Abstract
Neural correlates of social-cognition were assessed in 9- to- 17-year-olds (N = 34) using functional magnetic resonance imaging. Participants appraised how unfamiliar peers they had previously identified as being of high or low interest would evaluate them for an anticipated online chat session. Differential age- and sex-related activation patterns emerged in several regions previously implicated in affective processing. These included the ventral striatum, hippocampus, hypothalamus, and insula. In general, activation patterns shifted with age in older relative to younger females but showed no association with age in males. Relating these neural response patterns to changes in adolescent social-cognition enriches theories of adolescent social development through enhanced neurobiological understanding of social behavior.
Among the most marked changes in adolescence is a shift in patterns of social affiliation from one dominated by family to one dominated by peers (Rubin, Bukowski, & Parker, 2006; Steinberg & Morris, 2001). The desire for peer affiliation powerfully motivates adolescents in ways less apparent during earlier years, and this desire is reflected in the degree to which day-to-day thoughts, emotions, and behaviors focus on peer affiliation (Kaufman, Brown, Graves, Henderson, & Revolinski, 1993; Larson, Richards, Moneta, Holmbeck, & Duckett, 1996; Richards, Crowe, Larson, & Swarr, 1998; Wong & Csikszentmihalyi, 1991).
One common focus of peer-related cognitions and emotional reactivity in adolescence is concern about the opinions of others. This preoccupation relates specifically to social assessments: how the adolescent is viewed by his or her peers (Henker, Whalen, & O’Neil, 1995; La Greca & Lopez, 1998; La Greca & Stone, 1993; Muris, Meesters, Merckelbach, Sermon, & Zwakhalen, 1998; Silverman, La Greca, & Wasserstein, 1995; Urberg, 1992; Vasey, Crnic, & Carter, 1994). The heightened attention to the views of others can be adaptive, given that those who are attuned to the norms of peers are more likely to successfully navigate complex social scenarios and to form mature relationships (Baumeister & Leary, 1995).
However, excessive focus on peer opinions can be maladaptive. For example, peer rejection itself is related to social avoidance, loneliness, and depression (Burks, Dodge, & Price, 1995; Gazelle & Rudolph, 2004) as well as aggression, delinquency, and substance use (Dishion & Owen, 2002; Dodge et al., 2003; Laird, Jordan, Dodge, Pettit, & Bates, 2001). Furthermore, one’s appraisal of rejection by peers has been linked to various psychiatric problems even after accounting for actual peer rejection itself (Kistner, Balthazor, Risi, & Burton, 1999; Sandstrom, Cillessen, & Eisenhower, 2003).
Relational events appear particularly salient for adolescent females. Prior behavioral and neural data indicate that females, relative to males, become more sensitive to social signals in adolescence (McClure, 2000; McClure et al., 2004); at the same time, they evidence greater concerns about peer evaluation (La Greca & Lopez, 1998; La Greca & Stone, 1993; Rose & Rudolph, 2006; Rudolph & Conley, 2005; Storch, Zelman, Sweeney, Danner, & Dove, 2002). Hence, as adolescence progresses, females tend to become increasingly attuned to the affective dynamics of social interactions. This same pattern has not been reported in males, who tend to be focused more on group relationships and dominance-related processes than on interpersonal interactions (Maccoby, 1998; Rose & Rudolph, 2006). Indeed, the particularly marked increase in mood and anxiety disorders among adolescent females, more so than males, may relate in part to sex-specific changes in affective processing of social stimuli and response to interpersonal stress (Cyranowski, Frank, Young, & Shear, 2000; Hankin & Abramson, 2001; Nolen-Hoeksema & Girgus, 1994; Rudolph & Conley, 2005; Shih, Eberhart, Hammen, & Brennan, 2006; Stroud, Salovey, & Epel, 2002; Zahn-Waxler, Shirtcliff, & Marceau, 2008). Relative to males, females exhibit more sensitivity and responsiveness to social cues, particularly during and after adolescence (Hall, 1984; McClure, 2000), and demonstrate higher levels of concern about peer evaluation and social approval (La Greca & Lopez, 1998; La Greca & Stone, 1993; Rudolph & Conley, 2005; Storch et al., 2002). However, work is needed that specifically examines changes in social-cognitive factors among typically developing females and the associated changes in biological substrates that support such processes.
Few studies in this area have taken a neural systems approach. Incorporating biological, mechanism-based frameworks into the study of adolescent sex-specific social development will facilitate two advances. First, biological data can constrain theoretical perspectives. Although complex cultural and environmental interplay clearly affects behavioral development, such external influences on behavior must be mediated through the brain. Thus, comprehensive and accurate theories of adolescent social development need to incorporate observations from neuroscience. Second, emerging work demonstrates the manner in which the integration of neuroscience and developmental theory creates a fertile cross-fostering (Brotman, Gouley, Klein, Castellanos, & Pine, 2003; Nelson, Leibenluft, McClure, & Pine, 2005; Saxe, Carey, & Kanwisher, 2004). Embracing such a multilevel approach may lead to the construction of important bridges between models of the neural and social systems bases of behavior, thereby linking two rich traditions that are currently relatively unfamiliar with each other.
Modern neuroscience techniques allow sophisticated charting of brain development in a manner increasingly relevant to theories of adolescent social development. Several neuroimaging studies implicate developmental and / or sex-related factors in the neural response to social cues (Blakemore, den Ouden, Choudhury, & Frith, 2007; Guyer, Monk, et al., 2008; Killgore, Oki, & Yurgelun-Todd, 2001; Killgore & Yurgelun-Todd, 2004; McClure et al., 2004; Monk et al., 2003; Thomas et al., 2001; Yurgelun-Todd & Killgore, 2006). Nevertheless, these data extend findings initially generated among adults, focusing on neural responses to stimuli, such as passive viewing of adult actors, relatively removed from emotional events that are most salient to the social lives of adolescents. Given the distinctive nature of changes in the adolescent social landscape, work is needed that precisely tailors tasks to aspects of the adolescent social milieu. Rather than using methods from adult cognitive neuroscience, the current study employed a novel neuroscience approach that emerged explicitly from observations of salient social experiences, emotions, and cognitions that are common in adolescence.
Current theory suggests that social-neurocognitive processes related to social interaction change in adolescence. These changes can be interpreted in the context of a recent framework, the social information processing network (SIPN) model (Nelson et al., 2005), which views changes in adolescent social behavior as reflections of maturation in specific network-based nodes. The current study maps age- and sex-related associations in functional responses of the affective node across adolescence. Thus, our hypothesis is that peers become more polarized affectively with age during adolescence, as reflected in patterns of activation within affectively relevant brain circuits encompassing key brain structures, including the nucleus accumbens, insula, hypothalamus, hippocampus, and amygdala. These predictions are based not only on increased affective responses more generally in adolescence (Pine, Cohen, & Brook, 2001; Steinberg, 2005) but also on the specific increase in the salience of peers (Steinberg, 1989). We predicted that peer stimuli would elicit greater responses within specific regions of the affective node in older, relative to younger adolescents, and in females more than males (Nelson et al., 2005), as behavioral and neural data suggest that these age-related changes will differ by sex (Killgore & Yurgelun-Todd, 2004; Killgore et al., 2001; McClure, 2000; McClure et al., 2004; Rudolph & Conley, 2005).
Given adolescents’ increased self-focus (Steinberg, 2005) and their concern about peer social evaluation on their patterns of social-cognition and affect (Steinberg, 2005), the current study focused on adolescents’ appraisal of how peers view them in an evaluative context. Because functional magnetic resonance imaging (fMRI) does not provide an index of absolute neural activity, but rather a relative index of activity in one condition compared with another, fMRI analyses typically focus on specific, well-controlled contrasts that isolate a condition of interest. In this study, the main contrast was controlled on a variety of perceptual and cognitive demands, allowing comparison of activation to social stimuli that differed according to subject-specific parameters, as a function of participants’ self-generated ratings of social appeal. This contrast involved comparing neural responses to appraising how “peers” would evaluate them. The specific contrast involved appraisals of evaluations from peers participants rated as appealing (high in interest) versus those rated as unappealing (low in interest) for a chatroom interaction.
Accordingly, the current study tests the hypothesis that increasing age in adolescent females, more so than in adolescent males, predicts enhanced neural engagement within certain regions of the affective node along this dimension. This female-specific age-related enhanced neural engagement is expected when viewing high relative to low-desirable peers.
Method
Participants
Participants were 34 adolescents (16 females; majority were of Caucasian descent) ranging from 8.9 to 17.5 years of age (M = 13.60, SD = 2.4), recruited from the community with advertisements, and financially compensated for participation. All participants were deemed physically and psychiatrically healthy following a physical exam and semi-structured psychiatric interview using the Schedule for Affective Disorders for School-Aged Children–Present and Lifetime version (K–SADS–PL; J. Kaufman et al., 1997). A series of t tests confirmed no differences between males and females in age, full-scale intelligent quotient scores (Wechsler, 1999), parent education and annual income, and pubertal stage (Tanner, 1962; see Table 1).
Table 1.
Mean (Standard Deviations) for Sample Demographic Characteristics
| Age (years) | IQ | Parent education | Parent income | Tanner stage | |
|---|---|---|---|---|---|
| Male (n = 18) | 13.55 (2.60) | 117.55 (8.67) | 2.67 (0.59) | 4.31 (1.45) | 3.17 (1.15) |
| Female (n = 16) | 13.66 (2.18) | 115.44 (10.46) | 2.87 (1.06) | 4.60 (1.18) | 3.33 (1.29) |
| Total (N = 34) | 13.60 (2.38) | 116.56 (9.47) | 2.76 (0.83) | 4.45 (1.31) | 3.24 (1.20) |
Note. Parent education ranged from 1 (high school graduate) to 4 (graduate training). Parent annual income ranged from 1 ($15,000–24,999) to 6 (> $180,000).
Procedure
The institutional review board at the National Institute of Mental Health (NIMH) approved study procedures. All participants provided written assent and parents/legal guardians provided written informed consent for participation. Participants and their parents were informed during the consent process that they would receive misinformation at some point during the course of their testing; all participants were debriefed extensively at the conclusion of the study. No adverse reactions occurred.
Participants engaged in the “chatroom task,” designed to simulate adolescent social interactions across two phases. In Phase 1, participants were led to believe they were participating in a nationwide investigation of teenagers’ Internet-based communication through chatrooms. They were told that after an fMRI scan, they would chat online with another teenager from a collaborating institution. Participants then viewed on a laptop 40 photographs of peers (20 males) allegedly participating in the study and rated, on a 100-point scale from 0 = not interested at all to 100 = very interested, their interest in interacting with each peer (Figure 1a). Participants were also photographed and told that the “participants” they had rated would similarly evaluate their pictures and view the ratings they had received. Finally, they were told that they would later chat with a mutually high-interest participant, based on their ratings, interests, and hobbies. This deceptive approach was intended to increase task salience and followed Wendler’s (1996) recommendations for ethically permissible research using deception.
Figure 1.
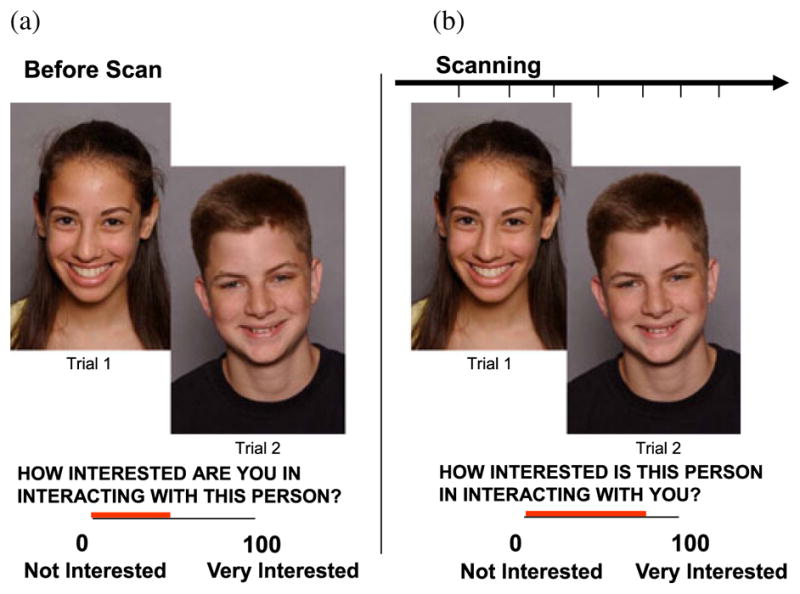
The chatroom paradigm required two visits to the laboratory. (a) During the first visit, approximately 2 weeks before functional magnetic resonance imaging (fMRI) scanning, participants viewed photographs of peers and rated how interested they were in chatting online with each peer. A median split of each participant’s ratings divided stimulus photographs into “peers of high interest” and “peers of low interest” conditions. Participants were also photographed, told that the same peers would rate their photos in a similar fashion, and informed that they would later learn how they had been rated. (b) During the second visit, while in the scanner, participants reviewed the photographs they had judged previously and rated how interested they expected each peer to be in chatting online with them (“appraisal” ratings).
The second phase occurred 2 weeks later, when participants underwent neuroimaging. In this phase, participants were scanned while reviewing the photographs they had rated 2 weeks previously. As they viewed each photograph, participants were asked to indicate how interested they thought each depicted peer would be in interacting with them (Figure 1b). This cognitive task involves appraisals about a pending social evaluation from peers for whom the participants themselves had made a prior social evaluation, designed to engage concerns about social evaluation and adolescents’ views of themselves. Using a handheld device inside the scanner, participants rated on a 100-point scale the degree to which the participant believed the depicted individual in each photograph would be in them (0 = not interested at all to 100 = very interested). Participants were debriefed postscan and told that no social evaluations were actually performed and no real interactions would occur. No adverse responses to deception were observed. E-Prime software (Psychological Software Tools, Pittsburgh, PA) presented the stimuli and recorded participants’ responses. Fifty participants were recruited; 13 were excluded for head motion greater than 3 mm and/or because they were not successfully deceived (i.e., did not believe they would actually interact with another participant). Three participants were excluded due to technical problems with the response box during scanning.
Measures
The chatroom task used a rapid, event-related design presented in a 7-min run. The appraisal task consisted of 40 face trials and 8 fixation trials. The face trials varied from 7.6 to 9.6 s in duration and consisted of two components: 3–5 s during which the face was presented without the rating screen and 4.6 s during which participants made their ratings. Stimulus presentation was random. Task stimuli were from the teen face emotion data set developed within our laboratory and included 40 digital headshots of 11- to 17-year-old actors (20 male) of varied ethnicities posing happy expressions with direct gaze under the direction of an acting coach (Nelson, 2004). Experienced face-processing researchers (E.B.M-T., E.E.N., and D.S.P.) selected from a set of 10 the picture for each actor that most overtly depicted happiness. Attractiveness of the actors was not controlled to maintain a stimulus set that reflected typical peers encountered by adolescents. Fixation crosses were displayed (4 s) randomly throughout the task to serve as a baseline. Interstimulus interval was 1 s.
fMRI Data Acquisition and Preprocessing
Scanning occurred in a General Electric (Waukesha, WI) Signa 3 Tesla magnet. Task stimuli were projected onto a screen at the foot of the scanner bed and viewed with a head coil-mounted mirror. Head movement was constrained by foam padding. Participants rated task stimuli using a handheld, two-button response box (Research Services Branch, NIMH, Bethesda, MD).
Functional scans were preceded by a localizer and a manual shim procedure. For functional image acquisition, each brain volume contained 29 contiguous 3.3 mm axial slices acquired parallel to the AC–PC line using a single shot gradient echo with T2* weighting with the following parameters: repetition time (TR) of 2,300 ms, echo time (TE) of 23 ms, voxel dimension of 3.3 × 3.75 × 3.75 mm, matrix size of 64 × 64, and field of view (FOV) of 24 cm. A high-resolution anatomical image was also acquired using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence to aid with spatial normalization using the following parameters: 124 1 mm axial slices, TR of 8,100 ms, TE of 32 ms, flip angle of 15°, number of excitations (NEX) = 1, matrix size of 256 × 256, bandwidth = 31.2 kHz, and FOV of 24 cm.
Data Analysis
Behavioral rating data collected before and during the scan were analyzed using SPSS 14.0 (Chicago, IL). fMRI data were preprocessed and analyzed using Analysis of Functional and Neural Images (AFNI) software version 2.56b (Cox, 1996). Standard preprocessing of echo-planar imaging data included slice time correction, reslicing to 1 mm isotropic voxels to place data in standard space, motion correction, spatial smoothing with a 6 mm full-width half-maximum Gaussian smoothing kernel, removal of signal deviations > 2.5 SD from the mean using an AFNI despiking algorithm applied on a voxel-wise basis, a bandpass filtering algorithm to remove cyclical fluctuations in signal (either > 0.011 or < 0.15 Hz) not temporally indicative of a hemodynamic response, and normalization of blood oxygen level-dependent (BOLD) signal intensity to percentage signal change using each subject’s voxel-wise time series mean as a baseline. Because total brain volume does not appear to change significantly after 9 years of age (Burgund et al., 2002; Wilke, Schmithorst, & Holland, 2002), preprocessing occurred using standard templates provided in AFNI that are normalized for adult brains. Movement artifact was mitigated by using motion correction parameters in the statistical model as nuisance covariates along with a covariate for mean intensity and linear drift. As noted above, 10 participants who moved more than 3 mm in any plane were excluded.
The statistical model was a gamma variate basis function convolved with the hemodynamic response function contained in AFNI. The basis function was set to the onset of each event type based on both the picture presentation and rating. These two subevents were modeled as separate events at the single-subject level but then combined into a single event to generate group-level contrasts. Because the same rating was always performed on every picture, the participant likely begins assessing the picture before actually rating it; thus, from a psychological perspective, picture presentation and rating are not distinguishable events. Event types consisted of two appraisal conditions. Appraisal events occurred when participants evaluated how peers would perceive them (Figure 1b), and were binned according to (a) peers of high interest and (b) peers of low interest to the participant for a chat session. To maximize statistical power in relevant analyses, these two “interest-in-peer” conditions were determined using a median split of each participant’s prescan interest ratings (Figure 1a). A general linear model was then used to determine the beta-value and t statistic for each event type at each voxel (Neter, Kutner, Machtsheim, & Wasserman, 1996). Contrasts of whole-brain BOLD activation were created for each individual for each event type. This was followed by a second group-level, random-effects analysis of individual contrast values using the AFNI 3dRegAna procedure. A regression analysis was included in the group-level analysis to assess the main effects of the contrasted event (peers of high interest vs. peers of low interest), as well as age, sex, and Age × Sex interaction effects. Coordinates were placed in Talairach space.
Results from the regression analysis were interpreted using a small volume correction (SVC) of p < .05 to protect against multiple comparisons for the five a priori regions of interest (ROIs) within the affective node (Forman et al., 1995; Poldrack, 2007). Given that we entered the study with regionally based a priori hypotheses, we restricted our analysis to these ROIs, thereby minimizing Type I errors. To conduct our correction, the standard approach in AFNI of running Monte Carlo simulations was used with the AlphaSim procedure, applied to anatomically defined ROIs based on Talairach Daemon software provided in AFNI. The ROIs included both left and right anatomy. The Monte Carlo simulations generated the combined probability of spatial extent and threshold required to surpass a corrected p < .05 within each ROI. AlphaSim parameters included connectivity radius of 4, threshold of 3.63 (based on the specified maxima threshold uncorrected at p < .001), and the anatomical masks provided by AFNI for each ROI (insula, nucleus accumbens, hippocampus, hypothalamus/thalamus, and amygdala). All supratheshold clusters were masked within each ROI so voxels that were part of a cluster but extended beyond the borders of the ROI were not included in calculation of spatial extent. Activation values in the ROIs that survived the SVC, as per our hypotheses, were extracted from the functionally defined ROIs identified in the whole-brain analysis and used to generate average contrast values for each participant. It is important to note that extracted estimates from the ROIs are taken from nonindependent voxels, which can lead to biases in additional statistical analyses. Thus, the extracted data are used solely for illustrative purposes and associated r values are used to depict the direction of any significant interaction effects from the initial analysis as a way to decompose the interaction.
Additionally, to explore age- and- sex-related variation in behavioral responses, a median split of age was used to create an age group variable (e.g., younger and older adolescents), thus facilitating analyses of between-group differences.
Results
Prescan Ratings of Peers
Initial analyses examined reliability of the peer-interest ratings. Cronbach’s alpha was calculated on the 40 stimuli for the 34 participants. Participants were highly consistent in their ratings across the stimulus set (alpha = .97); alpha estimates ranging from .93 to .99 were found for ratings given to male and female peers separately, and within younger/older male/female participant groups, as well as within males and females, and younger and older participants. No significant differences were found in mean-interest ratings given to the 20 male (M = 44, SD = 4) and 20 female (M = 43, SD = 6) stimuli, t(38) = −0.79, p = .43. As a whole, the variability and patterns of ratings from participant-to-participant did not differ dramatically. Finally, the number of occurrences that a given photo was rated low or high across all participants indicated that no single picture or set of pictures was consistently given high- or low-interest ratings across participants. Participants (40%–60%) selected most photos as high interest, indicating that about half the time a given photo was a high interest and half the time it was a low-interest stimulus.
A 2 × 2 (Age Group × Participant Sex) analysis of variance (ANOVA) assessed participants’ self-reported interest in peers based on prescan ratings. Age group, participant sex, and the Age Group × Participant Sex interaction were not significant. Thus, similar levels of interest in peers were found between female (M = 44, SE = 4) and male (M = 42, SE = 4) participants, as well as between younger (M = 44, SE = 4) and older (M = 42, SE = 4) participants.
A 2 × 2 × 2 (Age Group × Participant Sex × Peer Sex) repeated measures ANOVA compared the proportion of same- and opposite-sex peers in which participants indicated having high interest for a chatroom interaction, with age group and participant sex serving as between-group factors and peer sex (same and opposite) as a within-group factor. The main effect of peer sex, F(1, 30) = 45.36, p < .001, and the Age × Peer Sex interaction, F(1, 30) = 11.64, p < .005, were significant. Specifically, among both younger and older adolescents, high-interest ratings were given to more same- than opposite-sex peers. However, the proportion of same-sex peers selected was higher among younger than among older adolescents (younger: 76% vs. 24%; p < .001, and older: 59% vs. 41%; p < .01). Effects of participant sex were not significant.
Behavioral Ratings Made During Scanning
A 2 × 2 × 2 (Age Group × Participant Sex × Interest in Peer) repeated measures ANOVA assessed participants’ in-scanner appraisal ratings when considering how peers would perceive them, with age group and participant sex as between-group factors and self-reported interest in peer (high and low) as a within-group factor. As expected, a significant main effect of “interest ratings,” made at initial evaluation, emerged as a moderator of participants’ in-scanner appraisals (made 2 weeks later) of how peers would be expected to perceive the participant, F(1, 30) = 15.27, p < .001. Specifically, participants reported during scanning that they expected peers of high interest to be more interested in interacting with them (M = 59, SE = 3) than peers of low interest (M = 47, SE = 3). Interaction effects between age group, participant sex, and interest in peer on appraisal ratings were not significant. Finally, there was also a positive significant correlation between peer-interest ratings and appraisal ratings (r34 = .82, p < .001), suggesting that initial ratings captured stable aspects of participants’ reactions to peers while scanning.
Neural Activations During Appraisal of Evaluation
The multiple regression analysis conducted on the contrast of peers of high interest versus peers of low interest included a main effect of condition (high vs. low interest) and effects for age (treated continuously), participant sex, and an Age × Sex interaction. Relatively few suprathreshold clusters were found that encompassed the entire sample for the main effect of condition. No suprathreshold clusters were found as a function of participant sex alone. A number of clusters, however, varied as a function of age, and many of these were significantly associated with the Age × Sex interaction term, indicating differential age effects for each sex. Negative t values from the Age × Sex interaction effect indicated a greater age-related increase in activation in females relative to males, whereas positive values indicated greater change in males; no significant positive associations were found. Importantly, significant Age × Sex interaction effects were found for our a priori ROIs falling within key components of the SIPN affective node, including the nucleus accumbens, hypothalamus, hippocampus, and insula. For these regions, the Age × Sex interaction effect survived the SVC of p < .05 (Figures 2–5). All of these regions are involved in social-affective processing (social motivation–approach behavior, affective engagement, emotional memory, and subjective feelings), and, as hypothesized, all showed a greater age-related increase in activation in females than males when appraising peers of high interest relative to those of low interest.
Figure 2.
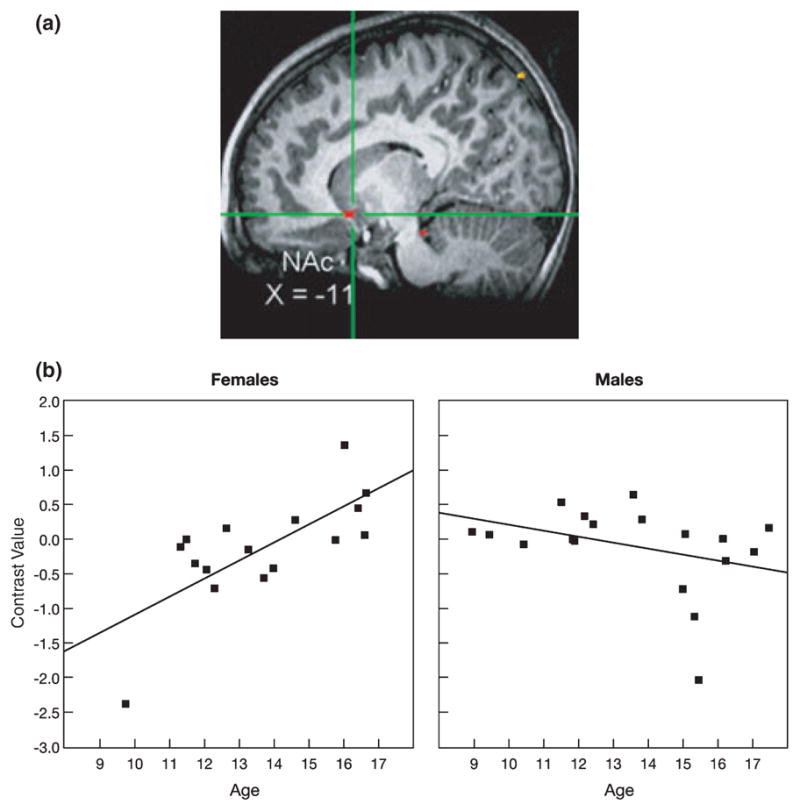
A significant Age × Sex interaction effect was found for activation of the nucleus accumbens (NAc) while participants appraised how they thought peers would evaluate them, specifically, while viewing peers of high interest versus peers of low interest. (a) Cross-hairs centered on the maximum intensity value (Talairach coordinates: x = −11, y = 11, z = −8), t(30) = −3.91, p < .001, for the cluster in the left NAc. (b) As age increased, NAc activation increased in females but did not change in males. Data were extracted at functional magnetic resonance imaging (fMRI) acquisition during appraisal ratings. Each participant’s data were converted to percentage signal change values using each participant’s voxel-wise time series mean as a baseline and averaged within the region.
Figure 5.
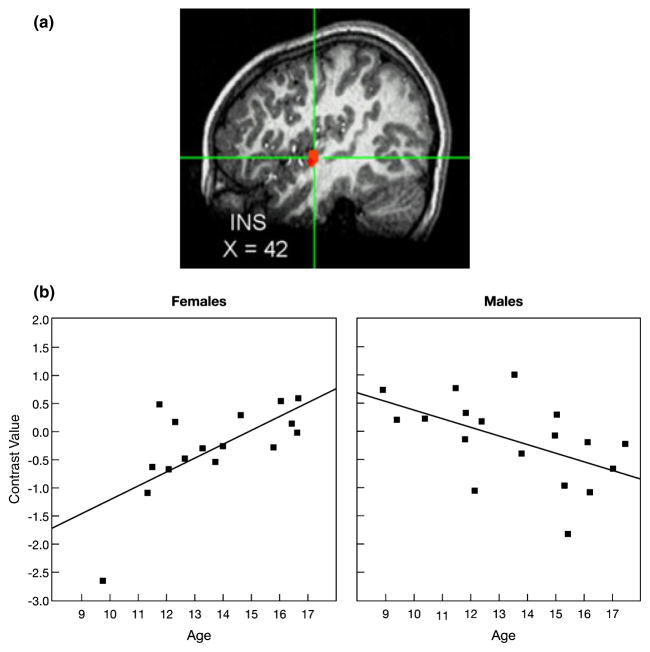
A significant Age × Sex interaction effect was found for activation of the insula (INS) while participants appraised how they thought peers would evaluate them, specifically, while viewing peers of high interest versus peers of low interest. (a) Cross-hairs centered on the maximum intensity value (Talairach coordinates: x = 42, y = −7, z = 1), t(30) = −4.13, p < .001, for the cluster the right INS. (b) As age increased, INS activation increased in females but decreased in males. Data were extracted at functional magnetic resonance imaging (fMRI) acquisition during appraisal ratings. Each participant’s data were converted to percentage signal change values using each participant’s voxel-wise time series mean as a baseline and averaged within the region.
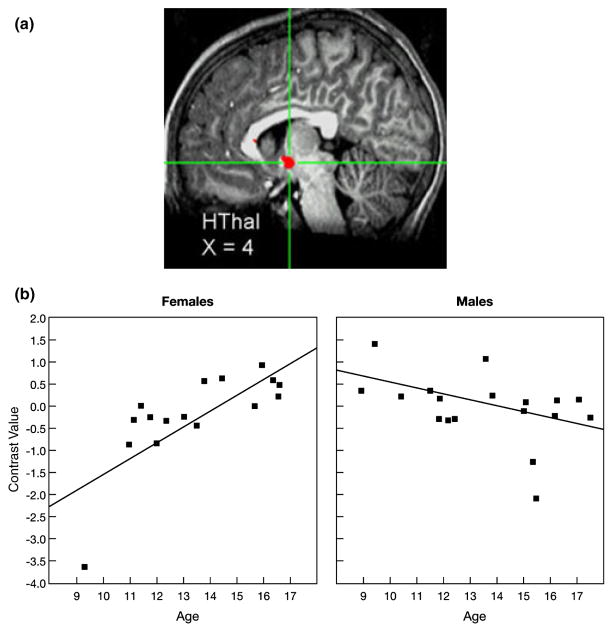
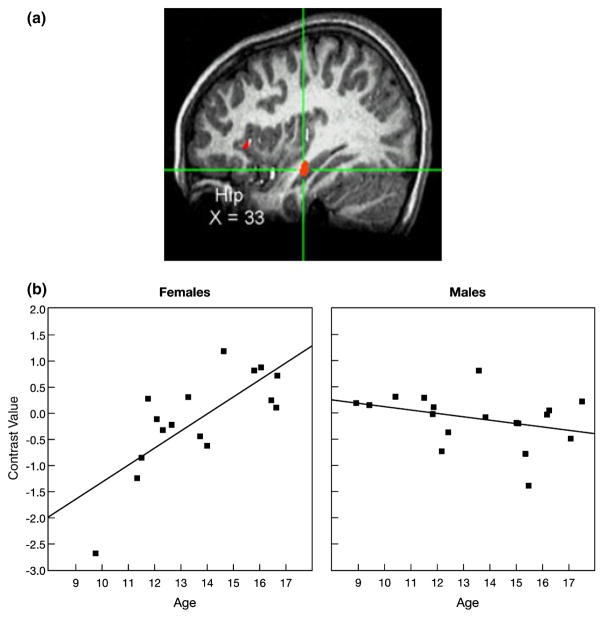
Figures 2a and 2b highlight the significant age-by-sex interaction effect on nucleus accumbens activation while participants appraised how peers of high versus low interest would evaluate them in return. The topography of the suprathreshold cluster is depicted in Figure 2a, and the post hoc scatter plot is depicted by sex in Figure 2b. As shown in Figure 2b, greater nucleus accumbens activation was found as age increased among adolescent females (r = .72) but not adolescent males (r = −.35). A similar pattern was found in the hypothalamus (Figures 3a and 3b) and hippocampus (Figures 4a and 4b), with increased activation of each region as age increased among females (hypothalamus: r = .74 and hippocampus: r = .75), but not males (hypothalamus: r = −.44 and hippocampus: r = −.33). A slightly different pattern emerged in the insula (Figures 5a and 5b). Again, as age increased among female adolescents, insula activation increased (r = .68); however, in contrast to females, insula activation decreased in males as age increased (r = −.53). Of note, the age-related change in females was not simply a change from baseline in older females relative to all other participants. Rather, the pattern shows a continuous shift from negative to positive activation in females across age. These scatter plots suggest both that younger females have greater activation within these regions when appraising low-rated versus high-rated peers and that older females have increased activation when appraising high-rated versus low-rated peers.
Figure 3.
A significant Age × Sex interaction effect was found for activation of the hypothalamus (HThal) while participants appraised how they thought peers would evaluate them, specifically, while viewing peers of high interest versus peers of low interest. (a) Cross-hairs centered on the maximum intensity value (Talairach coordinates: x = 4, y = −4, z = −5), t(30) = −5.08, p < .001, for the cluster in the left HThal. (b) As age increased, HThal activation increased in females but did not change in males. Data were extracted at functional magnetic resonance imaging (fMRI) acquisition during appraisal ratings. Each participant’s data were converted to percentage signal change values using each participant’s voxel-wise time series mean as a baseline and averaged within the region.
Figure 4.
A significant Age × Sex interaction effect was found for activation of the hippocampus (Hip) while participants appraised how they thought peers would evaluate them, specifically, while viewing peers of high interest versus peers of low interest. (a) Cross-hairs centered on the maximum intensity value (Talairach coordinates: x = 33, y = −16, z = −11), t(30) = −4.65, p < .001, for the cluster in the right Hip. (b) As age increased, hip activation increased in females but did not change in males. Data were extracted at functional magnetic resonance imaging (fMRI) acquisition during appraisal ratings. Each participant’s data were converted to percentage signal change values using each participant’s voxel-wise time series mean as a baseline and averaged within the region.
Discussion
This study probed patterns of brain activation in a group of psychiatrically healthy adolescents while they assessed how they expected individual peers to view them. The primary contrast compared brain activity while participants performed this assessment on peers in whom the participant initially expressed high interest versus those in whom the participant expressed low interest. Results of this study provide initial empirical support for hypothesized age- and sex-related changes in social information processing and brain–behavior relations (Nelson et al., 2005). Perceptions of potential social evaluation by high- versus low-interest peers induced activation within specific regions of the adolescent brain previously linked to social reward and motivation, visceral emotional response, hormonal interface, and social memory. As hypothesized, engagement of these neural regions varied as a function of age and sex.
Because fMRI is a relative rather than absolute measure, it is not possible to specify at an absolute level that an area is engaged or not engaged, only that it is more or less engaged in one versus another condition. In particular, compared with males, females displayed greater age-related increases in activation in the nucleus accumbens, hypothalamus, hippocampus, and insula, among other regions. For the nucleus accumbens, hypothalamus, and hippocampus, this interaction reflected an age-related increase in activation in females and little or no age-related change in males, whereas for the insula, the interaction was the result of a combined developmental increase in activation in females and a relative decrease in males. In females, the contrast of high- versus low-interest peers resulted in a change from negative to positive activation values. This pattern suggests a developmental shift from greater activation of the affective node by low-interest peers in young females to a greater activation of these same structures by high-interest peers in older females, possibly suggesting a change in socioemotional calculus from avoidance to approach. Considerable neuroscience research delineates the mechanisms through which each of these brain regions contributes to various complex, specific affective-cognitive processes. This understanding provides novel perspectives when evaluating components of adolescent social-evaluative mental processes as examined here.
The nucleus accumbens and associated ventral striatum have long been implicated in the processing of rewards (Schultz, 2006), including both primary, basic biological rewards, such as food and water, and more abstract rewards such as economic gains (Guyer et al., 2006) and social rewards such as cooperation (Aharon et al., 2001; Aron et al., 2005; Depue & Morrone-Strupinsky, 2005; Kampe, Frith, Dolan, & Frith, 2001; Rilling et al., 2002). Thus, the age-related increase in nucleus accumbens activity among female adolescents suggests that females’ reward-related circuitry may become increasingly polarized affectively with age during anticipation of how preferred versus non-preferred peers will feel about them. In male adolescents, however, little association with age was observed. Although the available literature reveals the limited work that compares males and females in their responses to social interactions of different emotional valences, behavioral and fMRI evidence indicates that adult females find positive social interactions rewarding and negative interactions aversive (Rilling et al., 2002); behavioral and non-fMRI physiological findings suggest that these responses may be stronger in females than in males (Feingold, 1994; Rose & Rudolph, 2006; Stroud et al., 2002). The present findings indicate that the reward-related, sex-specific activity in response to mutually positive social interactions may mature during adolescence.
The insula is implicated in a number of affect-related processes; notably, recent conceptualizations have suggested that it may play a key role in integrating visceral sensation and autonomic responses with cognitive appraisal responses to emotional and social stimuli (Craig, 2004; Insel & Fernald, 2004). In addition to receiving input from a variety of brain structures typically involved in affect, the insular cortex has some projections to the hypothalamus and other brain regions involved in somatic sensations and may be implicated in integrating affect with the bodily responses that accompany emotional experience (Davidson & Irwin, 1999). Thus, like the nucleus accumbens, the Age × Sex interaction in the insula is consistent with work that suggests engagement of neural structures involved in affective responses in dynamic social environments among females (Rilling et al., 2002).
Among males, findings of an age-related decrease in insula activation may suggest a reduced level of affective engagement, and particularly for somatic-related emotional responses in males through adolescence. It has been argued that the focus of adolescent male social relations shifts from the individual to the group in ways that are less common among females (Rose & Rudolph, 2006). Adolescent males increasingly value being part of a larger group and become more focused on status or competitive goals within a social group (e.g., members of a sports team with the goal of winning a game; Rose & Rudolph, 2006). Thus, these group-level processes may predominate, rendering differences between responses to high- and low-interest peers less pronounced among males. The reduced insula activity may reflect this process. In contrast, adolescent girls tend to place more emphasis on interpersonal engagement within dyadic friendships, with goals focused on making social connections rather than achieving competitive goals (Rose & Rudolph, 2006). As such, it is possible that the female focus on interpersonal engagement leads to heightened sensitivity about peer evaluation and the status of relationships, a hypothesis consistent with the patterns of neural activations found in this study, particularly with age among female adolescents.
As in the nucleus accumbens and insula, age-related changes in hypothalamus and hippocampus activation also likely reflect greater salience of high-versus low-interest peers in females. The hypothalamus plays a key role in the neuronal–endocrine interface, which may indicate a direct relation between hormonal shifts related to puberty and the sex differences in neural networks observed in this study. In addition to its role in sexual maturation of both brain and body, cell bodies from several neuropeptides linked to affiliative processes, such as oxytocin and endogenous opioids, can be found within the hypothalamus (Panksepp, 1998). Further, the hypothalamus plays a key role in regulating peripheral cortisol levels, which relates in part to fear and perceived stress level (Korte, 2001). Thus, as in the case of differential insula activation, increased hypothalamic engagement may indicate that during the appraisal of evaluation from socially desirable relative to undesirable peers, greater activity occurs in females in a neural network relating to widely distributed physiological systems throughout the body.
The hippocampus, although implicated in some emotional processes, is more closely associated with memory and related cognitive processes, such as spatial representation and context appraisal (Anderson, Moris, Amaral, Bliss, & O’Keefe, 2007). Thus, the relatively greater hippocampus activation during appraisal of high- versus low-interest peers among older females raises questions about sex differences in specific cognitive processes engaged during peer appraisal that might be examined in future behavioral studies. For example, the current data suggest that social evaluation in females relative to males may be associated with a stronger recall for, or greater effort to recall, the photograph being rated or perhaps the rating that the participant assigned to that photograph (Somerville, Wig, Whalen, & Kelley, 2006). Alternatively, the greater age-related hippocampal engagement in females may suggest that maturation in females involves an increasingly important role of context evaluations when making social-evaluative judgments. Therefore, the observed pattern of age- and sex-related changes in neuronal activity generates hypotheses for future behavioral studies on precise sex-specific social-cognitive processes that differentiate increasingly with age.
Beyond the patterns documented in this study, of interest is the absence of amygdala engagement during appraisal. One might have expected to see amygdala activation indicative of anxiety about pending peer evaluation (Nelson et al., 2005). Concerning the lack of amygdala response, the two events contributing to the specific contrast examined here may not have differed sufficiently in the particular psychological processes instantiated in this region within psychiatrically healthy adolescents. In contrast, using the same task, we have documented greater amygdala activation in response to low- versus high-interest peers in socially anxious adolescents relative to healthy controls (Guyer, Lau, et al., 2008). Differential activation in the amygdala appears to emerge among adolescents who have an extreme fear of potential social evaluation.
The current study found significant Age × Sex interactions for neural patterns but not for behavior. Specifically, as in the fMRI data, one might have expected females and males of increasing age to also show sex differences in their appraisal ratings for peers of high relative to low interest. Particularly for emotional events, the detection of hypothesized group differences in fMRI data, in the absence of hypothesized behavioral findings, represents a common observation (McClure et al., 2007; Wilkinson & Halligan, 2004). A few factors might account for the frequently observed greater sensitivity to detect between-group differences in fMRI relative to behavioral data. For example, the behavioral results were based on a single question and the sample was selected for psychological health, both of which may have obscured potential behavioral differences; prior work on sex-related differences in social-cognition rely on multiple questions in samples with varying levels of psychological health (La Greca & Lopez, 1998). The lack of sex-related differences in rating data, however, can be advantageous when interpreting fMRI data; in the absence of rating differences, observed age- and sex-related neural patterns cannot be attributed to an artifact of task performance differences during scanning.
Although the current study focused on psychiatrically healthy adolescents, this study may contribute to our understanding of adolescent sex differences in the emergence of psychopathology, particularly, the marked increase in clinically significant anxiety and depression among adolescent females (Zahn-Waxler et al., 2008). Behavioral and physiological evidence indicates that adult females find positive social interactions more rewarding and negative interactions more aversive than do males (Feingold, 1994; Rilling et al., 2002; Rose & Rudolph, 2006; Stroud et al., 2002) and that heightened sensitivity to interpersonal stress or high levels of social-evaluative concerns may contribute to internalizing problems among females (Cyranowski et al., 2000; Hankin & Abramson, 2001; Rudolph & Conley, 2005; Shih et al., 2006). In light of this evidence, the present findings suggest that sex-specific neural changes that first manifest during adolescence may increase females’ vulnerability to depression or anxiety. As mentioned above, however, this study did not find age- and sex-related variations in the activation of brain regions most commonly associated with adolescent mood and anxiety disorders such as the amygdala or pre-frontal cortices (Guyer, Lau, et al., 2008; McClure et al., 2007; Monk et al., 2006, 2008). This may reflect the focus on a psychiatrically healthy sample in this study, combined with a relatively unthreatening social-emotional process. The absence of significant activation in key regions implicated in adolescent mood and anxiety disorders among a group of psychiatrically healthy adolescent females may be an important indicator of resilience to psychosocial stress during this vulnerable period. Specifically, these findings may indicate that emotional responses to high- versus low-interest peers may be driven more by a brain network related to approach (e.g., nucleus accumbens) than to one related to fear and withdrawal (e.g., amygdala) among healthy female adolescents.
This study has some limitations, some of which may be addressed by future research. First, the complexity of social interaction makes it virtually impossible to maintain the integrity of a real-life social interchange while simultaneously isolating the cognitive and affective components that the interchange comprises, which introduces several potential interpretations of the current results. For example, it is currently unclear why some participants rated the depicted peers as socially desirable or undesirable. Neural response to the high-interest peers may reflect other processes, for example, attractiveness of peer. However, we documented a strong relation between participants’ initial ratings of each peer’s desirability and later ratings of appraisals of peer evaluation; this provides some evidence that participants’ initial impressions relate to a lasting aspect of the social-evaluative processes that can be engaged 2 weeks later. Further work is needed to delineate the precise feature of high-interest peers that elicits heightened activation in regions of the affective node with increased age during adolescence.
Second, task sensitivity to different cognitive or affective processes may also have been reduced because our key event incorporated two subcomponents rather than examining each component separately and additional “jitter” time was not interspersed between subcomponents. This limitation may have been offset by the advantages gained in task feasibility and maintained in psychological fidelity, particularly given confirmation of expected findings. Nonetheless, future studies should attempt to decompose subcomponents of such complex cognitive processes.
Third, although 34 participants is a relatively large sample for an fMRI study, the number of regressors included in our model could limit statistical power. This limitation is further confounded by our focus on interactions, which contrasted age-related brain activation patterns in two relatively smaller samples (n = 17) of males and females. Because results derived from small samples are associated more commonly with Type II rather than Type I error, the potential for masking true effects increases; however, observation of expected, significant findings reduces this possibility. Despite limitations in statistical power associated with small samples, greater caution is needed when interpreting negative rather than positive findings. Indeed, null results in fMRI studies can also be explained by nonoptimized image acquisition from specific brain regions and regional variations in the time course of BOLD response. In addition, we used ROIs for exploration as a way to depict patterns of signal change across conditions (Poldrack, 2007). We extracted contrast values from functionally defined ROIs and plotted the patterns for illustrative purposes. It is important to note that the r values computed on extracted data stem from nonindependent ROI defined by the contrast of interest, which introduces bias in the estimates and limits statistical conclusions. Future studies, based in larger, independent samples are therefore needed.
Finally, the deception and debriefing aspects of the task limit the research design to a cross-sectional versus longitudinal type, despite the ability of the latter to provide a deeper understanding of neurodevelopmental changes associated with adolescent social behavior and social-cognitive processes within an individual. On a related note, our results cannot speak to whether these age- and sex-related changes persist into adulthood because we did not include an adult control group. Adults were not included because we designed the task paradigm to engage emotions and cognitions experienced by a typical adolescent in their daily social lives. As such, the psychological implications of viewing the same stimuli (peers) would be quite different for an adolescent as opposed to an adult participant. While the task could incorporate both adolescent and adult photographs, this could significantly lengthen the task and possibly hinder young participants’ engagement in the task during scanning.
This study also has several strengths. First, the task paradigm is unusual in that it simulates social interactions and judgments that adolescents engage in routinely, and taps psychological processes central to typical adolescents’ heightened focus on social evaluation and approval, particularly among females (La Greca & Lopez, 1998; La Greca & Stone, 1993; Rudolph & Conley, 2005; Storch et al., 2002). Second, the results from this study provide an example of how merging neuroscience with traditional behaviorally focused approaches can yield valuable information for understanding development on multiple levels. Traditional developmental studies that documented the importance of peer social interactions to adolescent cognition and emotion (Steinberg & Morris, 2001) guided the age- and sex-related analyses employed here, while neuroscience research provided a framework for exploring and identifying activation within specific brain networks. The present results offer both support and constraints for existing theoretical approaches to adolescent development. For example, along with the numerous theoretical models that incorporate changes in psychosocial stressors to explain the rise in mood and anxiety disorders during adolescence (Cyranowski et al., 2000; Hankin & Abramson, 2001; Nolen-Hoeksema & Girgus, 1994), attention must be now be paid to accompanying mechanistic changes in brain systems related to affective and cognitive processing of social stimuli (Nelson et al., 2005). This underscores the importance of examining the role of the social-cognitive processes instantiated in these subcortical structures in the study of adolescent changes in emotional reactivity.
Finally, the current results generate additional questions to be targeted in both future neuroimaging and behavioral studies. For example, interpretations of the functional significance of activations presented here are necessarily speculative. Individual brain regions can participate in social-cognition in a variety of ways and different experiences or behaviors may elicit activation in the same regions for different reasons. Evaluating peers and anticipating peer responses to one’s self are complex activities that likely involve multiple, dynamically interacting, component subprocesses. Isolating these social-cognitive subprocesses and clarifying how they and their neural correlates change across development may elucidate the results presented here, as well as inform larger issues of adolescent development. Given the complex nature of the processes engaged here, future behavioral experiments might attempt to better dissociate the affective and nonaffective components of the social-cognitive process engaged in the current study. For example, future work could include two conditions that require participants to focus on emotional aspects (e.g., how interested are they in you?) and nonemotional, neutral aspects (e.g., how wide is their nose?) while rating depicted peers, as well as assess social perspective taking. Thus, the present fMRI findings can inform understanding of both behavioral and neural social-cognitive processes in a mutually reinforcing fashion.
In sum, we believe our use of the chatroom task exemplifies the interplay of analytic strategies between behavior- and brain-level approaches, representing an important integration of two fields. This is among the first studies to attempt to map neural processing engaged in ecologically valid social interactions among adolescents, using a novel paradigm to simulate social evaluation in which adolescents participate routinely. We hope this type of translational approach becomes more common in future research on adolescent development.
Acknowledgments
This research study was supported by the National Institute of Mental Health (NIMH) intramural research program and an NIMH career development grant to A.E.G. (K99 MH080076). We greatly acknowledge Harvey A. Iwamoto for task development and programming; Stephen J. Fromm, Richard Reynolds, and Gang Chen for statistical consultation on neuroimaging analysis; and Monique Ernst, Kenneth A. Towbin, Alan Zametkin, and Jennifer Cameron for medical oversight. We thank the families who participated.
Contributor Information
Amanda E. Guyer, National Institute of Mental Health
Erin B. McClure-Tone, Georgia State University
Nina D. Shiffrin, National Institute of Mental Health
Daniel S. Pine, National Institute of Mental Health
Eric E. Nelson, National Institute of Mental Health
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Anderson P, Moris R, Amaral D, Bliss T, O’Keefe J. Historical perspective: Proposed functions, biological characteristics, and neurobiological models of the hippocampus. In: Anderson P, Moris R, Amaral D, Bliss T, O’Keefe J, editors. The hippocampus book. New York: Oxford University Press; 2007. pp. 9–36. [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman LM, Gouley KK, Klein RG, Castellanos FX, Pine DS. Children, stress, and context: Integrating basic, clinical, and experimental prevention research. Child Development. 2003;74:1053–1057. doi: 10.1111/1467-8624.00589. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Burks VS, Dodge KA, Price JM. Models of internalizing outcomes of early rejection. Development and Psychopathology. 1995;7:683–695. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: Why are some more aware than others? Trends in Cognitive Science. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: A theoretical model. Archives of General Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuro-anatomy of emotion and affective style. Trends in Cognitive Science. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. Behavior and Brain Science. 2005;28:313–350. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Owen LD. A longitudinal analysis of friendships and substance use: Bidirectional influence from adolescence to adulthood. Developmental Psychology. 2002;38:480–491. doi: 10.1037//0012-1649.38.4.480. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Lansford JE, Burks VS, Bates JE, Pettit GS, Fontaine R, et al. Peer rejection and social information-processing factors in the development of aggressive behavior problems in children. Child Development. 2003;74:374–393. doi: 10.1111/1467-8624.7402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A. Gender differences in personality: A meta-analysis. Psychological Bulletin. 1994;116:429–456. doi: 10.1037/0033-2909.116.3.429. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gazelle H, Rudolph KD. Moving toward and away from the world: Social approach and avoidance trajectories in anxious solitary youth. Child Development. 2004;75:829–849. doi: 10.1111/j.1467-8624.2004.00709.x. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20:1–18. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA. Nonverbal sex differences: Communication accuracy and expressive style. Baltimore: Johns Hopkins University Press; 1984. [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Henker B, Whalen CK, O’Neil R. Worldly and workaday worries: Contemporary concerns of children and young adolescents. Journal of Abnormal Child Psychology. 1995;23:685–702. doi: 10.1007/BF01447472. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annual Review of Neuroscience. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K–SADS–PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman KL, Brown RT, Graves K, Henderson P, Revolinski M. What, me worry? A survey of adolescents’ concerns. Clinical Pediatrics. 1993;32:8–14. doi: 10.1177/000992289303200102. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12:427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Perceptual and Motor Skills. 2004;99:371–391. doi: 10.2466/pms.99.2.371-391. [DOI] [PubMed] [Google Scholar]
- Kistner J, Balthazor M, Risi S, Burton C. Predicting dysphoria in adolescence from actual and perceived peer acceptance in childhood. Journal of Clinical Child Psychology. 1999;28:94–104. doi: 10.1207/s15374424jccp2801_8. [DOI] [PubMed] [Google Scholar]
- Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neuroscience and Biobehavioral Review. 2001;25:117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Lopez N. Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology. 1998;26:83–94. doi: 10.1023/a:1022684520514. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Stone WL. Social Anxiety Scale for Children–Revised: Factor structure and concurrent validity. Journal of Clinical Child Psychology. 1993;22:17–27. [Google Scholar]
- Laird RD, Jordan KY, Dodge KA, Pettit GS, Bates JE. Peer rejection in childhood, involvement with antisocial peers in early adolescence, and the development of externalizing behavior problems. Development and Psychopathology. 2001;13:337–354. doi: 10.1017/s0954579401002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RW, Richards MH, Moneta G, Holmbeck G, Duckett E. Changes in adolescents’ daily interactions with their families from ages 10 to 18: Disengagement and transformation. Developmental Psychology. 1996;32:744–754. [Google Scholar]
- Maccoby EE. The two sexes: Growing up apart, coming together. Cambridge, MA: Harvard University Press; 1998. [Google Scholar]
- McClure EB. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychological Bulletin. 2000;126:424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biological Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Meesters C, Merckelbach H, Sermon A, Zwakhalen S. Worry in normal children. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:703–710. doi: 10.1097/00004583-199807000-00009. [DOI] [PubMed] [Google Scholar]
- Nelson EE. Adolescent facial expressions stimuli database. Section on development and affective neuroscience. Bethesda, MD: National Institute of Mental Health; 2004. [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Machtsheim CJ, Wasserman W. Applied linear statistical models. 4. Chicago: Irwin; 1996. [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: The foundations of human and animal emotions. Oxford, UK: Oxford University Press; 1998. [Google Scholar]
- Pine DS, Cohen P, Brook JS. Emotional reactivity and risk for psychopathology among adolescents. CNS Spectrum. 2001;6:27–35. doi: 10.1017/s1092852900022860. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MH, Crowe PA, Larson R, Swarr A. Developmental patterns and gender differences in the experience of peer companionship during adolescence. Child Development. 1998;69:154–163. [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: Potential tradeoffs for the emotional and behavioral development of girls and boys. Psychological Bulletin. 2006;132:98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH, Bukowski WM, Parker JG. Peer interactions, relationships, and groups. In: Eisenberg N, Damon W, Lerner RM, editors. Handbook of child psychology: Vol. 3. Emotional and personality development. 6. Hoboken, NJ: Wiley; 2006. pp. 571–652. [Google Scholar]
- Rudolph KD, Conley CS. The socioemotional costs and benefits of social-evaluative concerns: Do girls care too much? Journal of Personality. 2005;73:115–138. doi: 10.1111/j.1467-6494.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom MJ, Cillessen AH, Eisenhower A. Children’s appraisal of peer rejection experiences: Impact on social and emotional adjustment. Social Development. 2003;12:530–550. [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: Linking developmental psychology and functional neuroimaging. Annual Review of Psychology. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Shih JH, Eberhart NK, Hammen CL, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. Journal of Clinical Child and Adolescent Psychology. 2006;35:103–115. doi: 10.1207/s15374424jccp3501_9. [DOI] [PubMed] [Google Scholar]
- Silverman WK, La Greca AM, Wasserstein S. What do children worry about? Worries and their relation to anxiety. Child Development. 1995;66:671–686. doi: 10.1111/j.1467-8624.1995.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Wig GS, Whalen PJ, Kelley WM. Dissociable medial temporal lobe contributions to social memory. Journal of Cognitive Neuroscience. 2006;18:1253–1265. doi: 10.1162/jocn.2006.18.8.1253. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Pubertal maturation and parent-adolescent distance: An evolutionary perspective. In: Adams RMG, Gullota T, editors. Advances in adolescent behavior and development. Newbury Park, CA: Sage; 1989. pp. 71–97. [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Storch EA, Zelman E, Sweeney M, Danner G, Dove S. Overt and relational victimization and psychosocial adjustment in minority preadolescents. Child Study Journal. 2002;32:73–80. [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: Social rejection versus achievement stress. Biological Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence. Oxford, UK: Blackwell Scientific; 1962. [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, et al. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Urberg KA. Locus of peer influence: Social crowd and best friend. Journal of Youth and Adolescence. 1992;21:439–450. doi: 10.1007/BF01537896. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Crnic KA, Carter WG. Worry in childhood: A developmental perspective. Cognitive Therapy and Research. 1994;18:529–549. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Wendler D. Deception in medical and behavioral research: Is it ever acceptable? Milbank Quarterly. 1996;74:87–114. [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Human Brain Mapping. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nature Reviews Neuroscience. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- Wong MM, Csikszentmihalyi M. Affiliation motivation and daily experience: Some issues on gender differences. Journal of Personality and Social Psychology. 1991;60:154–164. [Google Scholar]
- Yurgelun-Todd DA, Killgore WD. Fear-related activity in the prefrontal cortex increases with age during adolescence: A preliminary fMRI study. Neuroscience Letters. 2006;406(3):194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: Gender and psychopathology. Annual Review Clinical Psychology. 2008;4:1–29. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]