Abstract
Toll-like receptors (TLRs) recognize pathogen associated molecular patterns (PAMPs) to detect the presence of pathogens. In addition to their role in innate immunity, TLRs also play a major role in the regulation of inflammation, even under sterile conditions such as injury and wound healing. This involvement has been suggested to be depend, at least in part, on the ability of TLRs to recognize several endogenous TLR ligands termed damage associated molecular patterns (DAMPs). The liver not only represents a major target of bacterial PAMPs in many disease states but also upregulates several DAMPs following injury. Accordingly, TLR-mediated signals have been implicated in a number of chronic liver diseases. Here, we will summarize recent findings on the role TLRs and TLR ligands in the pathophysiology of liver fibrosis and cirrhosis, viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease and hepatocellular carcinoma, and highlight the potential role of TLR agonists, antagonists and probiotics for the treatment of chronic liver disease.
Keywords: Bambi, hepatocyte, hepatic stellate cell, E5564, TAK-242
Introduction
Toll-like receptors (TLRs) are a highly conserved group of pattern recognition receptors that function as pathogen sensors in vertebrate and invertebrate species. The recognition of specific signature molecules termed pathogen associated molecular patterns (PAMPs) by TLRs is a cornerstone of the innate immune system, and enables it to rapidly mount protective responses against invading pathogens [1, 2, 3, 4]. In addition to their role in the innate immune system, TLRs contribute significantly to a number of other processes including adaptive immune responses, regulation of sterile inflammation, wound healing and promotion of epithelial regeneration and carcinogenesis [5, 6, 7]. Toll-like receptors play a major role in liver physiology and pathophysiology due to the liver’s anatomic association with the intestine and its exposure to relatively large amounts of intestinally derived PAMPs in healthy and disease states [8, 9, 10].
I. TLR signaling
Toll-like receptors and co-receptors
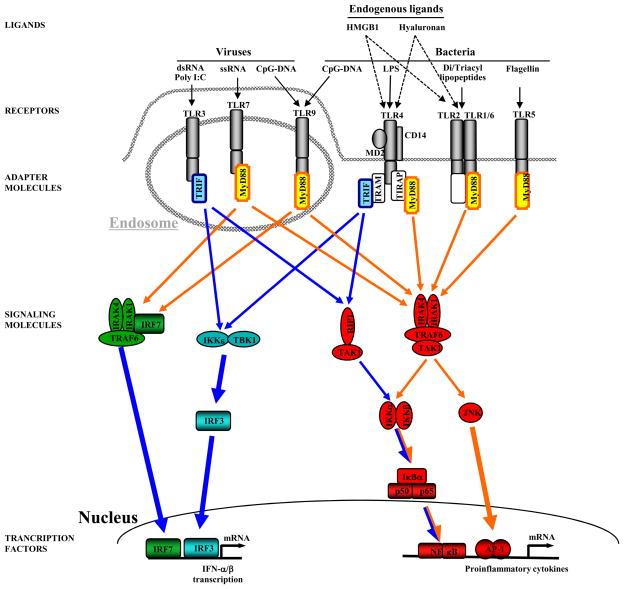
The human TLR family consists of currently 10 members, which are structurally characterized by the presence of a leucine-rich repeat (LRR) domain in their extracellular domain and a Toll/interleukin (IL)-1 receptor (TIR) domain in their intracellular domain [11]. The existence of a large number of TLRs enables the innate immune system to discriminate between PAMPs that are characteristic of different microbial classes and launch specific defense mechanisms [11, 12]. TLR4 is best known for sensing gram-negative bacteria by detecting lipopolysaccharide (LPS), a membrane component of gram-negative bacteria. TLR2 senses gram-positive infection by recognizing cell membrane components such as leipoteichoic acid, peptidoglycan, and various lipopeptides and lipoproteins. TLR3 and TLR7 sense viral infections by recognizing double-stranded and single-stranded RNA, respectively. TLR9 recognizes non-methylated CpG-containing DNA from bacteria and viruses. The molecular basis for ligand recognition is largely unknown but it has been suggested that leucine rich repeated domains in the extracellular domain of TLRs may be involved in ligand binding [13]. Activation of some TLRs such as TLR2 and TLR4 additionally requires the presence of co-receptors. Two of these co-receptors, CD14 and MD-2, also contain leucine rich repeat domains suggesting their involvement in ligand recognition and binding. Activation of TLR4 by LPS absolutely requires the presence of the co-receptor MD-2 for signaling, whereas some TLR4-mediated signals may still be generated in the absence of CD14 [14]. MD-2 is not only required for cell-surface expression of TLR4 [15], but also appears to be essential for the activation of the TLR4 signaling cascade [16]. The most recent model of LPS-mediated TLR4 activation suggests that CD14 catalyzes the binding of LPS to the MD-2-TLR4 complex. Binding of LPS to MD-2 induces a conformational change in MD-2 which then allows this complex to bind to a second TLR4 receptor thus achieving TLR4 homo-dimerization and signaling [17]. TLR2 ligands require the presence of CD36 for efficient signal induction by some ligands but the exact role of CD36 in TLR2 signaling remains to be elucidated [18]. Although each TLR detects specific ligands, many of the signaling molecules that mediate intracellular response are shared by the TLRs (see Figure 1). All TLRs signal through one or two adapter molecules termed MyD88 and Trif which explains why responses to different TLR ligands often generate similar downstream signals [11, 12]. Thus, it appears that the existence of a large number of TLRs mainly serves to enable an efficient and specific detection of a wide range of PAMPs rather than generating highly tailored immune responses.
Figure 1. TLR signaling.
TLRs that are predominantly activated by viral PAMPS are located within the endosome whereas TLRs that are predominantly activated by bacterial PAMPS are located on the cell surface. In addition to PAMPs, several endogenous mediators including hyaluronan and HMGB1 have been suggested to activate TLR2 and TLR4. TLRs mediate their signaling through two adapter molecules, MyD88 and Trif to induce up-regulation proin ammatory and antiviral genes. MyD88-induced signals (marked in orange) predominantly activate NF-κB, IRF-7 and JNK, Trif-dependent signals (marked in blue) predominantly activate NF-κB and IRF-3 (adapted from Schwabe et al, Gastroenterology 130:1886–900).
TLR-mediated signals are transduced through two major pathways, the “MyD88-dependent pathway” and the “MyD88-independent pathway”. MyD88 is an essential part of the signaling cascade of all TLRs except for TLR3. In contrast, Trif only interacts with TLR3 and TLR4, and is responsible for mediating MyD88-independent signals (see Figure 1). Both MyD88-dependent and MyD88-independent pathways initiate the transcription of a specific set of genes involved in proinflammatory, antiviral, and antibacterial responses [11]. In TLR4-signaling, MyD88 mediates an upregulation of inflammatory cytokines through activation of NF-κB whereas the MyD88-independent pathway contributes to both interferon upregulation through IRF-3, and inflammatory gene induction through NF-κB (see Figure 1). The MyD88-dependent and the MyD88-independent pathway mediate NF-κB and subsequent inflammatory cytokine production through different mechanisms and kinetics: NF-κB induction in the MyD88-dependent pathway occurs with fast kinetics whereas NF-κB activation in the MyD88-independent pathway occurs with slower kinetics. TLR3 interacts with Trif resulting in an upregulation of interferon and inflammatory cytokines through the MyD88-independent pathway in a similar fashion as the MyD88-independent pathway in TLR4 signaling (see above). In TLR7 and TLR9 signaling, MyD88 induces an IRF-7-dependent upregulation of interferon production and NF-κB-dependent upregulation of inflammatory cytokines. In TLR2 signaling, MyD88 upregulates inflammatory cytokines through an NF-κB dependent pathway.
Negative regulation of TLR signaling
Although strong proinflammatory and anti-viral responses after TLR stimulation may be beneficial in the short term to eradicate pathogens, a prolonged or exaggerated activation of TLR signaling may have deleterious effects. Therefore, several mechanisms have evolved that negatively regulate TLR-induced cellular responses [19]. These mechanisms act at the receptor level (RP105, ST2 and SIGGIR expression, TLR downregulation or degradation), at the level of adapter molecules such as MyD88 and TIRAP, or receptor proximal kinases such as IRAK. Mechanisms to achieve a reduction in TLR signaling include decreased TLR4 transcription [20], proteolytic degradation of TLR4, TLR 9 and TIRAP after being marked for degradation by the E3 ubiquitin ligase Triad3A [21] or SOCS-1 [22], expression of inhibitory TIR-domain containing receptors such as ST2 and SIGIRR [23, 24] or LRR-domain containing receptors such as RP105 [25], and expression of non-functional signaling molecules such as MyD88s [26, 27], IRAK-M [28], IRAK2c and IRAK2d [29].
II. TLR ligands
Pathogen associated molecular patterns (PAMPs)
Early detection of infection is advantageous in mounting an efficient defense against pathogens. Accordingly, even minuscule amounts of PAMPs such as lipopolysaccharide (LPS), lipopeptides, unmethylated DNA, and double-stranded RNA evoke intense inflammatory reactions. Many proinflammatory effects of PAMPs are a consequence of TLR-induced secretion of inflammatory mediators such as TNFα and IL-1β. Accordingly, PAMPs such as LPS, lipopeptides, dsRNA and unmethylated DNA are among the strongest inducers of TNFα and IL-1β release in vitro and in vivo [5]. As most bacteria reside in the extracellular space, TLRs that detect bacterial PAMPs such as LPS and lipoproteins are located on the cell surface. Viral RNA and bacterial DNA but not host DNA are present in late endosome-lysosomes. Therefore, TLR3, TLR7 and TLR9 are located in these cellular organelles [12]. The restriction of TLR ligand recognition to specific cellular compartments such as the cell membrane or lysosomes not only increases chances to encounter specific PAMPs but also decreases the chance of TLRs to be exposed to and aberrantly activated by host molecules, thus adding an additional level of control to ensure proper TLR activation.
Regulation of bacterial translocation by the intestinal barrier
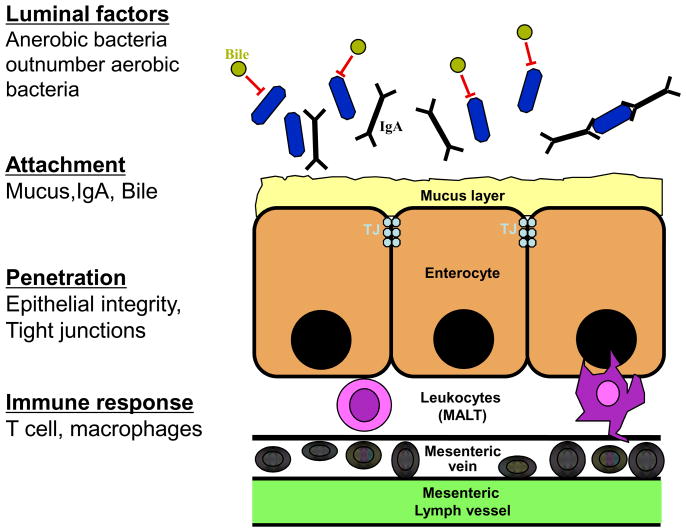
The intestinal microbiota hosts more than 99% of the bacterial mass in the body and is the principal source of bacterially derived PAMPs in health and many disease states. Several protective mechanisms ensure that only a minute amount of bacteria and bacterial products reaches the portal circulation under normal circumstances. These include a thick layer of mucins, secretion of IgA and antimicrobial factors, a tightly sealed epithelial surface and an active mucosa-associated lymphatic tissue (MALT) (see Figure 2) [30]. Accordingly, portal and systemic LPS levels are nearly undetectable in normal rats and healthy people, respectively [31, 32, 33]. In chronic liver disease, structural changes of the intestinal mucosa such as loss of tight junctions, widening of intercellular spaces, vascular congestion, and defects in the mucosal immune system promote the loss of barrier function and allow increased translocation of bacteria and bacterial PAMPs [30]. Whereas the upper gastrointestinal tract is only sparsely populated, microbial density gradually increases distally with about 105 colony-forming units/mL in the jejunum to 108 in distal ileum and cecum, and up to 1012 in the colon [34]. Although, intestinal anaerobic bacteria outnumber aerobic bacteria by a ratio of 100:1 to 1,000:1 [34], virtually all translocating bacteria are aerobic [30]. In fact, anaerobic bacteria suppress colonization and growth of potentially invasive microbes and thereby exert an important role in maintaining gastrointestinal health and in reducing the translocation of potentially harmful microbes [35]. Accordingly, selective elimination of anaerobic bacteria promotes intestinal bacterial overgrowth and translocation [35]. Gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, enterococci and streptococci not only represent the species that are most proficient at translocation, but also cause the large majority of infections in patients with cirrhosis [36, 37]. In cirrhosis, overgrowth of bacteria, especially in locations with low bacterial counts such as the proximal small intestine, and overgrowth of strains with a higher translocation capacity may occur, possibly due to changes in the intestinal motility and the decreased luminal levels of bile acid, a suppressor of bacterial growth [30]. Bacterial overgrowth together with the above described changes in the intestinal mucosal barrier result in an increased rate of bacterial translocation and endotoxemia.
Figure 2. Prevention of bacterial translocation by the intestinal epithelial barrier.
Under normal circumstances, a number of protective mechanisms at different levels ensure that only a minimal amount of bacterial transloction occurs: (i) Luminal factors such as the predominance of anaerobic bacteria which limit the growth and translocation of aerobic and facultative anaerobic bacteria; (ii) bile inhibits bacterial overgrowth; (iii) IgA prevents microbial entry and transports IgA-bound microbes from the lamina propria back to the lumen (iv) a thick mucus layer prevents bacterial contact and attachment (v) intact tight junctions prevent paracellular penetration (vi) the mucosa-associated lymphatic tissue (MALT) phagocytoses translocating bacteria. (adapted from Wiest et al., Hepatology 2005; 41:422–33.)
Damage associated molecular patterns (DAMPs)
Several TLRs not only have the ability to recognize more than one ligand, but often recognize ligands with completely different chemical structures [38]. The best examples for the high promiscuity of TLRs are TLR2 and TLR4. TLR4 recognizes lipids such as the lipid A portion of LPS as well as proteins from respiratory syncytial virus, vesicular stomatitis virus and mouse mammary tumor virus [39, 40, 41]. TLR2 recognizes a wide range of ligands including lipoteichoic acids, various proteins including lipoproteins and glycoprotein’s, zymosan, and peptidoglycan as well as lipopolysaccharides from specific bacterial strains [11, 38]. The ability of TLRs to recognize ligands that are chemically unrelated is believed to be the basis for the activation of TLRs by endogenous ligands. This activation does not occur under normal circumstances but only when there is a change in the environment that either leads to the release of endogenous ligands from a cellular compartment that is usually not in contact with TLRs, or to the modification of endogenous mediator that gives them the ability to activate TLRs [38, 42]. Due to the association of many endogenous ligands with tissue injury, the nomenclature of damage associated molecular pattern (DAMP) has been suggested. The best characterized DAMPs include HMGB1, hyaluronan, S100 proteins, the alternatively spliced extra domain A of fibronectin and heat shock protein 60. Most of these ligands act as agonists of TLR2 or TLR4, or both receptors. However, there is still ongoing controversy whether these DAMPs indeed are bona fide TLR ligands since many of these ligands have either been purified in bacterial systems or have a high affinity to bacterial products suggesting that bacterial products or other TLR activating substances such as lipids or DNA rather than the ligands themselves mediate their TLR activating effect [43]. Further studies including genetic inactivation of candidate molecules are required to characterize DAMPs as bona fide TLR ligands, and it is likely that only some of the above mentioned candidates will be confirmed as TLR ligands. Furthermore, it has been suggested that endogenous DNA can act as a TLR9 agonist to promote autoimmune reactions [5]. HMGB1 and hyaluronan from eukaryotic sources have been shown to activate TLRs thus largely excluding bacterial contaminants as mediators of TLR signals [44, 45]. Moreover, HMGB1 and hyaluronan are elevated in liver disease and HMGB1 plays a potential role in the pathophysiology of liver disease [46, 47]. For these reasons, we will limit further discussion on TLR-activating DAMPs to HMGB1 and hyaluronan.
Release of DAMPs
Release of DAMPs into the extracellular space is achieved by a number of different mechanisms that involve (i) leakage from necrotic cells, (ii) increased synthesis and post-translational modification in response to inflammation, and (iii) degradation of inactive precursors into TLR-mimetic degradation products in inflammatory environments.
HMGB1
HMGB1 is a DNA-binding protein that induces bends in the helical DNA structure to facilitate multiple physical interactions of DNA with transcription factors, recombinases and steroid hormone receptors, and to thus allow transcription and other nuclear transactions to take place [48]. In addition to this transcription factor-like function, HMGB1 also has cytokine-like effects that require its presence in the extracellular space [48, 49]. Release of HMGB1 into the extracellular space is mediated by two mechanisms: (i) Acetylation of many of its 43 lysine residues that lie in proximity to its two nuclear-localization signals thus reducing interaction with the nuclear importer protein complex, preventing nuclear re-entry and promoting secretion of HMGB1 [50]. This active HMGB1 secretion process seems to occur predominantly in inflammatory cells. (ii) The passive diffusion of HMGB1 from cells that undergo necrosis [51]. Importantly, HMGB1 release does not occur from apoptotic cells, presumably because HMGB1 is tightly bound to cruciform DNA and hypo-acetylated proteins within the apoptotic-cell nucleus whereas it is only loosely bound to DNA in necrotic cells [49]. Thus HMGB1 has been suggested to be a signature DAMP that signals the presence of necrosis, and subsequently triggers inflammation [51].
Hyaluronan
Hyaluronan is a negatively charged high molecular weight glycosaminoglycan, which is ubiquitously distributed in the extracellular matrix and a component of the basement membrane. At sites of inflammation and tissue destruction, high molecular weight hyaluronan can be broken down to lower molecular weight hyaluronan fragments via oxygen radicals and enzymatic degradation. In contrast to high molecular weight hyaluronan, low molecular weight hyaluronan has cytokine-like properties, and induces inflammatory gene expression in epithelial cells, endothelial cells, fibroblasts, dendritic cells (DCs), and macrophages [52]. In the extacellular space, hyaluronan is bound by the CD44 receptor which mediates some of its proinflammatory effects. However, hyaluronan is able to stimulate chemokine production in the absence of CD44 [45]. Since the disruption of basement membranes is typically associated with injury, it has been suggested that the recognition of low molecular weight hyaluronan by TLRs and other receptors is part of an injury recognition system.
III. TLR expression in the liver
Due to its anatomical links to the gut, the liver is constantly exposed to gut-derived bacterial products, and functions as a major filter organ and a first line of defense. 80% of intravenously injected endotoxin is detected in the liver within 20–30 minutes [53, 54]. Moreover, the liver is an important site for bacterial phagocytosis and clearance as it hosts more then 80% of the body’s macrophages. Kupffer cells, the resident macrophages of the liver, are able to efficiently take up endotoxin and phagocytose bacteria carried through the portal vein and are considered to play a major role in the clearance of systemic bacterial infection [55, 56]. The healthy liver contains low mRNA levels of TLRs such as TLR1, TLR2, TLR4, TLR6, TLR7, TLR8, TLR9, TLR10 and signaling molecules such as MD-2 and MyD88 in comparison to other organs [57, 58, 59], suggesting that the low expression of TLR signaling molecules may contribute to the high tolerance of the liver to TLR ligands from the intestinal microbiota to which the liver is constantly exposed.
Kupffer cells
Kupffer cells, the resident macrophages of the liver, play a crucial role in host defense which is linked to their ability to phagocytose, process and present antigen, and secrete various proinflammatory mediators including cytokines, prostanoids, nitric oxide, and reactive oxygen intermediates [55, 56, 60]. Kupffer cells are among the first cells in the liver to be hit by gut-derived toxins such as LPS and orchestrate inflammatory responses within the liver. Accordingly, Kupffer cells express TLR4 and are responsive to LPS [61]. Although some studies have demonstrated that Kupffer cells are involved in the uptake and hepatic excretion of LPS [62, 63], others have shown that Kupffer cell depletion does not reduce LPS clearance [64]. Moreover, Kupffer cells can inactivate LPS by deacetylation [65]. Following stimulation with LPS at concentrations between 0.1 and 1000 ng/ml, Kupffer cells produce TNFα, IL-1β, IL-6, IL-12, IL-18 and several chemokines [66]. Notably, Kupffer cells mediate the majority of cytokine and chemokine expression in liver after LPS injection (25 μg i.p.) as demonstrated by depletion experiments [67]. Kupffer cells also functionally express TLR2, TLR3 and TLR9 [68, 69, 70, 71]. In comparison to peripheral blood monocytes, Kupffer cells express low levels of CD14 [58]. Moreover, freshly isolated human Kupffer cells secrete the anti-inflammatory cytokine IL-10 in response to stimulation with LPS which contributes to the downregulation of proinflammatory cytokines [72]. Thus, Kupffer cells may have a higher LPS tolerance to adapt to the special circumstances in their anatomical location that is frequently hit with low levels of LPS even under normal conditions.
Hepatocytes
Hepatocytes fulfill metabolic and detoxifying functions in the liver, and are important mediators of the acute phase response. Hepatocytes express TLR4 receptors and are responsive to LPS, but this response is fairly weak with only two-fold elevated levels of serum amyloid A (SAA) and a less than 2-fold induction of most upregulated genes in a microarray after LPS. Moreover, doses of 100 ng/ml and higher were required to see significant effects in hepatocytes [73, 74]. Similarly, stimulation with TLR2 ligands induces NF-κB activation and a weak induction of SAA [74]. The expression of TLR2 in hepatocytes is upregulated by LPS, TNFα, bacterial lipoprotein and IL-1β in an NF-κB-dependent manner indicating that hepatocytes become more responsive to TLR2 ligands under inflammatory conditions [74]. In contrast, TLR4 expression in hepatocytes is not upregulated by proinflammatory mediators [75]. Hepatocytes play a major role in the uptake of LPS and its removal from the systemic circulation by secreting LPS into the bile [62, 64]. Clearance of LPS occurred at a similar rate in rats which had been depleted of Kupffer cells by gadolinium chloride indicating that hepatocytes are the principal mediators of this process [64]. A recent study demonstrated that TLR4, CD14 and MD-2 are required for the uptake of LPS by hepatocytes [76]. Interestingly, TLR4 signaling is not required for this process as hepatocytes from TLR4-deficient C3H/HeJ mice were as efficient as those isolated from TLR4-sufficient C3H/HeOuJ mice to take up LPS [76]. Although this study clearly demonstrated the ability of hepatocytes to take up LPS, the uptake of LPS in vivo through this TLR4-dependent mechanism remains to be proven.
Hepatic Stellate Cells
Following liver injury, hepatic stellate cells undergo an activation process and become the predominant extracellular matrix-producing cell type in the liver [77]. Activated human hepatic stellate cells express TLR4 and CD14 and respond to LPS with the activation of IKK/NF-κB and JNK as well as the secretion of proinflammatory cytokines [78]. Activated mouse hepatic stellate cells express TLR2, TLR4 and TLR9 and respond to LPS, lipoteichoic acid, N-acetyl muramyl peptide and CpG-DNA with an upregulation of Erk phosphorylation and IL-6, TGFβ1 and MCP-1 secretion [47, 79, 80, 81]. Quiescent murine hepatic stellate cells express as much TLR4 as in vivo-activated hepatic stellate cells and are highly responsive to LPS, even at doses as low as 1 ng/ml [47]. Moreover, quiescent HSCs activate NF-κB in response to LPS injection (0.1 mg/g intraperitoneally) in vivo [47]. Notably, LPS downregulates the TGFβ pseudoreceptor Bambi in quiescent hepatic stellate cells to promote TGFβ signaling and stellate cell activation [47].
Biliary epithelial cells
Biliary epithelial cells line the biliary tree which connects the liver with the intestinal lumen to deliver bile to the intestine. Mouse biliary epithelial cells express CD14, MD-2 and TLR2, TLR3, TLR4 and TLR5 [82] and display NF-κB activation and TNFα production after LPS (1 μg/ml) stimulation [82], and an increase in CDX2 and MUC2 following TLR2 or TLR4 stimulation [83]. Human biliary epithelial cells express TLR1–10 [84, 85]. In an in vitro model of biliary cryptosporidiosis, C. parvum recruits TLR2 and TLR4 to the host-cell-parasite interface, and induces NF-κB activation, IL-8 and human β-defensin 2 expression [84].
Sinusoidal endothelial cells
Sinusoidal endothelial cells form the fenestrated lining of the hepatic sinusoids and thus have an important function in hepatic perfusion and nutrient supply. Sinusoidal endothelial cells constitutively express TLR4 and CD14 as well as TLR9, and show an increase in NF-κB activation after LPS (10 ng/ml) stimulation [86, 87]. Moreover, mRNA for TLR1–9 was detected in sinusoidal endothelial cells and functional expression of TLR3 has been demonstrated by the ability of supernatants from poly-IC treated sinusoidal cells to reduce HBV replication in immortalized hepatocytes [88]. After repetitive LPS challenges, sinusoidal endothelial cells show reduced NF-κB activation, CD54 expression and a reduced ability to promote leukocyte adhesion [86]. In sinusoidal endothelial cells, LPS tolerance is not regulated at the level of TLR4 surface expression, but appears to be linked to prostanoid expression [86]. Although some authors propose sinusoidal endothelial cells to be involved in the hepatic uptake of LPS, other studies have not found such a role [62, 64].
Hepatic dendritic cells
Hepatic dendritic cells (DCs) are the professional antigen-presenting cells of the liver. During inflammation, dendritic cells are recruited into the liver sinusoids from where they can migrate to periportal and pericentral areas. Like other DCs, hepatic DCs express TLR2 and TLR4 [57, 89]. While one study found a lower expression of TLR4 on hepatic DCs in comparison to their splenic counterparts [57], another study reported a higher expression of TLR2 and TLR4 on hepatic cDCs and increased TNF-α and IL-6 after PGN and LPS (10 μg/ml) stimulation [89]. However, both studies reported a weaker stimulation of naive allogeneic CD4+ T cells by hepatic DCs in comparison to splenic DCs [57, 89].
IV. Role of TLRs in chronic liver disease
Alcohol-induced liver injury
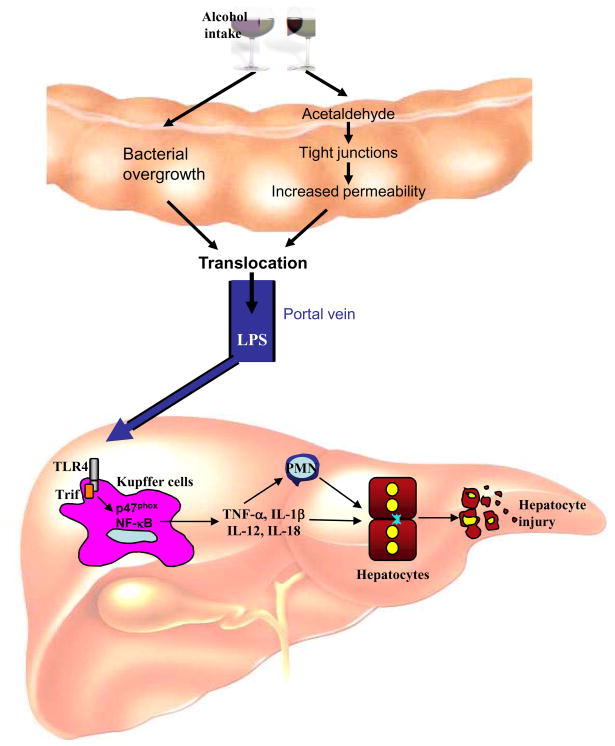
Acute and chronic ingestion of alcohol lead to a strong elevation of portal and systemic levels of endotoxin in animal models and humans [31, 32, 33]. Whereas healthy controls display only 2.5 pg/ml of endotoxin in peripheral blood, patients with alcoholic fatty liver, alcoholic hepatitis and alcoholic cirrhosis display 14 pg/ml, 16 pg/ml and 19 pg/ml endotoxin in peripheral blood [31]. Interestingly, acute ingestion of alcohol in rats leads to significantly higher levels of portal endotoxin than systemic levels with concentrations of 30–80 pg/ml in the portal vein 2 hours after gavage [33, 90]. Thus, peripheral measurements are likely to underestimate the amount of endotoxin that targets the liver, most likely due to the efficient clearance of endotoxin by the liver. Endotoxin is a crucial mediator of liver injury in alcoholic liver disease as demonstrated by the significant reduction of alcoholic liver injury following elimination of the gram-negative microbiota by antibiotics [91], and the sensitization to LPS-induced liver injury following long-term ethanol exposure [92]. The elevation of endotoxin appears to be predominantly caused by two mechanisms (see Figure 3): (i) Alcohol consumption leads to changes in the intestinal microbiota with upper gastrointestinal tract bacterial overgrowth being more than six times more frequent in alcoholics than in non-alcoholic [93, 94]. However, the effects of alcohol on the composition of the intestinal microbiota have not been studied in detail. (ii) A large body of literature has clearly documented that alcohol ingestion disrupts the intestinal epithelial barrier causing enhanced permeability [95, 96, 97] thus allowing increased levels of LPS to enter the portal circulation. It has been suggested that the intestinal microbiota converts ethanol into acetaldehyde which in turn disrupts tight junctions and increases paracellular permeability [98, 99].
Figure 3. Promotion of alcoholic liver injury by an LPS-TLR4 signaling cascade.
Orally ingested alcohol increase intestinal permeability leading to increased levels of LPS in the portal vein. In the liver, LPS binds to TLR4 on Kupffer cells to activate NF-κB, and NADPH oxidase, through a MyD88-independent pathway. Release of cytokine by Kupffer cells promotes hepatocyte injury through the recruitment of neutrophils through direct effects on hepatocytes.
Kupffer cells have been established as a crucial cellular target of LPS in ethanol-induced liver injury as demonstrated by a strong reduction of alcoholic liver injury following depeletion of Kupffer cells with gadolinium chloride [100]. Moreover, the activation of Kupffer cells in alcoholic liver disease largely depends on TLR4 since TLR4-mutated C3H/HeJ mice as well as TLR4-deficient mice display strongly reduced levels of proinflammatory mediators in the liver and blunted liver injury despite elevated endotoxin levels [101, 102]. The TLR4-mediated signal in alcoholic liver injury is mediated through a MyD88-independent pathway [102], most likely through the adapter molecule Trif (see Figure 3). NADP(H) oxidase is a crucial downstream mediator of TLR4 in Kupffer cells during alcohol-induced liver injury, as mice deficient in p47phox, the main cytosolic component of NADP(H) oxidase, show an absence of free radical production, NF-κB activation, TNFα mRNA induction and liver pathology after ethanol treatment [103].
Non-alcocoholic fatty liver and steatohepatitis
There is accumulating evidence that the intestinal microbiota plays an essential part in the promotion of hepatic fat accumulation. Ob/ob mice as well as obese humans have a different composition of their cecal microbiota in comparison to lean controls [104, 105]. One mechanism by which the altered composition of the intestinal microbiota in ob/ob mice as well as in humans promotes obesity and fatty liver is TLR-independent through an increased bacterial capacity for energy harvest [106]. A second mechanism by which the altered intestinal microbiota may promote obesity and fatty liver is an increase in LPS-containing microbiota [107] and increased intestinal permeability to LPS [108]. Accordingly, a 4-week high-fat diet increased plasma LPS concentration two to three times [107]. Notably, subcutaneous infusion of a low dose of LPS resulted in excessive weight gain, insulin resistanceand increase liver triglycerides in mice [107]. Vice versa, selective intestinal decontamination results in decreased endotoxin levels in mice on a high-fat diet [109], and improved glucose tolerance and reduced hepatic triglycerides in mice with diet-induced obesity as well as in ob/ob mice [110].
In addition to its role in hepatic fat accumulation, LPS is also involved in the development of non-alcoholic steatohepatitis (NASH). A crucial role for LPS signaling in NASH was first demonstrated by the increased hepatic sensitivity of genetically obese Fa/Fa rats and ob/ob mice to low doses of LPS [111]. This finding has been further confirmed in TLR4-mutant mice that display decreased injury and lipid accumulation following methionine-choline-deficient (MCD) diet, a model of non-alcoholic steatohepatitis [112]. In contrast, TLR2-deficient mice are not protected from steatohepatitis after MCD diet [113]. The predominant role of TLR4 signaling in NASH is further emphasized by the ability of the TLR4 ligand LPS but not the TLR2 ligand peptidoglycan to exacerbate liver injury in mice treated with a methionine-choline-deficient diet [113]. Notably, probiotics reduce hepatic injury and inflammation in ob/ob mice [114] suggesting that modulation of the gut microbiota by probiotics may represent a feasible approach for the prevention or treatment of NASH.
Hepatic fibrosis and cirrhosis
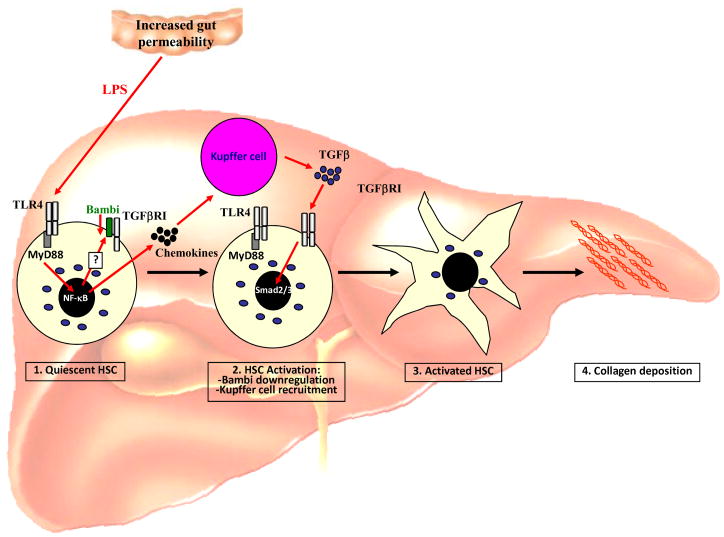
Chronic liver injury leads to the development of hepatic fibrosis, the increased accumulation of extracellular matrix in the liver, and hepatic cirrhosis, an advanced stage of hepatic fibrosis in which functional liver tissue is largely replaced by extracellular matrix and regenerating nodules. The development of hepatic fibrosis and cirrhosis occurs in virtually any type of chronic hepatic injury including viral hepatitis, alcohol, autoimmune and metabolic disease [77]. There is abundant data demonstrating that LPS is elevated in experimental models of hepatic fibrosis [47, 115, 116] and in patients with cirrhosis [31, 117, 118]. Whereas healthy subject displayed endotoxin levels of less than 3 pg/ml, patients with Child-Pugh class A, B and C had endotoxin levels of 4.9 pg/ml, 7.9 pg/ml and 10.2 pg/ml, respectively [118]. It is believed that changes in intestinal motility, subsequent alterations of the intestinal microbiota, decreased mucosal integrity and suppressed immunity in hepatic fibrosis contribute to a failure of the intestinal mucosal barrier and cause increases in bacterial translocation and LPS levels in later stages of hepatic fibrosis and cirrhosis [30, 119, 120, 121, 122, 123]. Studies from the 1950s have shown that antibiotics prevent hepatic injury and fibrosis induced by CCl4 treatment or a choline-deficient diet, and that endotoxin enhances hepatic fibrosis induced by a choline-deficient diet [124, 125]. Recent studies using TLR4-mutant as well as gut-sterilized, CD14- and LBP-deficient mice have demonstrated the crucial role for the LPS-TLR4 pathway in hepatic fibrogenesis [47, 126]. TLR4-mutant mice display a profound reduction in hepatic fibrogenesis in three different experimental models of biliary and toxic fibrosis [47]. The crucial role of LPS is supported by the finding that LBP-deficient as well as gut-sterilized mice also have a marked reduction of hepatic fibrosis [47, 126]. In addition to LPS, endogenous TLR ligands such as hyaluronan and HMGB1 are also elevated in murine fibrogenesis [47]. However, in view of the crucial role of the intestinal microbiota in hepatic fibrogenesis, their contribution is at best moderate. Accordingly, the inhibition of HMGB1 signaling by a blocking antibody did not improve biliary fibrogenesis (RFS, unpublished results). In contrast to TLR4, TLR2-deficient mice did not show a profound reduction in hepatic fibrosis following bile duct ligation [47]. TLR4 is expressed on two key mediators of hepatic fibrogenesis, Kupffer cells and hepatic stellate cells [47, 61, 78]. Kupffer cells initiate fibrogenesis by secreting proinflammatory and profibrogenic cytokines, while hepatic stellate stells are the predominant source of extracellular matrix production in the fibrotic liver [77]. Although Kupffer cells express the highest levels of TLR4 in the liver and are considered a prime target of LPS, TLR4 expressed on quiescent and activated hepatic stellate cells is the main mediator of fibrosis as demonstrated in TLR4-chimeric mice [47]. LPS directly targets hepatic stellate cells in vivo to upregulate chemokines and attract Kupffer cells. At the same time, TLR4 activation induces a downregulation of the TGFβ pseudoreceptor BAMBI on hepatic stellate cells. These two mechanisms work hand in hand to promote the activation of hepatic stellate cells by Kupffer cell released TGFβ, and subsequently hepatic fibrosis (see Figure 4) [126]. Notably, a recent study has identi ed a single nucleotide TLR polymorphism that results in a T399I substitution and confers a signi cantly reduced risk for brosis progression in patients with chronic hepatitis C virus infection [127]. This polymorphism is associated with a reduced TLR4 responsiveness thus confirming the profibrogenic role of TLR4 in a clinically relevant setting. Two recent studies also demonstrated that hepatic stellate cells express TLR9, and that TLR9-deficient mice display decreased hepatic fibrosis [80, 128]. The decrease in hepatic fibrosis was linked to the ability of TLR9-expressing hepatic stellate cells to recognize apoptotic hepatocytes resulting in stellate cell activation [128].
Figure 4. Promotion of hepatic stellate cell activation and fibrosis by LPS.
Following liver injury, alterations in the bacterial microbiota and the intestinal mucosal barrier cause an increase in the translocation of LPS. LPS directly targets quiescent hepatic stellate cells resulting in (i) downregulation of the TGFβ pseudoreceptor Bambi and (ii) upregulation of chemokines. These two signals complement each other to promote hepatic stellate cell activation: 1. Downregulation of Bambi through a TLR4-MyD88-NF-κB signaling cascade sensitizes hepatic stellate cells towards the effect of TGFβ. 2. Chemokines induce Kupffer cells, a main source of TGFβ in the injured liver, to migrate towards hepatic stellate cells. Together, these two mechanisms allow TGFβ-dependent activation of hepatic stellate cell by Kupffer cells resulting in increased deposition of extracellular matrix and liver fibrosis.
TLRs may also modulate several of the complications of hepatic fibrosis. Probiotics and selective intestinal decontamination decrease hepatic encephalopathy in rodent models as well as in patients [129, 130, 131]. However, it is not clear whether this effect is mediated through TLR-dependent mechanisms as probiotic treatment led not only to a significant reduction of endotoxin but also of the ammonia, a principal mediator of encephalopathy [132]. Moreover, LPS has been implied in the regulation of blood pressure in cirrhosis. In two studies, selective intestinal decontamination significantly decreased endotoxin levels and improved the hyperdynamic circulatory state of cirrhosis but did not significantly reduce the hepatic venous pressure gradient [133, 134]. In another study, there was no decrease in hepatic venous pressure gradient in patients with significant portal hypertension but none of the involved patients had detectable serum endotoxin levels [135].
Hepatocellular carcinoma
The liver is probably the best example for the link between chronic inflammation and cancer that was postulated by Rudolf Virchow more than 100 years ago [136]. Almost 80% of hepatocellular cancers in the western world develop as a consequence of chronic inflammation and arise in fibrotic or cirrhotic livers [137]. The involvement of TLR signaling in fibrosis-associated HCC has not yet been investigated. However, there is a high probability that fibrosis-associated HCC is mediated by TLR signaling as TLR4 and MyD88 are required for the development of fibrosis [47], a predisposing condition for HCC development. An important role for the TLR signaling molecule MyD88 was shown in DEN-induced hepatocellular carcinoma (HCC) in male mice [138], but this tumor-promoting pathway is mediated by IL-1R, another upstream activator of MyD88 [139]. Therefore, it is possible that DEN-induced non-fibrotic HCC and fibrosis-associated HCCs may both be MyD88-dependent but mediated by different signaling cascades.
HCV infection
Hepatitis C affects approximately 3.2 million people within the United States and 170 million people worldwide [140]. About 30% of patients chronically infected with Hepatitis C virus (HCV) show signs of active hepatic inflammation, and are at risk of developing fibrosis, cirrhosis and hepatocellular carcinoma. Accordingly, Hepatitis C continues to be one of the leading indications for liver transplantation in the United States [141].
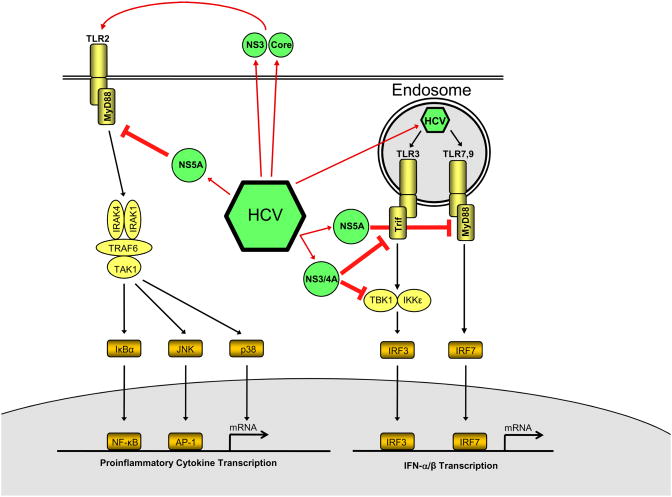
Patients with chronic HCV infection display more than 10-fold-increased serum levels of endotoxin with <5 pg/ml in healthy controls and > 50 pg/ml in chronic HCV patients with mild to moderate hepatic inflammation and less than 25% incidence of hepatic fibrosis [142]. Moreover, several HCV ligands act as TLR agonists [143, 144]. However, HCV has developed strategies to avoid activation of anti-viral pathways by TLRs and their ligands: HCV selectively impairs innate immune pathways that limit HCV replication such as type I interferons (see Figure 5) while at the same time generating a chronic inflammatory state that causes persistent liver injury [145, 146]. When double-stranded RNA from HCV binds to TLR3 within the endosome, the expected response would be activation of TRIF and subsequent upregulation of IFN-β through TBK-1/IKKε and IRF-3. However, the IFN-β response is strongly inhibited by a mechanism that involves the cleavage of TRIF by nonstructural HCV protein NS3/NS4A [147]. Furthermore, NS3 binds directly to TBK-1, disrupting the activation of IRF-3 and the upregulation of IFN-β [148]. Thus, HCV inhibits the ability of the TLR-3 pathway to upregulate IFN-β and hinders viral clearance by two distinct mechanisms. In addition to disrupting the function of TLR3, HCV also affects the function of other TLRs integral to the viral and immune response. In vitro studies utilizing a murine macrophage line that stably express HCV proteins, demonstrate that expression of HCV proteins inhibits the signaling pathways of TLR2, TLR4, TLR7 and TLR9. Specifically, these studies showed that the NS5A inhibits TLR signaling by binding to the adaptor protein MyD88 and inhibiting the recruitment of IRAK-1 leading to a decrease in MyD88-dependent signals [149]. Myeloid dendritic cells from patients with chronic HCV display an increased expression of TLR2 and TLR4, but a decrease in the cytokine response to TLR agonists [150] thus confirming the impairment of TLR signaling by HCV in primary cells. Cell culture produced HCV impairs TLR9-induced IFNα production in plasmacytoid dendritic cells, but has no effect on TLR3- and TLR4- myeloid and monocyte derived dendritic cells suggesting that HCV may specifically target plasmacytoid dendritic cells [151]. Accordingly, TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection [152]. Thus restoration or boosting of TLR signaling pathways that are related to HCV eradication may be a promising treatment strategy. Accordingly, one week of treatment with the TLR7 agonist isatoribine caused a significant reduction of plasma HCV RNA, an increase in the levels of OAS, a marker of antiviral immunity, and an increase in the levels of the chemokine IP-10 and neopterin, a marker of macrophage activation [153].
Figure 5. Positive and negative regulation of TLR signaling by HCV.
Double-stranded RNA from HCV binds to TLR3 within the endosome. However, efficient TLR3 signaling is prevented by 2 mechanisms: (i) Degradation of Trif by HCV NS3/4A and (ii) by NS3 by binding to TBK1 and blocking the association between TBK1 and IRF3. Moreover, HCV NS5A also blocks TLR9-induced levels at the level of MyD88. The interference of HCV with these anti-viral pathways HCV prevents eradication of HCV by the immune system. At the same time, some HCV proteins promote inflammatory signals through TLR2 and TLR4. These may be dampened by inhibition of MyD88 signaling by HCV NS3 but are likely to contribute to chronic inflammation and potentially the progression to fibrosis and cirrhosis. (Figure based on [8]).
While certain TLR pathways, especially those that are related to viral clearance, are inhibited by HCV, other TLR signaling pathways may be activated by HCV and thus promote chronic inflammation. For example, Hepatitis C core protein and NS3 were found to activate TLR signaling via TLR2, a response that was abrogated in monocytes isolated from MyD88 or TLR2 deficient mice [143]. Another study utilizing B cells from patients infected with HCV as well as an immortalized lymphoma cell line, demonstrated increased TLR4 expression and higher levels of IFN-β and IL-6 in HCV cells in comparison to controls [154]. HCV core and NS3 proteins have also been shown to produce both pro and anti-inflammatory cytokines (TNF-α and IL-10) in human macrophages through the TLR2/1 and TLR2/TLR6 heterodimers [144]. Moreover, macrophages from HCV patients do not display tolerance to LPS after pretreatment with different TLR ligands, leading to persistent macrophage activation and cytokine release [142]. In summary, the current data supports the hypothesis that there is a decreased activation of TLR signaling pathway related to virus eradication but an increased activation of TLR signaling pathways related to inflammation. It is likely that this constellation promotes virus expansion, inflammation and potentially the progression to fibrosis and cirrhosis.
Hepatitis B
Hepatitis B virus (HBV) causes a chronic infection in about 10% of adults exposed to the virus affecting about 1.25 million within the United States and 370 million people worldwide [155]. One study reported a 24-fold induction in endotoxin levels in acute HBV infection, and a 72-fold induction of endotoxin levels in chronic HBV infection [156]. Moreover, it has been suggested that HBV contains TLR ligands [157]. Several TLRs block HBV replication through their ability to upregulate interferons. In HBV transgenic mice, activation of TLR3 by poly-I:C leads to an IFN-dependent inhibition of HBV replication [158]. These findings have been extended in two recent studies which demonstrated reduced HBV replication after injection of TLR3, TLR4, TLR5, TLR7 and TLR9 ligands into HBV transgenic mice [159], and the ability of supernatants from non-parenchymal cells stimulated with TLR3 and TLR4 agonists to repress HBV replication [88]. In a recent clinical study, TLR 1–10 receptor expression was quantified in patients with chronic Hepatitis B and compared to healthy controls. This study demonstrated that TLR1, TLR2, TLR4 and TLR6 were down regulated in HBV infected peripheral blood monocytes, and these cells also had a decreased cytokine response to TLR2 and TLR4 ligands [160]. Interestingly, HBeAg-positive chronic hepatitis B decreases TLR2 expression in peripheral monocytes, Kupffer cells and hepatocytes whereas HBeAg-negative chronic hepatitis B is associated with increased TLR2 expression suggesting a direct effect of the HBeAg on TLR2 expression [161]. Thus, it appears that HBV has developed abilities to downregulate TLRs and thus avoid anti-viral pathways, but that prolonged infection and loss of HBeAg may upregulate TLR signaling pathways such as TLR2 that are not involved in anti-HBV responses but promote hepatic inflammation and disease progression. Independently of their effect on HBV replication, TLR7 and TLR9 agonists may also have a role as adjuvants in vaccines in Hepatitis B as they booster edcellular and humoral responses to HBsAg as single agents or in combination in mice [162, 163, 164]. However, these results still require confirmation in humans.
Hepatic autoimmune diseases
The liver is known to be a classical immunoprivileged site. Notably, activation of TLR3 is required for infiltration of CD8+ T cells and liver disease after injecting mice with activated liver-specific effector CD8+T cells, suggesting that TLR signals may represent an important trigger to overcome this immunoprivilege and to induce hepatic autoimmune disease [165].
Primary biliary cirrhosis (PBC) is an autoimmune disorder usually affecting middle aged adult women which causes severe injury to the interlobular bile ducts [166]. Biliary epithelial cells are known to express TLRs, but under normal conditions are tolerant to antigenic exposures [85]. Although biliary epithelial cells isolated from livers from patients with PBC express similar levels of TLRs compared to controls, they secreted higher levels of chemokines when stimulated with TLR3 agonist poly I:C and co-cultured with liver-infiltrating mononuclear cells [167]. Moreover, TLR3 and IFN-α/β have been found to be elevated in the portal tracts and liver parenchyma of patients with PBC when compared to control patients with autoimmune hepatitis and Hepatitis C [168]. In addition, peripheral blood monocytes isolated from patients with PBC have been found to be hyperresponive to TLR ligands [169, 170]. TLRs may also play a role in B cell proliferation in PBC. Peripheral blood monocytes taken from PBC patients and stimulated with the TLR9 ligand, CpG, have been shown to induce IgM-producing B cells and an increased expression of TLR9 on these cells [171, 172]. Although high IgM levels are a typical feature of PBC, the relevance of the induction of IgM-producing B cells by CpG in PBC needs to be further investigated. There is only limited data on the role of TLRs in primary sclerosing cholangitis (PSC), an autoimmune disease that leads to fibro-obliterative changes within the intrahepatic and extrahepatic biliary tract. Stimulating isolated biliary epithelial cells with anti-biliary epithelial cell antibodies from patients with PSC leads to upregulation of TLR4 and TLR9, and a dramatically increased secretion of inflammatory cytokines under baseline conditions, and in the presence of LPS or CpG DNA [173].
V. Targeting TLRs in chronic liver disease
In recent years, a number of different approaches have been developed to modulate TLR signaling. These approaches include modulation of TLR ligand release from the intestinal microbiota by probiotics and antibiotics, activation of TLR signaling by synthetic TLR ligands and inhibition of TLR activation by small molecule inhibitors. Many of these recently developed agents have undergone evaluation in tissue culture experiments, mouse models and clinical trials, but their efficacy and clinical usefulness have not been proven beyond doubt in large sets of patients with liver disease.
TLR4-MD2 antagonists
Several small molecule inhibitors of TLR4 have been discovered and are currently being tested in human studies. Some of these inhibitors are lipid A mimetics which bind to the TLR4-MD2 complex but lack intrinsic activity, and thus prevent binding of the lipid A portion of LPS and subsequent TLR4 activation. The crystal structure of the TLR4-MD2 complex with bound “TLR4 antagonist” E5564 (Eisai Co., Ltd.) was recently published suggesting that the mechanism of action of E5564 is binding through a large internal pocket in MD-2 [17]. Thus, lipid A mimetic small molecule inhibitors should actually be classified as MD-2 inhibitors. In vitro, E5564 dose-dependently inhibited LPS-mediated activation of primary cultures of human myeloid cells and mouse tissue culture macrophage cell lines as well as human or animal whole blood at nanomolar concentrations as measured by production of tumor TNF- and other cytokines. In vivo, E5564 blocked induction of LPS-induced cytokines and LPS or bacterial-induced lethality in primed mice, and cytokine induction and symptoms after endotoxin injection in healthy volunteers [174, 175]. CRX-526 (Corixa Corporation) is another lipid A mimetic antagonist of TLR4 signaling that has been shown to inhibit IL-6 and MIP-1α production after LPS stimulation in vitro, and to almost completely suppress LPS-induced TNFα release in vivo [176]. Moreover, CRX-526 reduced colitis in the dextran sodium sulfate and MDR1a-deficiency models of colitis [176]. TAK-242 (Takeda Pharmaceutical Company Ltd.) represents a second class of TLR4 antagonists [177]. TAK-242 exerts its inhibitor effects at the intracellular domain of TLR4 as demonstrated by a study in which the extracellular domain of TLR4 was replaced by CD4 to create a constitutively active receptor chimera [178]. Activity of this receptor chimera was inhibited by TAK-242 providing evidence that its inhibitory effect does not require the presence of the TLR4 extracellular domain [178]. TAK-242 prevented increases in serum levels of wide range of cytokines in mice injected with LPS, and protected mice from LPS-induced lethality [179]. Interestingly, TAK-242 showed beneficial effects even when administered after LPS challenge [179]. Both E5564 and TAK-242 are currently tested in phase III clinical trials in patients with septic shock [180]. To the best of our knowledge, none of the TLR4/MD-2 inhibitors have been tested in chronic liver disease. Based on the involvement of TLR4 in fibrogenesis, alcoholic liver injury and NASH, small molecule inhibitors of TLR4 might be attractive candidates for the treatment or prevention of these diseases.
TLR7 agonists
The guanosine analogue isatoribine (Anadys Pharmaceuticals, Inc) is a small molecule ligand of TLR7 [181]. In a recent phase 1 trial in patients with chronic HCV infection, isatoribine induced a significant reduction of plasma HCV [153]. Eight out of 12 patients treated with 800 mg intravenous injection of isatoribine once daily for 7 days displayed a decrease of more than 0.5 log10 units with a mean change of -0.76 log in all 12 patients [153]. The reduction of viral load was correlated with an induction of markers of a heightened immune antiviral state, including a 7.6-fold induction of 2-, 5- oligoadenylate synthetase levels. This upregulation is comparable to that reported during the first week after the start of treatment with pegylated interferon alpha-2b. Side effects of isatoribine were mild to moderate and similar to those observed in patients treated with interferon-based therapies. Thus, TLR7 agonists such as isatoribine may represent good candidates for anti-viral therapy, potentially in combination with other anti-viral compounds. Recently, an oral prodrug of isatoribine, ANA975, was developed and may thus represent a novel oral treatment approach for patients with chronic HCV infection [182]. In contrast to isatoribine, the TLR7 and TLR8 agonist resiquimod appears to lack antiviral effects in HCV-infected patients as reported in a preliminary study [183].
TLR9 agonists
CPG 10101 (Actilon; Coley Pharmaceutical Group, Inc) is a synthetic oligodeoxynucleotide (ODN) that has been optimized to stimulate human TLR9. In a multicenter Phase 1b trial involving 60 patients with HCV infection, CPG 10101 induced a decrease in HCV RNA a greater than 1 log reduction in 22 of 40 patients who received more than 1 mg CPG 10101, and was thus similar to that reported for pegylated interferon monotherapy [184]. Moreover, CPG 10101 dose-dependently induced surrogate markers of anti-viral immunity such as (IFN)-gamma-inducible protein 10 (IP-10) and IFN-alpha as well as a sustained increase 2′5′-oligoadenylate synthetase [185]. CPG 10101 was well tolerated, and adverse events were consistent with CPG 10101’s mechanism of action. Although data from this study suggests CPG 10101 to be a good candidate for further studies in chronic HCV infection, development of CPG 10101 as therapy for HCV in conjunction with PEG-IFN and/or ribavirin has been temporarily suspended for unknown reasons [185].
Probiotics and Antibiotics
Modulation of the intestinal microbiota is an emerging strategy to reduce bacterial translocation and circulating endotoxin levels, and potentially those of other bacterially derived TLR agonist. A number of different approaches have been taken to achieve this goal. Modulation of the enteric microbiota by probiotics or synbiotics, a combination of probiotics with prebiotics, has been demonstrated to reduce bacterial translocation [186, 187, 188], circulating endotoxin levels in animal models [132], and bacterial infection, a surrogate marker for bacterial translocation, in patients with hepatic cirrhosis [189, 190]. In liver cirrhosis, probiotics have shown positive effects on several parameters including the improvement of liver function, prevention of infection, improvement of the hyperdynamic circulation and prevention of hepatic encephalopathy [132]. Other areas in which probiotics have positive effects on liver injury and ALT levels are (i) NASH with positive effects on ALT levels and liver histology in one mouse and one human study [114, 191], (ii) alcoholic liver injury [192] and (iii) LPS-induced liver injury [187, 188]. One problem is that a number of studies are relatively small and many of these are uncontrolled studies. The large number of probiotic strains and combinations of strains represents a second important problem, and it will require additional studies to confirm and ideally compare the efficacy of these probiotic strains or combinations for specific liver diseases. Among the most commonly used strains and combinations of strains are: (i) VSL#3, a combination of Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei and Lactobacillus bulgaricus has shown efficacy in reducing liver injury in patients with forms of chronic liver disease [191], in a mouse model of NASH [114], in a mouse model of LPS-induced liver failure [187]. (ii) Synbiotic cocktail 2000, a combination of Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei F19, L. plantarum 2592 plus betaglucan, inulin, pectin and resistant starch that has been shown to improve Child-Turcotte-Pugh classification and reduce encephalopathy [132], and to reduce bacterial infections after orthotopic liver transplantation [190]. (iii) L. plantarum 299V that has been demonstrated to prevent infection after orthotopic liver transplantation in combination with fiber [189]. A second approach to reduce TLR ligands is the treatment with antibiotics to achieve selective intestinal decontamination of gram-negative bacteria, the predominant source of LPS. Selective intestinal decontamination has been shown to reduce bacterial translocation in many but not all studies performed in rats [193, 194, 195]. Importantly, norfloxacin administration reduced the 1-year probability of developing spontaneous bacterial peritonitis, hepatorenal syndrome, and improved the 3-month and 1-year probability of survival compared with placebo [196]. While the reduction of spontaneous bacterial peritonitis in norfloxacin-treated patients is a direct consequence of reducing bacterial strains in the microbiota responsible for spontaneous peritonitis, some of the positive effect on mortality are likely SBP-independent and related to reducing bacterial translocation and circulating levels of TLR ligands [196, 197]. However, in view of the severe consequences of long-term antibiotics treatment, these treatment regimens will be reserved for selected high-risk patients with hepatic cirrhosis, and are not suited to reduce TLR agonists in patients with early stages of liver disease.
V. Conclusion
There is increasing evidence that TLRs play a key role in the pathophysiology of many chronic liver diseases. The possibility of targeting TLR signaling at different levels such as the intestinal microbiota, the level of TLRs, co-receptors such as MD2 and CD14 and downstream signaling molecules will open up a new therapeutic options for the treatment of chronic liver disease. However, a number of issues regarding the role of the TLRs and their ligands in liver disease need to addressed before TLRs can seriously be considered as pharmacological targets in liver disease: (i) The role of endogenous TLR ligands and their role in chronic liver disease need to be investigated more thoroughly. Well-designed in vitro and in vivo studies using mice in which the release of these ligands is blocked or induced by genetic methods are required to avoid any issues with contaminating bacterial ligands. (ii) As a large number of cell types in the liver express TLRs, a better understanding of cell-specific functions is required, e.g. by using cell-specific knockout strategies. (iii) The majority of studies on the role of TLRs in liver disease is based on animal models. Further translational research is required to firmly establish the role of TLRs in human liver disease. (iv) The potential therapeutic effect of TLR agonists, TLR antagonists and probiotics needs to be further assessed in well-controlled animal and human studies. It is possible that targeting of TLRs by small molecule antagonists may have immunosuppressive effects, and they may have to be restricted to selective patient groups and exclude patient groups with advanced liver disease and immunosuppression. Therefore, probiotics appear to be ideal candidates for the treatment of chronic liver disease such as alcoholic liver disease, NAFLD and NASH and liver fibrosis due to their high tolerability and limited side effects. However, it needs to be established whether the old pharmacologic teaching that “no effect is to be expected from a drug that does not have side effects” is a rule with exceptions. Moreover, it would be interesting to determine whether the suppression of TLR signaling pathways by HCV can be blocked pharmacologically, and whether TLR agonists may have some effects on HBV and HCV that differ from those of interferon, and could thus provide a useful addition to current interferon-based therapies. We can anticipate that further research on TLR signaling in chronic liver disease will help to shape new concepts with the intestinal microbiota, TLRs and their downstream signaling mediators as pharmacological targets.
Summary Box 1
Toll-like receptors are a cornerstone of the innate immune system and provide an almost instant anti-microbial response to fight pathogens.
Toll-like receptors are pattern recognition receptors that detect the presence of minute amounts of signature molecules present in pathogens (pathogen associated molecular patterns=PAMPs).
Activation of Toll-like receptors activate anti-viral and pro-inflammatory signaling pathways.
Toll-like receptors signal through the adapter molecules MyD88, Trif or both to activate the “MyD88-dependent” and “MyD88-independent” signaling pathways.
It has been suggested that Toll-like receptors may also be activated by endogenous ligands. Many of these endogenous ligands are associated with injury and inflammation and belong to a group of molecules termed damage associated molecular patterns (DAMPs). However, none of these molecules has been proven a bona fide TLR ligand beyond doubt.
Summary Box 2
The liver is a target of bacterial TLR ligands due to its anatomic connection to the intestine.
Under normal circumstances, the liver is exposed to small amounts of bacterial PAMPs but does not show signs of inflammation due to its higher tolerance to PAMPs and its ability to efficiently excrete PAMPs such as LPS.
In many types of chronic liver disease, levels of PAMPs are elevated. Most research has focused on LPS, and has shown increased LPS levels in chronic viral hepatitis, liver fibrosis and cirrhosis, and alcoholic liver disease.
LPS promotes liver injury and fibrogenesis under many circumstances. Blocking LPS release from the intestinal microbiota, or inhibiting activation and signaling of the LPS receptor TLR4 may therefore represent a feasible strategy for the prevention or treatment of chronic liver disease.
TLRs and downstream signaling molecules play a role in chronic viral hepatitis. Chronic HCV infection leads to a downregulation of anti-viral signaling pathways. Activation of specific TLR signals may booster anti-viral immunity, and therefore represent a novel treatment approach for chronic viral hepatitis.
Acknowledgments
This study was supported by National Institute of Health grant R01 DK076920 (to RFS) and an American Liver Foundation postdoctoral research awared (to JK).
References
- 1.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre B, Nicolas E, Michaut L, et al. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 4.Roach JC, Glusman G, Rowen L, et al. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–82. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler BA. TLRs and innate immunity. Blood. 2008 doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 7.Rakoff-Nahoum S, Medzhitov R. Role of toll-like receptors in tissue repair and tumorigenesis. Biochemistry (Mosc) 2008;73:555–61. doi: 10.1134/s0006297908050088. [DOI] [PubMed] [Google Scholar]
- 8.Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology. 2006;44:287–98. doi: 10.1002/hep.21308. [DOI] [PubMed] [Google Scholar]
- 10.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–35. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 13.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–37. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Z, Georgel P, Du X, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–70. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 15.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–72. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 16.Schromm AB, Lien E, Henneke P, et al. Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J Exp Med. 2001;194:79–88. doi: 10.1084/jem.194.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–17. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Hoebe K, Georgel P, Rutschmann S, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–7. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 19.Liew FY, Xu D, Brint EK, et al. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 20.Nomura F, Akashi S, Sakao Y, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–9. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 21.Chuang TH, Ulevitch RJ. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol. 2004;5:495–502. doi: 10.1038/ni1066. [DOI] [PubMed] [Google Scholar]
- 22.Mansell A, Smith R, Doyle SL, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–55. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 23.Brint EK, Xu D, Liu H, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–9. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 24.Wald D, Qin J, Zhao Z, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–7. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 25.Divanovic S, Trompette A, Atabani SF, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6:571–8. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssens S, Burns K, Vercammen E, et al. MyD88S, a splice variant of MyD88, differentially modulates NF-kappaB- and AP-1-dependent gene expression. FEBS Lett. 2003;548:103–7. doi: 10.1016/s0014-5793(03)00747-6. [DOI] [PubMed] [Google Scholar]
- 27.Burns K, Janssens S, Brissoni B, et al. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–8. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi K, Hernandez LD, Galan JE, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 29.Hardy MP, O’Neill LA. The murine IRAK2 gene encodes four alternatively spliced isoforms, two of which are inhibitory. J Biol Chem. 2004;279:27699–708. doi: 10.1074/jbc.M403068200. [DOI] [PubMed] [Google Scholar]
- 30.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–33. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 31.Fukui H, Brauner B, Bode JC, et al. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–9. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 32.Parlesak A, Schafer C, Schutz T, et al. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–7. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 33.Mathurin P, Deng QG, Keshavarzian A, et al. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–17. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 34.Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology. 1984;86:174–93. [PubMed] [Google Scholar]
- 35.Wells CL, Maddaus MA, Reynolds CM, et al. Role of anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun. 1987;55:2689–94. doi: 10.1128/iai.55.11.2689-2694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988;157:1032–8. doi: 10.1093/infdis/157.5.1032. [DOI] [PubMed] [Google Scholar]
- 37.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 38.Beutler B. Neo-ligands for innate immune receptors and the etiology of sterile inflammatory disease. Immunol Rev. 2007;220:113–28. doi: 10.1111/j.1600-065X.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 39.Georgel P, Jiang Z, Kunz S, et al. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–13. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Jude BA, Pobezinskaya Y, Bishop J, et al. Subversion of the innate immune system by a retrovirus. Nat Immunol. 2003;4:573–8. doi: 10.1038/ni926. [DOI] [PubMed] [Google Scholar]
- 41.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 42.Lotze MT, Zeh HJ, Rubartelli A, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsan MF, Baochong G. Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J Endotoxin Res. 2007;13:6–14. doi: 10.1177/0968051907078604. [DOI] [PubMed] [Google Scholar]
- 44.Park JS, Svetkauskaite D, He Q, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 45.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 46.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 48.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 49.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 50.Bonaldi T, Talamo F, Scaffidi P, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–60. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 52.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 53.Mathison JC, Ulevitch RJ. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979;123:2133–43. [PubMed] [Google Scholar]
- 54.Ruiter DJ, van der Meulen J, Brouwer A, et al. Uptake by liver cells of endotoxin following its intravenous injection. Lab Invest. 1981;45:38–45. [PubMed] [Google Scholar]
- 55.Benacerraf B, Sebestyen MM, Schlossman S. A quantitative study of the kinetics of blood clearance of P32-labelled Escherichia coli and Staphylococci by the reticuloendothelial system. J Exp Med. 1959;110:27–48. doi: 10.1084/jem.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Egmond M, van Garderen E, van Spriel AB, et al. FcalphaRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med. 2000;6:680–5. doi: 10.1038/76261. [DOI] [PubMed] [Google Scholar]
- 57.De Creus A, Abe M, Lau AH, et al. Low TLR4 Expression by Liver Dendritic Cells Correlates with Reduced Capacity to Activate Allogeneic T Cells in Response to Endotoxin. J Immunol. 2005;174:2037–45. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 58.Lichtman SN, Wang J, Lemasters JJ. LPS receptor CD14 participates in release of TNF-alpha in RAW 264.7 and peritoneal cells but not in kupffer cells. Am J Physiol. 1998;275:G39–46. doi: 10.1152/ajpgi.1998.275.1.G39. [DOI] [PubMed] [Google Scholar]
- 59.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 60.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–86. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 61.Su GL, Klein RD, Aminlari A, et al. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932–6. doi: 10.1053/he.2000.5634. [DOI] [PubMed] [Google Scholar]
- 62.Van Bossuyt H, De Zanger RB, Wisse E. Cellular and subcellular distribution of injected lipopolysaccharide in rat liver and its inactivation by bile salts. J Hepatol. 1988;7:325–37. doi: 10.1016/s0168-8278(88)80005-9. [DOI] [PubMed] [Google Scholar]
- 63.Fox ES, Thomas P, Broitman SA. Clearance of gut-derived endotoxins by the liver. Release and modification of 3H, 14C-lipopolysaccharide by isolated rat Kupffer cells. Gastroenterology. 1989;96:456–61. doi: 10.1016/0016-5085(89)91571-0. [DOI] [PubMed] [Google Scholar]
- 64.Mimura Y, Sakisaka S, Harada M, et al. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology. 1995;109:1969–76. doi: 10.1016/0016-5085(95)90765-3. [DOI] [PubMed] [Google Scholar]
- 65.Shao B, Lu M, Katz SC, et al. A host lipase detoxifies bacterial lipopolysaccharides in the liver and spleen. J Biol Chem. 2007;282:13726–35. doi: 10.1074/jbc.M609462200. [DOI] [PubMed] [Google Scholar]
- 66.Seki E, Tsutsui H, Nakano H, et al. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166:2651–7. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- 67.Kopydlowski KM, Salkowski CA, Cody MJ, et al. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163:1537–44. [PubMed] [Google Scholar]
- 68.Seki E, Tsutsui H, Tsuji NM, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–8. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 69.Schuchmann M, Hermann F, Herkel J, et al. HSP60 and CpG-DNA-oligonucleotides differentially regulate LPS-tolerance of hepatic Kupffer cells. Immunol Lett. 2004;93:199–204. doi: 10.1016/j.imlet.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 70.Jiang W, Sun R, Wei H, et al. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of toll-like receptor 4 expression on macrophages. Proc Natl Acad Sci U S A. 2005;102:17077–82. doi: 10.1073/pnas.0504570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thobe BM, Frink M, Choudhry MA, et al. Src family kinases regulate p38 MAPK-mediated IL-6 production in Kupffer cells following hypoxia. Am J Physiol Cell Physiol. 2006;291:C476–82. doi: 10.1152/ajpcell.00076.2006. [DOI] [PubMed] [Google Scholar]
- 72.Knolle P, Schlaak J, Uhrig A, et al. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–9. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, Gallo DJ, Green AM, et al. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002;70:3433–42. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsumura T, Degawa T, Takii T, et al. TRAF6-NF-kappaB pathway is essential for interleukin-1-induced TLR2 expression and its functional response to TLR2 ligand in murine hepatocytes. Immunology. 2003;109:127–36. doi: 10.1046/j.1365-2567.2003.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsumura T, Ito A, Takii T, et al. Endotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TLR4 gene expression in murine liver and hepatocytes. J Interferon Cytokine Res. 2000;20:915–21. doi: 10.1089/10799900050163299. [DOI] [PubMed] [Google Scholar]
- 76.Scott MJ, Billiar TR. beta 2-integrin induced p38MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of LPS by hepatocytes. J Biol Chem. 2008 doi: 10.1074/jbc.M803905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paik YH, Schwabe RF, Bataller R, et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–55. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 79.Brun P, Castagliuolo I, Pinzani M, et al. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G571–8. doi: 10.1152/ajpgi.00537.2004. [DOI] [PubMed] [Google Scholar]
- 80.Gabele E, Muhlbauer M, Dorn C, et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008 doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 81.Thirunavukkarasu C, Uemura T, Wang LF, et al. Normal rat hepatic stellate cells respond to endotoxin in LBP-independent manner to produce inhibitor(s) of DNA synthesis in hepatocytes. J Cell Physiol. 2005;204:654–65. doi: 10.1002/jcp.20366. [DOI] [PubMed] [Google Scholar]
- 82.Harada K, Ohira S, Isse K, et al. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Lab Invest. 2003;83:1657–67. doi: 10.1097/01.lab.0000097190.56734.fe. [DOI] [PubMed] [Google Scholar]
- 83.Ikeda H, Sasaki M, Ishikawa A, et al. Interaction of Toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. Lab Invest. 2007;87:559–71. doi: 10.1038/labinvest.3700556. [DOI] [PubMed] [Google Scholar]
- 84.Chen XM, O’Hara SP, Nelson JB, et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J Immunol. 2005;175:7447–56. doi: 10.4049/jimmunol.175.11.7447. [DOI] [PubMed] [Google Scholar]
- 85.Harada K, Isse K, Nakanuma Y. Interferon gamma accelerates NF-kappaB activation of biliary epithelial cells induced by Toll-like receptor and ligand interaction. J Clin Pathol. 2006;59:184–90. doi: 10.1136/jcp.2004.023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uhrig A, Banafsche R, Kremer M, et al. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J Leukoc Biol. 2005;77:626–33. doi: 10.1189/jlb.0604332. [DOI] [PubMed] [Google Scholar]
- 87.Martin-Armas M, Simon-Santamaria J, Pettersen I, et al. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J Hepatol. 2006;44:939–46. doi: 10.1016/j.jhep.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Wu J, Lu M, Meng Z, et al. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology. 2007;46:1769–78. doi: 10.1002/hep.21897. [DOI] [PubMed] [Google Scholar]
- 89.Shu SA, Lian ZX, Chuang YH, et al. The role of CD11c(+) hepatic dendritic cells in the induction of innate immune responses. Clin Exp Immunol. 2007;149:335–43. doi: 10.1111/j.1365-2249.2007.03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rivera CA, Bradford BU, Seabra V, et al. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am J Physiol. 1998;275:G1252–8. doi: 10.1152/ajpgi.1998.275.6.G1252. [DOI] [PubMed] [Google Scholar]
- 91.Adachi Y, Moore LE, Bradford BU, et al. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–24. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 92.Hansen J, Cherwitz DL, Allen JI. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20:461–74. [PubMed] [Google Scholar]
- 93.Bode JC, Bode C, Heidelbach R, et al. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–4. [PubMed] [Google Scholar]
- 94.Hauge T, Persson J, Danielsson D. Mucosal bacterial growth in the upper gastrointestinal tract in alcoholics (heavy drinkers) Digestion. 1997;58:591–5. doi: 10.1159/000201507. [DOI] [PubMed] [Google Scholar]
- 95.Worthington BS, Meserole L, Syrotuck JA. Effect of daily ethanol ingestion on intestinal permeability to macromolecules. Am J Dig Dis. 1978;23:23–32. doi: 10.1007/BF01072571. [DOI] [PubMed] [Google Scholar]
- 96.Draper LR, Gyure LA, Hall JG, et al. Effect of alcohol on the integrity of the intestinal epithelium. Gut. 1983;24:399–404. doi: 10.1136/gut.24.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–82. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 98.Ferrier L, Berard F, Debrauwer L, et al. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148–54. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Purohit V, Bode JC, Bode C, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–61. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adachi Y, Bradford BU, Gao W, et al. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–60. [PubMed] [Google Scholar]
- 101.Uesugi T, Froh M, Arteel GE, et al. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–8. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 102.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008 doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kono H, Rusyn I, Yin M, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–72. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 106.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 107.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 108.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–25. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 109.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 110.Membrez M, Blancher F, Jaquet M, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416–26. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 111.Yang SQ, Lin HZ, Lane MD, et al. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–62. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rivera CA, Adegboyega P, van Rooijen N, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–9. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szabo G, Velayudham A, Romics L, Jr, et al. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res. 2005;29:140S–5S. doi: 10.1097/01.alc.0000189287.83544.33. [DOI] [PubMed] [Google Scholar]
- 114.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–50. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 115.Nolan JP, Leibowitz AI. Endotoxin and the liver. III. Modification of acute carbon tetrachloride injury by polymyxin b--an antiendotoxin. Gastroenterology. 1978;75:445–9. [PubMed] [Google Scholar]
- 116.Grinko I, Geerts A, Wisse E. Experimental biliary fibrosis correlates with increased numbers of fat-storing and Kupffer cells, and portal endotoxemia. J Hepatol. 1995;23:449–58. doi: 10.1016/0168-8278(95)80204-5. [DOI] [PubMed] [Google Scholar]
- 117.Chan CC, Hwang SJ, Lee FY, et al. Prognostic value of plasma endotoxin levels in patients with cirrhosis. Scand J Gastroenterol. 1997;32:942–6. doi: 10.3109/00365529709011206. [DOI] [PubMed] [Google Scholar]
- 118.Lin RS, Lee FY, Lee SD, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–72. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 119.Chesta J, Defilippi C. Abnormalities in proximal small bowel motility in patients with cirrhosis. Hepatology. 1993;17:828–32. [PubMed] [Google Scholar]
- 120.Guarner C, Runyon BA, Young S, et al. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997;26:1372–8. doi: 10.1016/s0168-8278(97)80474-6. [DOI] [PubMed] [Google Scholar]
- 121.Ramachandran A, Prabhu R, Thomas S, et al. Intestinal mucosal alterations in experimental cirrhosis in the rat: role of oxygen free radicals. Hepatology. 2002;35:622–9. doi: 10.1053/jhep.2002.31656. [DOI] [PubMed] [Google Scholar]
- 122.Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252–62. doi: 10.1002/hep.1840060217. [DOI] [PubMed] [Google Scholar]
- 123.Garcia-Tsao G, Lee FY, Barden GE, et al. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 1995;108:1835–41. doi: 10.1016/0016-5085(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 124.Luckey TD, Reyniers JA, Gyorgy P, et al. Germfree animals and liver necrosis. Ann N Y Acad Sci. 1954;57:932–5. doi: 10.1111/j.1749-6632.1954.tb36472.x. [DOI] [PubMed] [Google Scholar]
- 125.Rutenburg AM, Sonnenblick E, Koven I, et al. The role of intestinal bacteria in the development of dietary cirrhosis in rats. J Exp Med. 1957;106:1–14. doi: 10.1084/jem.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Isayama F, Hines IN, Kremer M, et al. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1318–28. doi: 10.1152/ajpgi.00405.2005. [DOI] [PubMed] [Google Scholar]
- 127.Huang H, Shiffman ML, Friedman S, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 128.Watanabe A, Hashmi A, Gomes DA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–18. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 129.Bajaj JS, Hafeezullah M, Franco J, et al. Inhibitory Control Test for the Diagnosis of Minimal Hepatic Encephalopathy. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 130.Bajaj JS, Saeian K, Christensen KM, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103:1707–15. doi: 10.1111/j.1572-0241.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 131.Loguercio C, Abbiati R, Rinaldi M, et al. Long-term effects of Enterococcus faecium SF68 versus lactulose in the treatment of patients with cirrhosis and grade 1–2 hepatic encephalopathy. J Hepatol. 1995;23:39–46. doi: 10.1016/0168-8278(95)80309-2. [DOI] [PubMed] [Google Scholar]
- 132.Liu Q, Duan ZP, Ha DK, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–9. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 133.Albillos A, de la Hera A, Gonzalez M, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–17. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 134.Rasaratnam B, Kaye D, Jennings G, et al. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann Intern Med. 2003;139:186–93. doi: 10.7326/0003-4819-139-3-200308050-00008. [DOI] [PubMed] [Google Scholar]
- 135.Kemp W, Colman J, Thompson K, et al. Norfloxacin treatment for clinically significant portal hypertension: results of a randomised double-blind placebo-controlled crossover trial. Liver Int. 2008 doi: 10.1111/j.1478-3231.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- 136.Virchow R. Aetiologie der neoplastischen Geschwulste/Pathogenie der neoplastischen Geschwulste. In: Virchow R, editor. Die krankhaften Geschwulste. Berlin: Verlag von August von Hirschwald; 1863. pp. 57–101. [Google Scholar]
- 137.Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 138.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 139.Sakurai T, He G, Matsuzawa A, et al. Hepatocyte Necrosis Induced by Oxidative Stress and IL-1alpha Release Mediate Carcinogen-Induced Compensatory Proliferation and Liver Tumorigenesis. Cancer Cell. 2008;14:156–65. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 141.Mukherjee S, Sorrell MF. Controversies in liver transplantation for hepatitis C. Gastroenterology. 2008;134:1777–88. doi: 10.1053/j.gastro.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 142.Dolganiuc A, Norkina O, Kodys K, et al. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627–36. doi: 10.1053/j.gastro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dolganiuc A, Oak S, Kodys K, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–24. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 144.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479–87. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 145.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 146.Ishii S, Koziel MJ. Immune responses during acute and chronic infection with hepatitis C virus. Clin Immunol. 2008;128:133–47. doi: 10.1016/j.clim.2008.03.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li K, Foy E, Ferreon JC, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005 doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Otsuka M, Kato N, Moriyama M, et al. Interaction between the HCV NS3 protein and the host TBK1 protein leads to inhibition of cellular antiviral responses. Hepatology. 2005;41:1004–12. doi: 10.1002/hep.20666. [DOI] [PubMed] [Google Scholar]
- 149.Abe T, Kaname Y, Hamamoto I, et al. Hepatitis C virus nonstructural protein 5A modulates the toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J Virol. 2007;81:8953–66. doi: 10.1128/JVI.00649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miyazaki M, Kanto T, Inoue M, et al. Impaired cytokine response in myeloid dendritic cells in chronic hepatitis C virus infection regardless of enhanced expression of Toll-like receptors and retinoic acid inducible gene-I. J Med Virol. 2008;80:980–8. doi: 10.1002/jmv.21174. [DOI] [PubMed] [Google Scholar]
- 151.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385–95. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]
- 152.Yonkers NL, Rodriguez B, Milkovich KA, et al. TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J Immunol. 2007;178:4436–44. doi: 10.4049/jimmunol.178.7.4436. [DOI] [PubMed] [Google Scholar]
- 153.Horsmans Y, Berg T, Desager JP, et al. Isatoribine, an agonist of TLR7, reduces plasma virus concentration in chronic hepatitis C infection. Hepatology. 2005;42:724–31. doi: 10.1002/hep.20839. [DOI] [PubMed] [Google Scholar]
- 154.Machida K, Cheng KT, Sung VM, et al. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006;80:866–74. doi: 10.1128/JVI.80.2.866-874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–45. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 156.Sozinov AS. Systemic endotoxemia during chronic viral hepatitis. Bull Exp Biol Med. 2002;133:153–5. doi: 10.1023/a:1015546821875. [DOI] [PubMed] [Google Scholar]
- 157.Cooper A, Tal G, Lider O, et al. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J Immunol. 2005;175:3165–76. doi: 10.4049/jimmunol.175.5.3165. [DOI] [PubMed] [Google Scholar]
- 158.McClary H, Koch R, Chisari FV, et al. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74:2255–64. doi: 10.1128/jvi.74.5.2255-2264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Isogawa M, Robek MD, Furuichi Y, et al. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–72. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen Z, Cheng Y, Xu Y, et al. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128:400–8. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 161.Visvanathan K, Skinner NA, Thompson AJ, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45:102–10. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 162.Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 163.Weeratna RD, Makinen SR, McCluskie MJ, et al. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848) Vaccine. 2005;23:5263–70. doi: 10.1016/j.vaccine.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 164.Ma R, Du JL, Huang J, et al. Additive effects of CpG ODN and R-848 as adjuvants on augmenting immune responses to HBsAg vaccination. Biochem Biophys Res Commun. 2007;361:537–42. doi: 10.1016/j.bbrc.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 165.Lang KS, Recher M, Junt T, et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–45. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 166.Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet. 2003;362:53–61. doi: 10.1016/S0140-6736(03)13808-1. [DOI] [PubMed] [Google Scholar]
- 167.Shimoda S, Harada K, Niiro H, et al. Biliary epithelial cells and primary biliary cirrhosis: the role of liver-infiltrating mononuclear cells. Hepatology. 2008;47:958–65. doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 168.Takii Y, Nakamura M, Ito M, et al. Enhanced expression of type I interferon and toll-like receptor-3 in primary biliary cirrhosis. Lab Invest. 2005 doi: 10.1038/labinvest.3700285. [DOI] [PubMed] [Google Scholar]
- 169.Mao TK, Lian ZX, Selmi C, et al. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42:802–8. doi: 10.1002/hep.20859. [DOI] [PubMed] [Google Scholar]
- 170.Honda Y, Yamagiwa S, Matsuda Y, et al. Altered expression of TLR homolog RP105 on monocytes hypersensitive to LPS in patients with primary biliary cirrhosis. J Hepatol. 2007;47:404–11. doi: 10.1016/j.jhep.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 171.Kikuchi K, Lian ZX, Kimura Y, et al. Genetic polymorphisms of toll-like receptor 9 influence the immune response to CpG and contribute to hyper-IgM in primary biliary cirrhosis. J Autoimmun. 2005 doi: 10.1016/j.jaut.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 172.Kikuchi K, Lian ZX, Yang GX, et al. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–12. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 173.Karrar A, Broome U, Sodergren T, et al. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology. 2007;132:1504–14. doi: 10.1053/j.gastro.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 174.Mullarkey M, Rose JR, Bristol J, et al. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther. 2003;304:1093–102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- 175.Rossignol DP, Lynn M. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J Endotoxin Res. 2002;8:483–8. doi: 10.1179/096805102125001127. [DOI] [PubMed] [Google Scholar]
- 176.Fort MM, Mozaffarian A, Stover AG, et al. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J Immunol. 2005;174:6416–23. doi: 10.4049/jimmunol.174.10.6416. [DOI] [PubMed] [Google Scholar]
- 177.Ii M, Matsunaga N, Hazeki K, et al. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol. 2006;69:1288–95. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- 178.Kawamoto T, Ii M, Kitazaki T, et al. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40–8. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 179.Sha T, Sunamoto M, Kitazaki T, et al. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571:231–9. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 180.Kanzler H, Barrat FJ, Hessel EM, et al. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–9. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 181.Lee J, Chuang TH, Redecke V, et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:6646–51. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xiang AX, Webber SE, Kerr BM, et al. Discovery of ANA975: an oral prodrug of the TLR-7 agonist isatoribine. Nucleosides Nucleotides Nucleic Acids. 2007;26:635–40. doi: 10.1080/15257770701490472. [DOI] [PubMed] [Google Scholar]
- 183.Pockros P, Tong M, Wright T. A phase IIa placebo-controlled, double bind trial to determine the safety, tolerability and pk/pd of an oral interferon inducer. Gastroenterology. 2003;124:76A. [Google Scholar]
- 184.Pappas SC. Good science behind hepatitis C virus antiviral drug development: necessary but not sufficient. Hepatology. 2007;46:1317–8. doi: 10.1002/hep.22053. [DOI] [PubMed] [Google Scholar]
- 185.McHutchison JG, Bacon BR, Gordon SC, et al. Phase 1B, randomized, double-blind, dose-escalation trial of CPG 10101 in patients with chronic hepatitis C virus. Hepatology. 2007;46:1341–9. doi: 10.1002/hep.21773. [DOI] [PubMed] [Google Scholar]
- 186.Adawi D, Kasravi FB, Molin G, et al. Effect of Lactobacillus supplementation with and without arginine on liver damage and bacterial translocation in an acute liver injury model in the rat. Hepatology. 1997;25:642–7. doi: 10.1002/hep.510250325. [DOI] [PubMed] [Google Scholar]
- 187.Ewaschuk J, Endersby R, Thiel D, et al. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology. 2007;46:841–50. doi: 10.1002/hep.21750. [DOI] [PubMed] [Google Scholar]
- 188.Osman N, Adawi D, Ahrne S, et al. Endotoxin- and D-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis. 2007;39:849–56. doi: 10.1016/j.dld.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 189.Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74:123–7. doi: 10.1097/00007890-200207150-00021. [DOI] [PubMed] [Google Scholar]
- 190.Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. Am J Transplant. 2005;5:125–30. doi: 10.1111/j.1600-6143.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 191.Loguercio C, Federico A, Tuccillo C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–3. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 192.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–7. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 193.Runyon BA, Borzio M, Young S, et al. Effect of selective bowel decontamination with norfloxacin on spontaneous bacterial peritonitis, translocation, and survival in an animal model of cirrhosis. Hepatology. 1995;21:1719–24. [PubMed] [Google Scholar]
- 194.Llovet JM, Bartoli R, Planas R, et al. Selective intestinal decontamination with norfloxacin reduces bacterial translocation in ascitic cirrhotic rats exposed to hemorrhagic shock. Hepatology. 1996;23:781–7. doi: 10.1002/hep.510230419. [DOI] [PubMed] [Google Scholar]
- 195.Bauer TM, Fernandez J, Navasa M, et al. Failure of Lactobacillus spp. to prevent bacterial translocation in a rat model of experimental cirrhosis. J Hepatol. 2002;36:501–6. doi: 10.1016/s0168-8278(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 196.Fernandez J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818–24. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 197.Runyon BA. A pill a day can improve survival in patients with advanced cirrhosis. Gastroenterology. 2007;133:1029–31. doi: 10.1053/j.gastro.2007.07.017. [DOI] [PubMed] [Google Scholar]