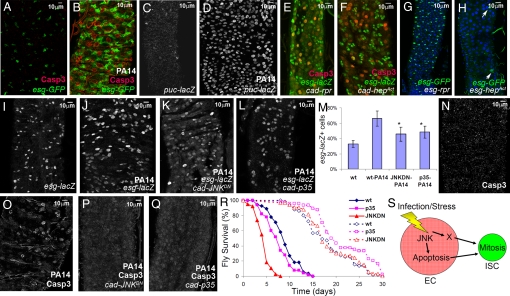
Fig. 3.
P. aeruginosa infection triggers proliferation of SCs and progenitors via JNK-mediated apoptosis of enterocytes. (A and B) Uninfected (A) and PA14-fed (B) midgut enterocytes of esg-GAL4 UAS-srcGFP flies show substantial activation of caspase-3 (red) with concomitant accumulation of GFP (esg+ cells) in their neighboring SCs and progenitors. (C, D) JNK pathway activation in uninfected (C) and PA14-fed (D) midgut cells of puc-lacZ flies. (E and F) Overexpression of either the apoptotic gene reaper (E) or the activated form of the JNK activating kinase HepAct (F) in the all posterior midgut activates caspase-3 (red) in enterocytes neighboring esg+ cells. (G and H) Expression of reaper (G) or HepAct (H) in the progenitor population does not increase their numbers (arrows in H). (I–R) JNK pathway inhibition via overexpression of JNKDN or inhibition of cell death by p35 overexpression in all posterior midgut cells exhibited a reduced number of esg+ cells (P < 0.05, n = 5 intestines, >1,000 cells; I–M), absence of strong apoptotic caspase-3 staining (N–Q) and increased mortality (solid lines in R, P < 0.001, n = 40) following infection compared to the isogenic control flies. Longevity of these flies (dashed lines in R) is not restricted. (S) Model illustrating the JNK-apoptosis cassette and a putative factor X acting in parallel, or sequentially, to induce SC mitosis upon enterocyte (EC) infection or stress.
Infection Induces JNK Pathway Activation
Since apoptosis and inflammation are often induced via JNK pathway activation in flies and mammals (20), we monitored the intestines of uninfected and PA14-fed flies for the expression of a reporter of JNK pathway activation, puc-lacZ. Only PA14-infected flies expressed this reporter in the majority of the posterior midgut cells (Fig. 3 C and D). Moreover, puc-lacZ positive cells showed no overlap with esg+ cells (n = 7 intestines). These findings indicate that apoptosis and induction of the JNK pathway are specifically activated in mature cells, while undifferentiated esg+ cells are resistant to apoptosis and JNK pathway activation.
Enterocyte Apoptosis Induces SC or Progenitor Proliferation in the Absence of Infection.
Given that infection causes death of enterocytes, we examined whether this was sufficient to stimulate increased proliferation of SCs and progenitors. Overexpression of the apoptotic gene reaper (rpr) (Fig. 3E) or the activated form of the JNK kinase, HepAct (Fig. 3F), in the posterior midgut cells induced enterocyte apoptosis and the concomitant appearance of supernumerary esg+ cells. Control experiments driving reaper or HepAct expression specifically in esg+ cells revealed that the % of these cells did not increase (Fig. 3 G and H), demonstrating that apoptosis and JNK activation in mature cells, rather than in SCs and progenitors can lead to SC or progenitor multiplication.
Inhibition of Apoptosis During Infection Inhibits Proliferation.
To address whether the death of enterocytes during infection is the driving signal for esg+ cell expansion, we expressed the baculovirus protein p35, a universal apoptosis inhibitor, in all cells of the posterior midgut. As a result, the increase in the SC and progenitor population due to infection was inhibited (Fig. 3 G, L, and M). In addition, when a dominant-negative form of JNK (JNKDN) was expressed in all cells of the posterior midgut, the percentage of esg+ cells and the strong activated caspase-3 staining were both reduced upon infection (Fig. 3 G, K, and M–Q) compared with wt controls (Fig. 3 B and D). Importantly, these isogenic p35 or JNKDN expressing flies died faster after being infected (Fig. 3R) (P < 0.001), indicating that increased intestinal regeneration during infection is important for host homeostasis. hep1 flies, which are also defective in JNK activation, showed 50 and 100% mortality post infection at approximately 5 and 9 days, respectively, which is similar to that of JNKDN flies and significantly faster than wt Oregon R flies (Fig. 3R). These results collectively show that induction of apoptosis in the mature enterocytes is both necessary and sufficient to induce supernumerary SCs or progenitors, and that at least one consequence of the JNK pathway in the midgut is to induce mature cell apoptosis, which ultimately contributes to preserve intestinal homeostasis (Fig. 3S).
Synergism between Infection and Oncogenic Predisposition in Intestinal Pathology.
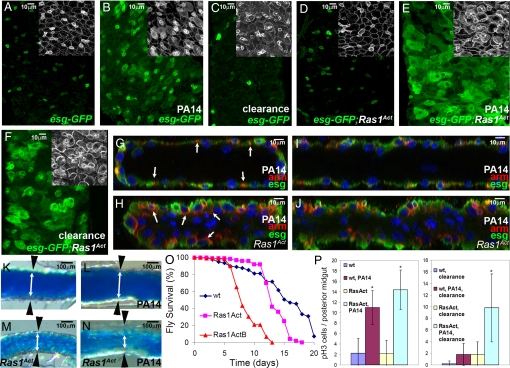
To assess the synergism between infection and oncogenic predisposition, we expressed a latent oncogenic form of the Drosophila ortholog of mammalian K-Ras (Ras1Act) (21) in the progenitor compartment. Expression of Ras1Act under semipermissive conditions (see Materials and Methods) is cytologically innocuous because the gut diameter and the number and shape of esg+ cells appear normal (Fig. 4D and Fig. S3B). However upon infection, a number of intestinal dysplastic phenotypes became apparent. First, essentially all cells in the posterior midgut were esg+, with heightened Armadillo (Arm) staining (Fig. 4E, Inset), a property of SCs and progenitors of wt uninfected flies (Fig. 4A, Inset) (22). Second, supernumerary esg+ cells that were present predominantly in the posterior midgut upon infection of wt infected flies, were present in both the anterior and posterior midgut of genetically predisposed flies (Fig. S2 B and D). Third, there was a higher number of Dl+ cells in the intestine (57 ± 4% vs. 30 ± 3% for the wt control; P = 0.0002, n = 4), indicative of more SCs. Fourth, Arm staining, which was restricted to the lateral region of the wt midgut cells (Fig. 4 G and I), was found both laterally and apically in RasAct expressing flies (Fig. 4 H and J), indicating perturbation of apicobasal cell polarity. Fifth, the epithelial monolayer of RasAct flies developed additional layers with more apical cells that lack detectable Arm staining, many of which had nuclei of irregular shape (Fig. 4 G–J and Fig. S5). Sixth, infected RasAct-expressing flies had a reduced midgut luminal space, increased defecation rate upon infection and succumbed faster to the infection (Fig. 4 K–O and Fig. S6).
Fig. 4.
Ras1Act oncogene synergizes with infection to induce SC-mediated intestinal dysplasia. (A–F) Control tub-GAL80ts esg-GAL4 UAS-GFP flies (A–C) alone or in combination with UAS-Ras1Act (D–F) that are either uninfected (A and D), PA14-fed (B and E), or cleared from infection (C and F). Midgut esg+ cells are shown in green (A–F) and Arm staining in white in insets. (G–J) Cross-sections (G and H) and sagittal sections (I and J) of PA14-infected wild-type (wt) (G and I) and Ras1Act (H and J) midguts indicating the difference in epithelial structure. DAPI marks the nuclei and red Arm staining. Arm is localized in lateral cell junctions, but in Ras1Act there is mislocalization of Arm in the apical domain (arrows in G and H). (K–N) Posterior midguts of wt (K and L) and Ras1Act (M and N) flies fed on sucrose (K and M) or PA14 (L and N) for 5 days, followed by ingestion of blue dye cornmeal to highlight the gut lumen. White double arrows and black arrowheads delineate the borders of the intestinal epithelium. (O) Control flies survive longer compared to flies expressing two different insertions of Ras1Act (P < 0.001; n = 40). (P) pH3+ cells per posterior midgut are significantly and similarly increased during infection in both control and Ras1Act flies, but differ following clearance (*, P < 0.05; n = 7).
Interestingly, the synergy between the genetic background and infection in causing perturbations of the progenitor cell population is not limited to the Ras1Act oncogene. We assessed the effect of expression in the esg+ cells of a dominant negative form of Notch (NotchDN), a pathway relevant to intestinal SC control and cancer in mammals (23, 24). It has previously been shown that reduction of Notch expression autonomously increases SC numbers and inhibits differentiation, thus resulting in supernumerary SCs in Drosophila (17, 18). Under semipermissive NotchDN expression conditions, no such effect on esg+ cells was apparent, but following infection, clusters of esg+ cells covering most of the surface of the posterior midgut, rendered these flies hypersusceptible to infection (Fig. S7). Similarly, weak RNAi knockdown of the tumor suppressor gene discs large (dlgRNAi) (25) was found to have no effect in the absence of infection, but in the presence of infection it resulted in extensive expansion of esg+ cells, covering most of the surface of the gut (Fig. S8).
Importantly, Ras1Act and dlgRNAi flies, but not the wt controls, retained a significant overabundance of esg+ cells 5 days following bacteria clearance (Fig. 4 C and F and Fig. S8), while dlgRNAi flies also retained a significant increase in gut diameter (Fig. S3C). In addition, 5 days following bacterial clearance, Ras1Act (Fig. 4P) and dlgRNAi flies, but not the wt controls, had increased numbers of proliferating (pH3+) cells (>5-fold increase vs. controls). Although assessment of the phenotype at time points further beyond clearance is not feasible, due to the effect of aging in the SC compartment (26), these data demonstrated that genetically predisposed SCs and progenitors exhibit prolonged pathology.
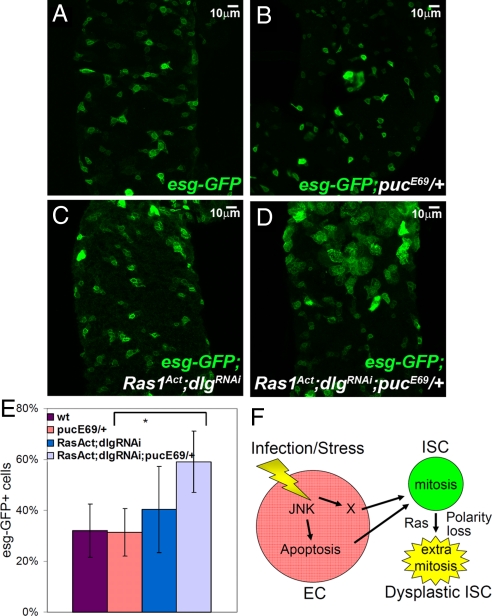
Since our earlier results demonstrated that JNK activation is necessary and sufficient for full induction of SC or progenitor growth upon infection, we also tested whether JNK signaling can synergize with Ras1Act and dlgRNAi in the absence of infection to cause an increase in the progenitor population. To weakly increase JNK activation to a level below the threshold that would trigger an alteration in the progenitor population, we used a genetic background containing only one copy of the JNK pathway inhibitor, puckered (puc). Expression of Ras1Act and dlgRNAi (under semipermissive conditions) did not produce significant increase in the esg+ cell population (Fig. 5 A, C, and E). However, in the pucE69/+ genetic background, Ras1Act and dlgRNAi expression resulted in supernumerary esg+ cells (Fig. 5 D and E), indicating that alterations in these genes can synergize to increase the progenitor cell population.
Fig. 5.
Synergism between JNK activation and Ras1Act expression and dlg downregulation. Posterior midgut SCs and progenitors marked with GFP (green) from flies of the genotype tub-GAL80ts esg-GAL4 UAS-GFP without (A) or with JNK activation due to the pucE69 mutation (B) or with Ras1Act and dlgRNAi expression (C) or with Ras1Act and dlgRNAi expression in the pucE69 background (D). (E) Ras1Act and dlgRNAi only increased the percent of esg+ cells in pucE69 flies (P < 0.05, n = 5 intestines). (F) Model of the diversion of homeostasis (as described in Fig. S3) toward dysplasia due to Ras and polarity gene alterations causing intestinal SC predisposition.
Conclusions
The findings presented here support a model (Fig. 3S), according to which JNK driven apoptosis is partly responsible for the compensatory proliferation of SCs and progenitors. It is likely that a stress or growth signaling pathway acts in parallel or sequentially to JNK to induce SC and progenitor proliferation. Indeed, recent findings suggest that the JAK/STAT pathway may be a JNK and apoptosis-independent mediator of this response (27, 28). Nonetheless, the signals that trigger these pathways in enterocytes, and those that signal intestinal SC to proliferate in the presence of microbes remain to be discovered.
These signals appear important to preserve intestinal homeostasis by replenishing dying cells and can be subverted toward a dysplasia-like phenotype in genetically predisposed animals (Fig. 5F). It is possible that in addition to the Upd cytokines (27, 28) other growth factors secreted from injured enterocytes increase intestinal SC fate proliferation rate. During chronic exposure to microbes, such factors might promote irreversible alterations in cell polarity and tissue architecture. Cell aging, infection by Erwinia carotovora, Serratia marcescens, and Pseudomonas entomophila, and exposure to xenobiotics, have recently been shown to induce supernumerary intestinal progenitor cells (26, 27, 29–31). Therefore, it will be important to systematically analyze whether specific environmental factors and intestinal microbiota can also contribute to dysplasia-like phenotypes, as observed in our synergy experiments.
In conclusion, while JNK signaling and SC or progenitor proliferation appear to be beneficial for the host upon infection, an imbalance in the regenerative process can be detrimental for genetically predisposed hosts. This potential risk is especially relevant in cancer patients undergoing chemotherapy, as such individuals may have a genetic predisposition for cancer interacting with an immunocompromised state, making them particularly susceptible to both intestinal infections and cancer recurrence. Hence, it may be beneficial to identify and target bacterial factors that promote SC proliferation. Once such factors are known, cancer screening methods based on the responsiveness of SCs to infection may be developed. In this respect, the ability of bacterial factors to overstimulate the regenerative process, could be interpreted as an index of an individual's risk of developing cancer.
Materials and Methods
Fly Strains
cad-Gal4md509, UAS-srcGFPM7E, esg-lacZB7–2-22, UAS-p35BH2, UAS-bskDN, and UAS-HepAct were obtained from the Bloomington Stock Center; Myo1A-GAL4 UAS-GFP (27); esg-GAL4 (NP5130, Drosophila Genomics Resource Center); puc-lacZE69 (M. Arias, 1993); tub-GAL80ts esg-GAL4 UAS-GFP, UAS-NotchDN (17); UAS-Ras1Act (21); hep1 (M. Miura) and UAS-dlg RNAi (25).
Transgene Overexpression
esg-GAL4 UAS-srcGFP flies were reared on normal fly food at 25 °C. For temporal overexpression of transgenes, the tub-GAL80ts esg-GAL4 UAS-GFP (progenitor cell-specific) and tub-GAL80ts;cad-GAL4 UAS-GFP esg-lacZ (posterior midgut-specific) flies were reared on normal fly food at the restrictive temperature (18 °C), where the Gal80 repressor is active and Gal4 expression is suppressed, then transferred onto infection media and incubated at the semipermissive (25 °C) or fully-permissive (29 °C) temperature, respectively. For flip-out clones tub-GAL80ts esg-GAL4 UAS-GFP females were crossed with UAS-FLP;act-FRTstopFRT-lacZ (19) males at 18 °C and progeny were transferred to infection media at 25 °C for 5 days.
Antibodies
Primary antibodies included mouse anti-Arm (1:50; Developmental Studies Hybridoma Bank), mouse anti-β-gal (1:400; Promega), rabbit anti-β-gal (1:10,000; Cappel), rabbit anti-caspase-3 (1:200; Cell Signaling Technologies), rabbit anti-phospho-histone-H3 (1:2,000; Millipore), mouse anti-Delta (DI) (1:100; DSHB), and guinea pig anti-Dl (1:5,000; a gift from M. Muskavitch). Secondary antibodies conjugated to Alexa Fluor-488, 555, and 647 were diluted to 1:1,000 (Molecular Probes). Standard immunohistochemical methods were used (17, 22). Images were collected on a Leica TCS SP2-AOBS confocal microscope. Additional information can be found in SI Text.
Supplementary Material
Acknowledgments.
We thank Nikolaos Giagtzoglou, Michele Markstein, and Richard Binari for critical reading of the manuscript. This work was partially supported by National Institutes of Health (NIH) Grant AI063433 and Shriners Grant no. 8892 (to L.G.R.). N.P. was supported by the NIH and is an Investigator of the Howard Hughes Medical Institute, and Y.A. received a Shriners Fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911797106/DCSupplemental.
References
- 1.Loktionov A. Cell exfoliation in the human colon: Myth, reality, and implications for colorectal cancer screening. Int J Cancer. 2007;120:2281–2289. doi: 10.1002/ijc.22647. [DOI] [PubMed] [Google Scholar]
- 2.El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743–747. doi: 10.1136/gut.48.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quante M, Wang TC. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda) 2008;23:350–359. doi: 10.1152/physiol.00031.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selgrad M, et al. The role of viral and bacterial pathogens in gastrointestinal cancer. J Cell Physiol. 2008;216:378–388. doi: 10.1002/jcp.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakatos PL, Fischer S, Lakatos L, Gal I, Papp J. Current concept on the pathogenesis of inflammatory bowel disease-crosstalk between genetic and microbial factors: Pathogenic bacteria and altered bacterial sensing or changes in mucosal integrity take “toll”? World J Gastroenterol. 2006;12:1829–1841. doi: 10.3748/wjg.v12.i12.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smakman N, Borel Rinkes IH, Voest EE, Kranenburg O. Control of colorectal metastasis formation by K-Ras. Biochim Biophys Acta. 2005;1756:103–114. doi: 10.1016/j.bbcan.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.van Es JH, Clevers H. Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends Mol Med. 2005;11:496–502. doi: 10.1016/j.molmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Boman BM, Huang E. Human colon cancer stem cells: A new paradigm in gastrointestinal oncology. J Clin Oncol. 2008;26:2828–2838. doi: 10.1200/JCO.2008.17.6941. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 10.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 11.Fukata M, Abreu MT. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234–243. doi: 10.1038/sj.onc.1210908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin DC. Intestinal morphogenesis. Curr Opin Gastroenterol. 2007;23:111–114. doi: 10.1097/MOG.0b013e3280145082. [DOI] [PubMed] [Google Scholar]
- 13.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: Lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 14.Donskey CJ. Antibiotic regimens and intestinal colonization with antibiotic-resistant gram-negative bacilli. Clin Infect Dis. 2006;43(Suppl 2):S62–S69. doi: 10.1086/504481. [DOI] [PubMed] [Google Scholar]
- 15.Pena C, et al. Effects of carbapenem exposure on the risk for digestive tract carriage of intensive care unit-endemic carbapenem-resistant Pseudomonas aeruginosa strains in critically ill patients. Antimicrob Agents Chemother. 2007;51:1967–1971. doi: 10.1128/AAC.01483-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apidianakis Y, et al. Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc Natl Acad Sci USA. 2005;102:2573–2578. doi: 10.1073/pnas.0409588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 18.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 19.Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- 20.Kanda H, Miura M. Regulatory roles of JNK in programmed cell death. J Biochem. 2004;136:1–6. doi: 10.1093/jb/mvh098. [DOI] [PubMed] [Google Scholar]
- 21.Gisselbrecht S, Skeath JB, Doe CQ, Michelson AM. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 1996;10:3003–3017. doi: 10.1101/gad.10.23.3003. [DOI] [PubMed] [Google Scholar]
- 22.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 23.Fre S, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 24.van Es JH, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 25.Ni JQ, et al. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods. 2008;5:49–51. doi: 10.1038/nmeth1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cronin SJ, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.