SUMMARY
Cowpox virus is considered ancestral to orthopoxviridae since CPXV encodes the most extensive array of putative immunomodulators that likely contribute to its wide host range including zoonotic infections in humans. Unlike vaccinia virus, CPXV prevents stimulation of CD8+ T cells and this correlated with retention of MHC-I in the endoplasmic reticulum by CPXV203. However, deletion of CPXV203 did not restore MHC-I transport or T cell stimulation. Here, we demonstrate that the type II transmembrane protein, CPXV012, additionally interferes with MHC-I/peptide complex formation by inhibiting peptide translocation by TAP. CPXV012 thus represents the first non-herpesvial TAP inhibitor. Importantly, human and mouse MHC-I transport and T cell stimulation was restored upon deletion of both CPXV012 and CPXV203 suggesting that these unrelated proteins independently mediate T cell evasion in multiple hosts. Interestingly, CPXV012 is a truncated version of a putative NK cell ligand indicating that poxviral gene fragments can encode new unexpected functions.
INTRODUCTION
The eradication of Variola virus (VARV), the cause of smallpox, in 1977 left cowpox virus (CPXV) and monkeypox virus (MPXV) as the predominant remaining infectious orthopoxviruses (OPXV) causing human disease through zoonosis (Lewis-Jones, 2004). MPXV is second to VARV with regard to virulence, with symptoms similar to smallpox and mortality rates reaching almost 10%. The less virulent CPXV is endemic in Europe with occasional transmission via direct contact with infected domestic animals (Baxby and Bennett, 1997). In contrast to VARV, which was restricted to humans, both MPXV and CPXV infect many different mammal species, which renders their eradication impossible. This wide host range implies that these viruses are particularly adept at evading immune responses of many species.
We previously reported that CD8+ T cells obtained from mice infected with CPXV were not stimulated in the presence of CPXV-infected target cells (Dasgupta et al., 2007). In contrast, T cell stimulation was observed in the presence of Vaccinia virus (VACV)-infected targets suggesting a CPXV-specific immune evasion mechanism. This mechanism was not restricted to rodents since T cells from vaccinated humans were similarly stimulated by VACV but not by CPXV. T cell evasion correlated with the observation that major histocompatibility complex class I molecules (MHC-I) were retained in the endoplasmic reticulum (ER) by CPXV, whereas maturation was unimpaired in VACV-infected cells. However, it remained to be demonstrated whether MHC-I retention was responsible for T cell evasion, particularly since we did not observe such a correlation for MPXV which inhibited T cell stimulation independent of MHC-I downregulation (Hammarlund et al., 2008).
The differential T cell stimulation between CPXV and VACV suggested that CPXV encodes a specific immunomodulator absent from the genome of VACV. Indeed, the CPXV-specific open reading frame (ORF) 203 retains MHC-I in the ER via a carboxyterminal “KTEL” ER-retrieval motif (Byun et al., 2007). However, deletion of CPXV203 only partially restored MHC-I trafficking suggesting that CPXV expressed at least one other gene product inhibiting MHC-I maturation. Here, we identify CPXV012 as the second ORF responsible for MHC-I inhibition. We demonstrate that the combined deletion of CPXV012 with CPXV203 restores both MHC-I expression and T cell stimulation by CPXV-infected cells suggesting that interference with MHC-I maturation is responsible for T cell evasion by CPXV. We further demonstrate that CPXV012 retains MHC-I by inhibiting TAP-dependent peptide translocation and thus assembly with peptides in the ER. Interestingly, CPXV012 of the Brighton Red (BR) strain studied here is a truncated version of D10L, a C-type lectin domain-containing protein encoded by the GRI and Ger91 strains of CPXV. However, only the truncated version interferes with MHC-I whereas the full-length version is a putative ligand for the NK cell inhibitory receptor NKR-P1B. Our data thus identify the first poxviral TAP inhibitor and the first TAP-inhibitor outside the herpesvirus family. The data further imply that truncated ORFs found in many poxviral genomes can have novel, unexpected functions.
RESULTS
CPXV012 downregulates MHC-I
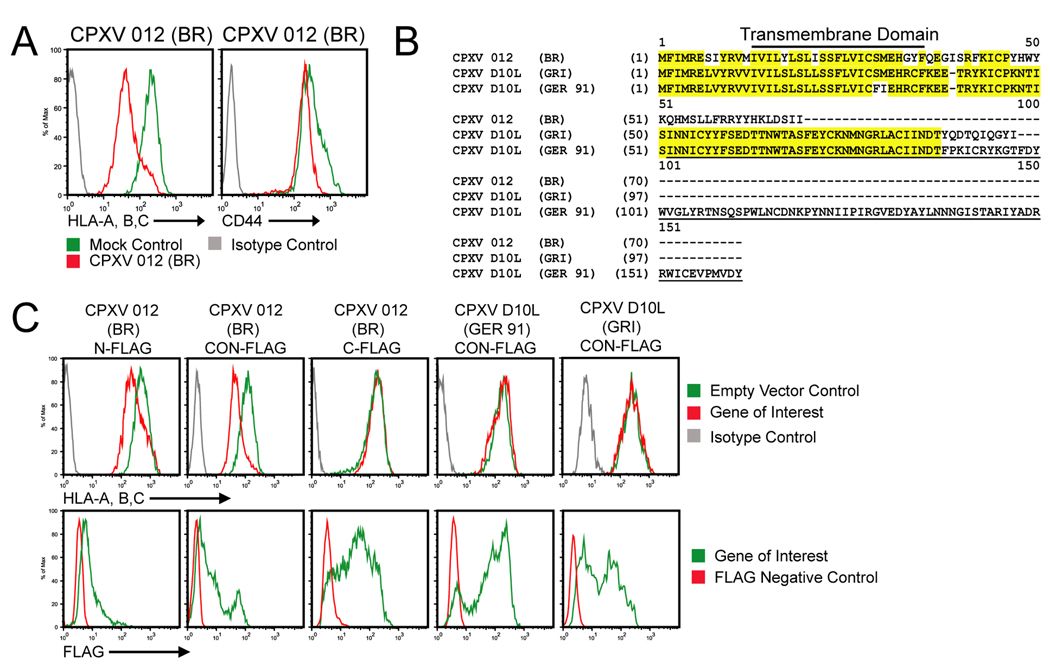
Given our finding that MPXV does not retain MHC-I (Hammarlund et al., 2008), despite the presence of a CPXV203 orthologue (Byun et al., 2007), we hypothesized that additional MHC-I inhibitors of CPXV should be absent from both VACV and MPXV. We further hypothesized that the new ORF should contain a transmembrane domain (TM) since MHC-I was retained in the ER of CPXV-BR (Dasgupta et al., 2007) and the CPXV203-deleted virus Δ203 (Byun et al., 2007). Genomic comparison of MPXV-Zaire1979 and VACV-WR with CPXV-BR revealed a short list of TM-containing ORFs only found in CPXV: 001, 007, 012, 047, 063, 214 (www.poxvirus.org). Upon transient expression of each ORF in HeLa cells, CPXV012 significantly reduced MHC-I surface levels (Fig. 1A) whereas all other transfectants showed unaltered MHC-I expression (data not shown). We further observed that CD44 levels were not reduced consistent with CPXV012 being MHC-I specific (Fig. 1A).
Figure 1. CPXV012 downregulates MHC-I surface expression.
(A) Flow cytometry of MHC-I surface levels in the presence of CPXV012. HeLa cells were transiently transfected with either pCPXV012 (red) or empty vector (green). At 48 hpt, cells were stained with anti-HLA-A, B, C or anti-CD44 and analyzed by flow-cytometry. Solid grey line represents isotype control. (B) Amino acid alignment of CPXV012 and its orthologs, CPXVD10L (GRI) and CPXVD10L (GER 91). Identical residues are highlighted in yellow. Underlined residues represent the C-type lectin like domain. (C) Flow cytometry of MHC-I surface levels in the presence of CPXV012 and CPXVD10L. pCPXV012-N-FLAG, pCPXV012-C-FLAG, pCPXV012CO-N-FLAG, pCPXVD10L (GER)-N-FLAG or pCPXVD10L (GRI)-N-FLAG were transiently transfected into HeLa cells. The cells were incubated for 48 h and stained with either HLA-specific antibodies (unpermeabilized cells) or anti-FLAG antibodies (permeabilized cells).
CPXV012 encoded by the CPXV-BR strain represents a 69 amino-acid (AA) truncated version of the ORF D10L that comprises 96 AA in strain GRI and 160 AA in strain GER-91 (www.poxvirus.org) (Fig. 1B). The 160AA version of this protein has similarity to C-type lectin ligands for NKR-P1 NK cell inhibitory receptors (Voigt et al., 2007). However, the C-type lectin domain is missing from the truncated CPXV012 of BR which only shares the N-terminal 46 AA with D10L of GER-91 and GRI strains (Fig. 1B). Given this limited overlap, we wanted to know whether the full-length D10Lprotein from strain GER-91 or the 96 AA variant in GRI inhibited MHC-I expression. N-terminally epitope-tagged, codon-optimized (CO-N-FLAG) versions of CPXV012, D10L-GER or D10L-GRI were transfected into HeLa cells and surface expression of MHC-I as well as intracellular expression of the viral proteins was determined by flow-cytometry. As shown in Fig. 1C, while the CO-N-FLAG version of CPXV012 (BR) downregulated MHC-I, D10L of GRI or GER had no effect despite higher expression levels. Thus, the short ORF expressed by BR acquired a new function that is different from its full-length ancestral protein. Since the N-terminal 46AA are highly conserved (70% identity) between the D10L and CPXV012, these data further suggested that the N-terminus, including the TM, is not sufficient to downregulate MHC-I. Instead, the mutations acquired in the 3’-end seem to be essential for MHC-I interference. Moreover, epitope-tagging of the C-terminus (C-FLAG) rendered CXPV012 non-functional despite increased protein levels compared to the N-terminally tagged, native sequence (N-FLAG) (Fig. 1C).
CPXV012 is an ER-resident type II transmembrane protein
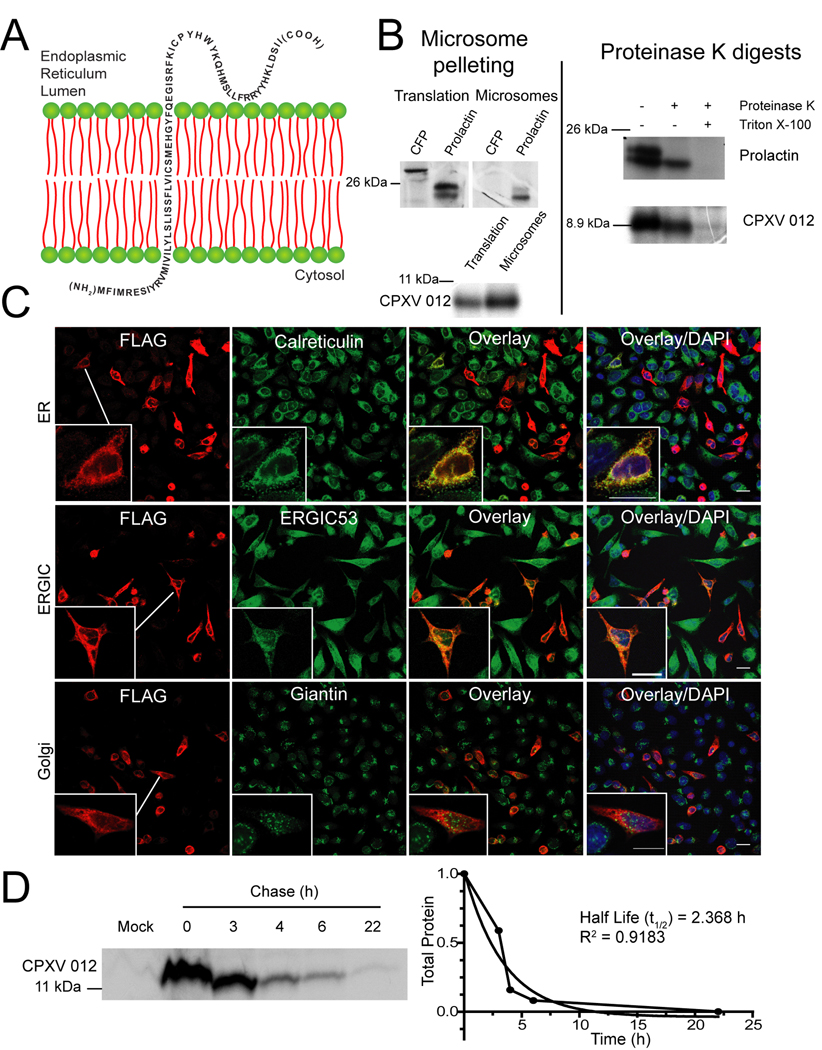
The full-length D10L protein is expected to be surface-expressed with a type II transmembrane topology similar to related proteins of mammalian hosts and of rat cytomegalovirus (Voigt et al., 2007). Since the N-terminus, including the TM, is highly conserved in CPXV012, the type II topology is expected to be conserved (Fig. 2A). To test this hypothesis, we performed a protease protection assay by in vitro translating CPXV012 in the presence of microsomes. Pre-prolactin was used as control, since it undergoes a characteristic shift in electrophoretic mobility upon translocation due to cleavage of the leader peptide by the signal sequence peptidase (Fig. 2B). As expected, only the cleaved prolactin co-sedimented with the microsomes, whereas pre-prolactin as well as the cytosolic cyan fluorescent protein (CFP) were not enriched in microsome pellets.
Figure 2. CPXV012 is a short lived, ER resident type II transmembrane protein.
(A) Model of CPXV012 topology in the ER membrane. (B) In vitro translation of CPXV012 mRNA in the presence of microsomes. In vitro transcribed mRNAs encoding CPXV012 and control proteins, CFP and prolactin were added to a rabbit reticulocyte translation system in the presence of canine microsomal membranes. Left panel: Total translation products or products recovered after microsome pelleting. Right panel: Following translation, pelleted microsomal fractions were treated with proteinase K and separated by SDS-PAGE. Molecular weight markers (kDa) are shown. (C) Subcellular localization of CPXV012. HeLa cells were transiently transfected with pCPXV012-N-FLAG. After 48 h, cells were fixed, permeabilized, and stained with antibodies specific to calreticulin, ERGIC53, giantin, or FLAG. (D) CPXV012 half-life by pulse-chase. pCPXV012-CON-FLAG and empty vector control (Mock) were transiently transfected into HeLa cells. At 24 hpt, cells were pulse-labeled for 1 h and the label was chased for the indicated time. Lysates were immunoprecipitated with anti-FLAG, separated by SDS-PAGE, and visualized by autoradiography (left panel). Optical density of CPXV012-specific bands was measured and plotted against the chase time (right panel).
Similar to prolactin, CPXV012 co-sedimented with microsomes and was protected from protease digestion (Fig. 2B). However, the MW did not change in the presence of microsomes consistent with a non-cleavable signal sequence. In contrast, a small MW reduction was observed upon protease treatment suggesting that the short N-terminus is exposed to the cytosol. We therefore conclude that CPXV012 most likely displays a type II trans-membrane topology (Fig. 2A).
To determine the subcellular localization of CPXV012, we performed immunofluorescence analysis (IFA) of CPXV012 in transfected HeLa cells. CPXV012 N-FLAG was co-stained with markers of the ER, ER-Golgi intermediate compartment (ERGIC) or the Golgi. While the Golgi protein giantin stained a subcellular structure that was distinct from CPXV012, ERGIC-53 partially overlapped whereas the ER-protein calreticulin completely overlapped with FLAG-staining (Fig. 2C). Similarly, the ER-proteins calnexin and protein-disulfide isomerase co-localized with CPXV012 (data not shown). Therefore, we conclude that CPXV012 locates to the ER. Pulse-chase experiments further revealed that the FLAG-CPXV012 has a half-life of approximately 2 hours suggesting that CPXV012 is short-lived (Fig. 2D). Taken together, these data suggest that CPXV012 is a short-lived, ER-resident type II transmembrane protein.
CPXV012 and CPXV203 are responsible for MHC-I downregulation by CPXV
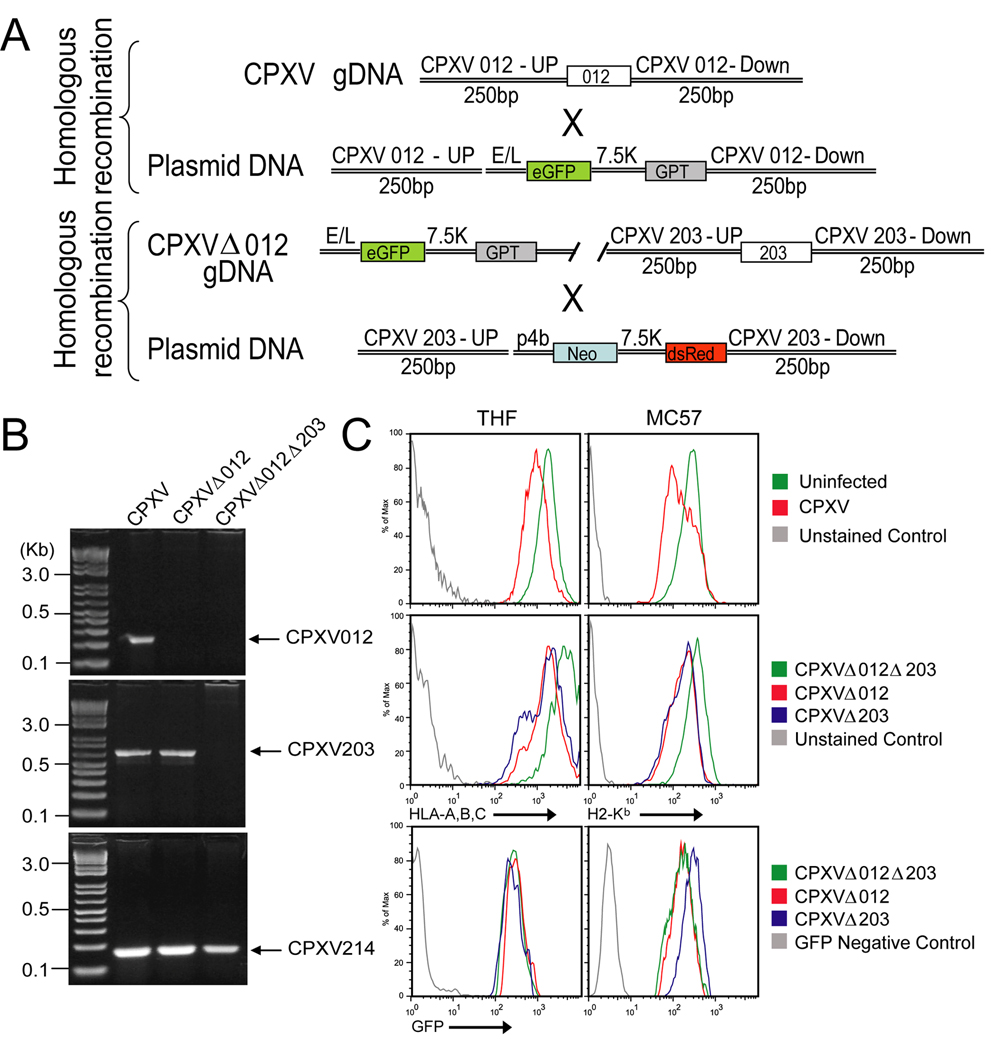
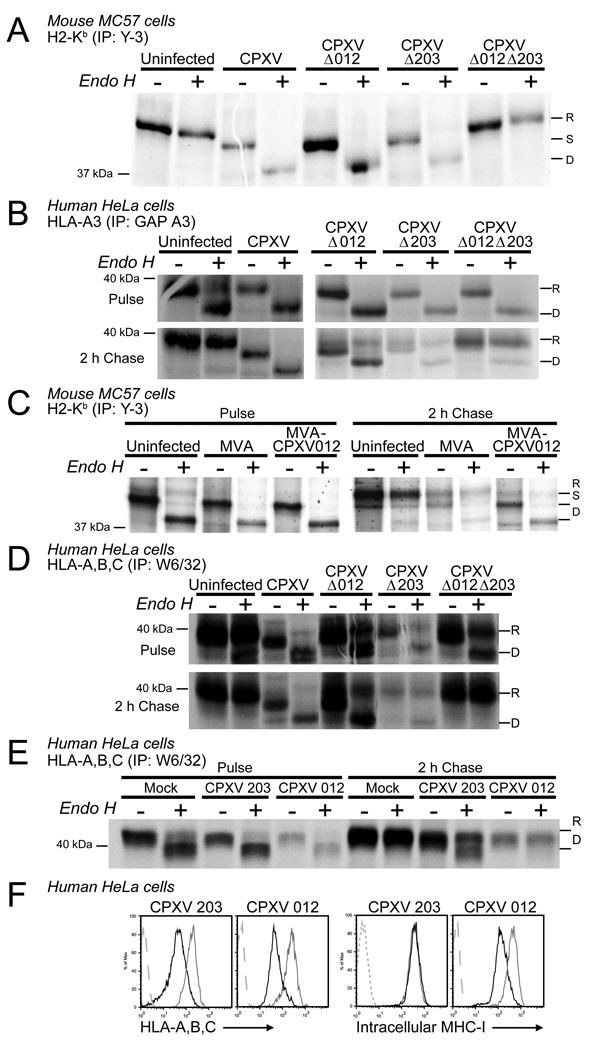
To determine whether CPXV012 was responsible for the residual MHC-I retention reported for CPXV lacking ORF203 (Δ203) (Byun et al., 2007), we generated a recombinant virus lacking both CPXV203 and CPXV012 (Fig. 3A, B) (supplemental data). The double knockout (DKO) virus did not show any growth defect in vitro (data not shown). We compared MHC-I surface levels in mouse and human cells infected with wildtype (WT) CPXV-BR, the single knockout (SKO) viruses Δ012 or Δ203 (Byun et al., 2007), and Δ012Δ203. As described previously (Dasgupta et al., 2007), WT CPXV reduced MHC-I surface levels compared to uninfected human (THFs) or mouse (MC57) fibroblasts (Fig. 3C). Decreased MHC-I was also observed on cells infected with the SKO viruses. In contrast, MHC-I levels remained high in cells infected with the DKO virus despite comparable infection as monitored by GFP expression. These observations are consistent with CPXV012 and CPXV203 each independently interfering with MHC-I surface expression.
Figure 3. CPXV012 contributes to MHC-I downregulation by CPXV.
(A) Schematic design of Δ012 and Δ012Δ203 mutant viruses. (B) PCR- analysis of Δ012 and Δ012Δ203 mutants. Purified viral genomic DNA of WT CPXV, Δ012 and Δ012Δ203 was PCR amplified with primers specific for CPXV012, CPXV203, or CPXV214. PCR products were separated by gel electrophoresis. (C) Flow cytometry of MHC-I surface expression. THF (left) and MC57 (right) cells were infected with WT CPXV, Δ012, Δ203, and Δ012Δ203. At 24hpi, cells were stained with either HLA- or H2-Kb-specific antibodies.
Deletion of CPXV012 and CPXV203 restores T cell stimulation
We previously demonstrated that ER-retention of MHC-I by CPXV correlated with a lack of stimulation of T cells derived from either VACV-immune humans or CPXV-infected mice (Dasgupta et al., 2007). In contrast, infection of antigen presenting cells (APC) by VACV-WR stimulated both human and murine OPXV-specific CD8+ T cells and VACV did not interfere with MHC-I transport. To determine if MHC-I downregulation by CPXV203 and CPXV012 was responsible for inhibiting T cell stimulation, we monitored cytokine production by VACV-specific T cells in the presence of APC infected with WT or recombinant CPXV, or VACV-WR.
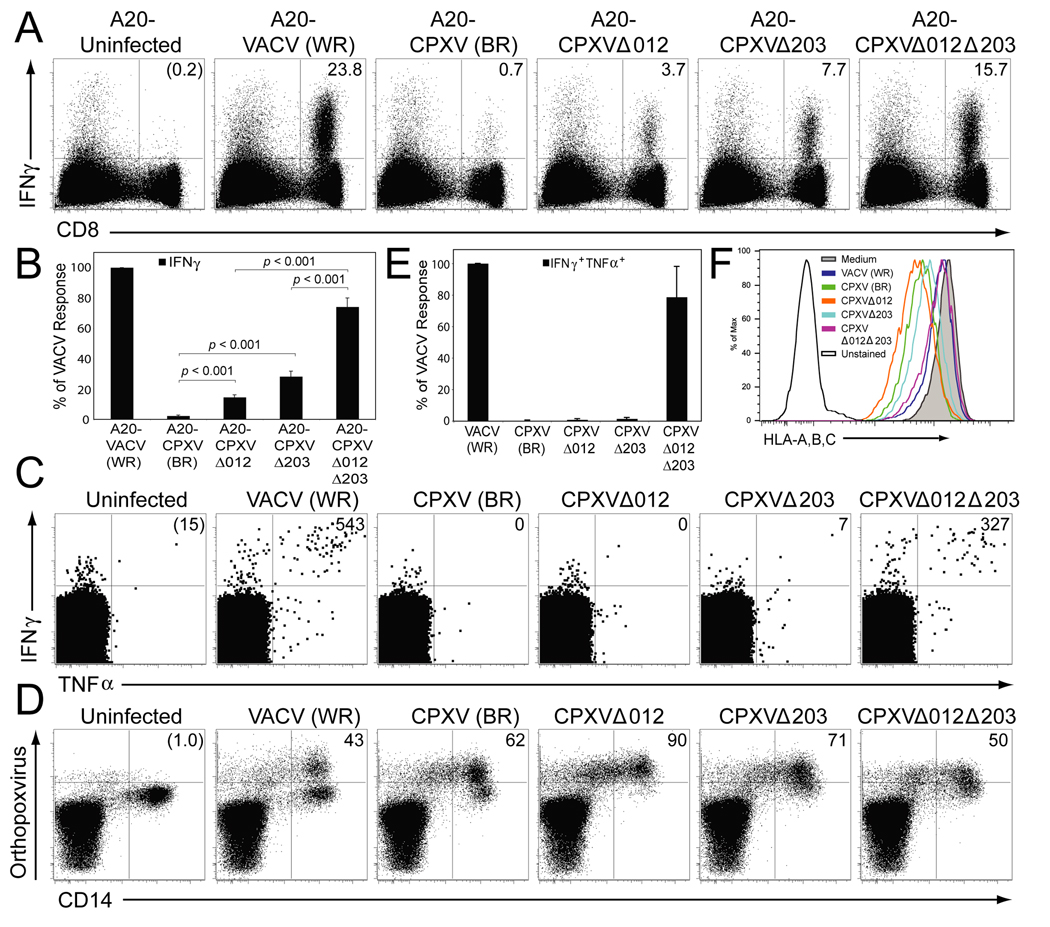
BALB/c mice were infected with VACV and the splenic CD8+ T cell response against target cells (A20 cells) infected with CPXV, Δ012, Δ203, Δ012Δ203 or VACV was analyzed 8 days later by intracellular cytokine staining (ICCS). A representative example is shown in Fig. 4A, whereas (Fig. 4B) shows the average results from six mice. As reported previously (Dasgupta et al., 2007), a large percentage of CD8+ T cells produced IFNγ in response to VACV-infected targets whereas less than 1% of CD8+ T cells responded to CPXV-infected targets. Similarly, both SKO viruses showed a diminished T cell response, although at a significantly higher frequency than WT virus, particularly Δ203 that expresses CPXV012 only. However, more than 70% of CD8+ T cells responding to VACV-infected cells also responded to A20 cells infected with Δ012Δ203(Fig. 4B) indicating that the majority of VACV-specific T cells were able to recognize DKO-infected targets. Thus, deletion of both MHC-I inhibitors restored T cell stimulation.
Figure 4. CD8+ T cell activation by CPXV is restored upon deletion of both CPXV012 and CPXV203.
(A) The antiviral CD8+ T cell response in BALB/c mice measured by ICCS. Splenocytes (8 dpi) were incubated with uninfected A20 cells, or infected with the indicated viruses. The percentage of IFNγ producing CD8+ T cells (upper right quadrant) was determined after background subtraction (uninfected control). (B) The average CD8+ T cell response of 6 mice was determined by ICCS and normalized to 100% based on the response to VACV. The data are representative of 2 experiments with 3 mice per group. Statistical differences were calculated using the 2-tailed paired Student t-test. (C) Human virus-specific CD8+ T cell responses in the presence of indicated viruses. PBMC of VACV-immune subjects were gated on CD8+CD4− T cells and the number of IFNγ +TNFα+CD8+ T cells per 106 CD8+ T cells (upper right quadrant) was determined after background subtraction (uninfected control). The CD8+ T cell responses from a representative subject at 2 months after VACV infection is shown. D) The number of virus-infected CD14+ monocytes in a representative PBMC sample at 16 hpi was determined by staining with polyclonal antibodies against OPXV antigens. The numbers in the upper right quadrants represent the percentage of virus-infected CD14+ cells after background subtraction (uninfected). (E) The average antiviral CD8+ T cell response from 4 subjects determined by ICCS at 2 months post-VACV infection and normalized to 100% based on the response to VACV. (F) HLA-A, B, C-expression on virus-infected primary CD14+ monocytes at 16 h p.i.. Monocytes were identified based on forward and side scatter characteristics and CD14 expression.
To determine whether CPXV012 and CPXV203 were also responsible for the previously reported evasion of T cells obtained from vaccinated humans (Dasgupta et al., 2007), we infected peripheral blood mononuclear cells (PBMC) from VACV-immune subjects with CPXV, Δ012, Δ203, Δ012Δ203 or VACV for 15 h and determined the frequency of stimulated virus-specific CD8+ T cells by ICCS. (Fig. 4C) depicts the ICCS results for one individual whereas (Fig. 4E) shows the average results from four individuals. Comparable infection of monocytes within the PBMC by all strains was verified by staining for OPXV-proteins (Fig. 4D). VACV-infected cells induced significant CD8+ T cell responses, as monitored by IFNγ and TNFα production, in all tested individuals, whereas T cell responses against CPXV, Δ012 and Δ203-infected cells was sharply reduced (Fig. 4C, E). In contrast, T cell stimulation was restored when PBMC were infected with Δ012Δ203. These observations correlated with MHC-I downregulation measured on CD14+ OPXV-infected APC by CPXV and the SKO viruses but not by Δ012Δ203 (Fig. 4F). Taken together, these data indicate that T cell evasion is caused by MHC-I downregulation mediated independently by CPXV203 and CPXV012. Remarkably, each of these gene products inhibits T cell stimulation of both human and mouse T cells, consistent with the ability of CPXV to infect many mammalian hosts.
CPXV012 inhibits MHC-I transport
CPXV retains MHC-I in the ER of human cells (Dasgupta et al., 2007) and murine MHC-I was retained by Δ203 (Byun et al., 2007). To determine whether CPXV012 was responsible for MHC-I retention by Δ203 we examined MHC-I assembly and maturation in murine and in human cells infected with single or double KO viruses.
Murine MC57 fibroblasts, mock-infected or infected with CPXV, Δ012, Δ203, or Δ012Δ203 for 6 hours, were pulse/chase labeled and immunoprecipitated with an antibody specific for H2-Kb. As shown in Fig. (5A), H2-Kb remained in the ER, as evident by EndoH-sensitivity, in cells infected with WT and both SKO viruses. In contrast, MHC-I acquired EndoH resistance in DKO-infected cells similar to the mock-infected cells. ER-retention of HLA-A3 by SKO viruses was also observed in transfected HeLa cells whereas the DKO virus did not impede its intracellular transport (Fig. 5B). Thus, each CPXV203 and CPXV012 independently interfere with MHC-I maturation. Since MHC-I transport was restored upon deletion of both ORFs we conclude that CPXV012 was responsible for the remaining MHC-I retention reported for Δ203 (Byun et al., 2007). To further examine whether CPXV012 was sufficient for MHC-I retention, we inserted CPXV012 into Modified Vaccinia Ankara (MVA) (supplemental Fig. S1). MC57 cells infected with MVA or MVA-CPXV012 were labeled and Kb was immunoprecipitated. As shown in Fig. (5C) , Kb remained EndoH sensitive in MVA-CPXV012 during the 2h chase period compared to MVA or uninfected cells, consistent with CPXV012 being sufficient for MHC-I retention.
Figure 5. CPXV012 impaires intracellular transport and steady state levels of MHC-I.
(A) H2-Kb expression in the presence of CPXV012. MC57 cells were infected with indicated viruses, pulse labeled for 20 min at 6hpi followed by 2 h chase. Immunoprecipitated samples were EndoH-treated as indicated, separated by SDS-PAGE, and visualized by autoradiography. “R”, “S”, and “D” refer to EndoH-resistant, sensitive, and digested HC. The reduced amount of MHC-I in EndoH-treated samples is due to reduced protein recovery after EndoH treatment. B) Intracellular transport of HLA-A3 transfected into HeLa cells and infected with WT or recombinant CPXV. At 6 hpi, cells were pulse labeled for 20 min followed by 2h chase. HLA-A3 was immunoprecipitated and EndoH-treated as indicated. C) H2-Kb immunoprecipitation from MC57 cells infected with MVA or MVA expressing CPXV012. Cells were labeled and immunoprecipitated as in A. D) Immunoprecipation of endogenous HLA-A,B,C in HeLa cells infected with indicated viruses. Cells were pulse labeled for 20 min at 6h p.i. and chased for 2h. HLA-A,B,C was immunoprecipitated and then treated with EndoH as in A.. E) HeLa cells transiently transfected with CPXV012, CPXV203 or vector control (Mock) were pulse-labeled for 20 min at 24 hpt followed by 2h chase and immunoprecipitation with anti-HLA-A,B,C. F) Intracellular and cell surface levels of MHC-I in the presence of transiently expressed CPXV012 measured by flow cytometry at 48 h after HeLa cells were transfected with pCPXV203 and pCPXV012. Permeabilized cells (intracellular staining) or unfixed cells were stained with anti-HLA-A, B, C. Vector-transfected and isotype control samples are represented by grey and dashed grey lines, respectively.
In addition to ER-retention, we observed reduced amounts of MHC-I reactive with conformation-specific antibodies recognizing fully assembled, peptide loaded H2-Kb (Y3), HLA-A3 (GAP) or HLA-A,B,C (W6/32) in the presence of CPXV012. This was particularly pronounced for endogenous MHC-I when HeLa cells were infected with CPXV012-expressing viruses (Fig. 5D) or transfected with CPXV012-expressing plasmid (Fig. 5E,F). In contrast, viruses that only expressed CPXV203 or transfection with CPXV203 did not affect the total levels of peptide-loaded MHC-I molecules (Fig. 5F), although cell surface expression was highly reduced. These results suggested that, unlike CPXV203 that retains MHC-I by physical association and continuous retrieval via the KDEL-receptor, CPXV012 impaired the assembly of peptide-loaded MHC-I.
CPXV012 inhibits MHC-I assembly with peptides
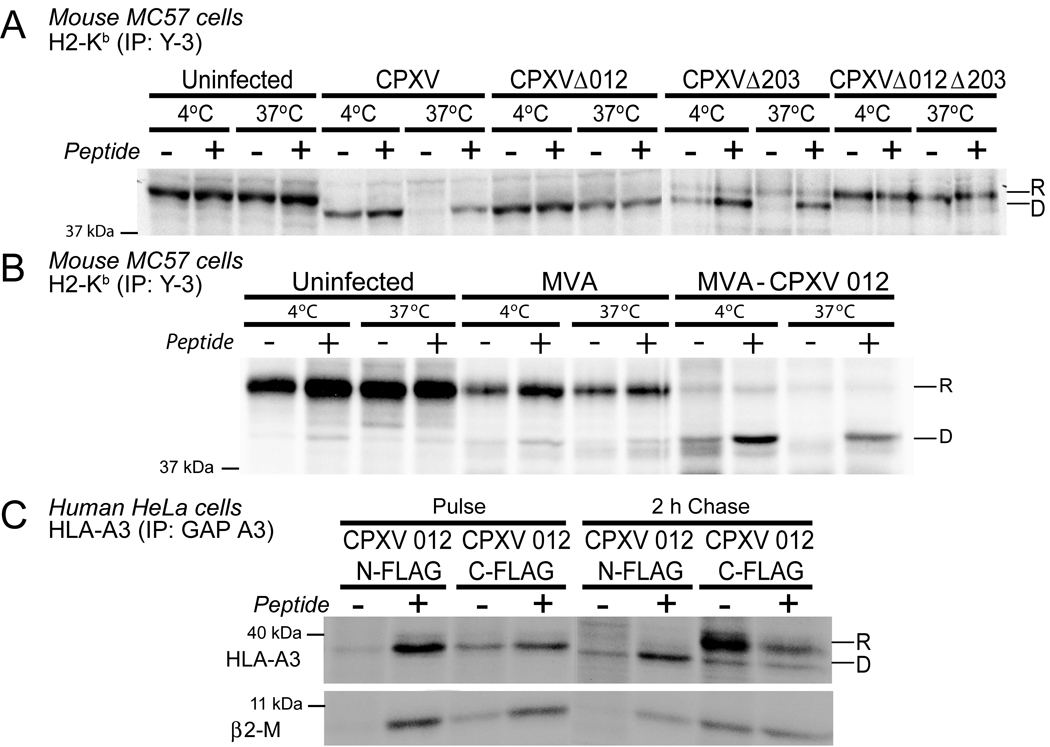
MHC-I molecules are hetero-trimers consisting of heavy chain (HC), β2-microglobulin (β2m) and peptide. The reduced amount of MHC-I recovered by conformation-dependent antibodies in the presence of CPXV012 could thus be either due to increased degradation of the HC or a decreased heterotrimer assembly. Interference with MHC-I assembly, particularly reduced peptide loading, results in heat-labile hetero-dimers (Ljunggren et al., 1990). To examine whether MHC-I molecules were heat-labile in CPXV012 expressing cells, we immunoprecipitated H2-Kb from MC57 cells infected with CPXV, Δ012, Δ203, or Δ012Δ203 and pulse/chase labeled for 2h. Cell lysates were either incubated at 37°C or kept at 4°C prior to immunoprecipitation with antibody Y3 and EndoH treatment. EndoH resistant, heat-stable Kb heterodimers were recovered from both uninfected cells and cells infected with the DKO virus (Fig. 6A). Heat-stable MHC-I were also recovered from Δ012-infected cells, although they were EndoH sensitive, consistent with CPXV203 retaining fully folded, peptide-loaded MHC-I molecules. In contrast, we did not recover thermostable EndoH-sensitive Kb from cells infected with either WT or Δ203. To determine whether this instability was due to lack of peptides, we added the Kb-binding peptide SIINFEKL prior to heat-challenge. As shown in Fig. 6A, peptide addition prevented disassembly of Kb in lysates from WT and Δ203-infected MC57 cells whereas adding peptide did not increase Kb-recovery from uninfected cells or cells infected with Δ012Δ203 or Δ012 (Fig. 6A). Similarly, MVA-CPXV012-infected MC57 cells (Fig. 6B) contained unstable Kb that was stabilized by SIINFEKL. To extend these observations to human MHC-I, we co-transfected HLA-A3 with N-terminally or C-terminally FLAG-tagged CPXV012 and studied its thermostability in the presence or absence of the Flu-NP peptide ILRGSVAHK (Bakker et al., 2008) (Fig. 6C). In N-FLAG transfectants, the conformation-specific antibody GAP precipitated HLA-A3 from lysates incubated at 37°C only in the presence of peptide. As expected, we observed that C-terminally tagged CPXV012 did not destabilize HLA-A3. These data present strong evidence that CPXV012 prevents peptide loading of MHC-I and acts by a distinct mechanism from that of CPXV203 which retains peptide-loaded MHC-I.
Figure 6. CPXV012 inhibits peptide loading of MHC-I.
(A) and (B) H2-Kb thermostability in the presence of virus expressed CPXV012. MC57 cells were infected with indicated viruses. At 6 hpi, cells were pulse-labeled for 20 min followed by 2 h chase. Lysates were incubated at indicated temperatures in the presence or absence of the peptide SIINFEKEL. H2-Kb was immunoprecipitated with mab Y3 and EndoH-treated. (C). Thermostability of HLA-A3 transfected in HeLa together with CPXV012-N-FLAG or CPXV012-C-FLAG. Cells were labeled as in A and incubated at indicated temperature in the presence or absence of peptide ILRGSVAHK. “R” and “D” refer to EndoH-resistant and digested MHC-I.
CPXV012 inhibits TAP-dependent peptide translocation
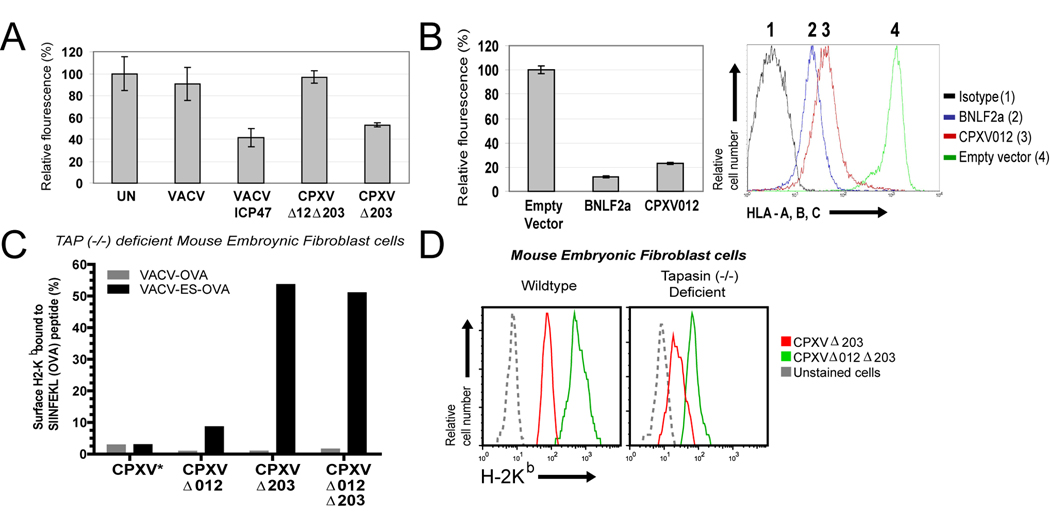
CPXV012-mediated inhibition of peptide loading suggested that ER-retention by CPXV012 was an indirect consequence of CPXV012 preventing assembly of MHC-I with peptides. Several steps are involved in the peptide loading process that occurs in a multi-protein complex consisting of the peptide transporter TAP, the MHC-I-specific chaperone Tapasin, the ER-resident chaperone calnexin, the thiol-reductase ERp57 and protein-disulfide isomerase (Raghavan et al., 2008). Since the most critical step is the translocation of cytosolic peptides by TAP we hypothesized that CPXV012 inhibits TAP. We examined peptide translocation across the ER-membrane using a fluorescent assay that recovers glycosylated, fluorescently labeled peptides using the lectin concanavalin A. Upon permeabilization of the plasma membrane with streptolysin O (SLO), a fluorescently labeled nonameric peptide is added carrying the N-linked glycosylation sequence NXT/S. Using such peptides, TAP-dependent peptide translocation can be observed independent of MHC-I binding since N-linked glycosylation occurs exclusively in the ER (Neefjes et al., 1993). We examined TAP transport in HeLa cells infected with Δ203 or Δ012Δ203. For control, we included VACV-expressing ICP47 (Banks et al., 1994), a TAP inhibitor of herpes simplex virus 1 (HSV-1) (Früh et al., 1995; Hill et al., 1995). Compared to uninfected cells or cells infected with VACV, infection with VACV-ICP47 reduced fluorescent peptide recovery by approximately 60% (Fig. 7A). While peptide recovery from cells infected with Δ012Δ203 was comparable to uninfected or VACV-infected, there was a significant reduction of peptide transport by Δ203 by approximately 50% suggesting that CPXV012 impedes TAP transport. To determine whether CPXV012 is sufficient for TAP-inhibition, we stably expressed CPXV012 in the human melanoma cell line MJS. Functional expression of CPXV012 was verified by monitoring MHC-I surface expression (Fig. 7B). For comparison we used the TAP-inhibitor BNLF2a from EBV (Hislop et al., 2007; Horst et al., 2009). As shown in Fig. 7B, CPXV012 reduced peptide transport by approximately 80% whereas BNLF2a reduced peptide transport by about 90%. Thus, we conclude that CPXV012 inhibits peptide transport by TAP.
Figure 7. CPXV012 inhibits TAP-dependent peptide transport.
(A) Peptide transport in the presence of CPXV012. HeLa cells were infected with indicated viruses and at 6 hpi permeabilized with SLO followed by incubation with peptide FITC~CVNKTERAY in the presence or absence of ATP in transport buffer. Recovered fluorescence was background (−ATP) adjusted and is shown as percent relative to uninfected cells. (B) Peptide transport in CPXV012-expressing cells. Peptide transport was measured in MJS cells stably transduced with GFP-expressing control lentiviruses or lentiviruses expressing either BNLF2a or CPXV012 (left panel). Transduction efficiency was verified by monitoring MHC-I surface levels by flow cytometry (right panel). CPXV012, BNLF2a, GFP control, and isotype control are represented by red, blue, green, and black lines, respectively. (C) CPXV012 does not inhibit TAP-independent peptide loading. TAP−/− MEFs were infected with VACV-OVA or VACV-ES-OVA together with indicated CPXV recombinants (MOI=10 each) and simultaneously treated with 25 U/ml IFN-γ. At 24 hpi, SIINFEKL/H2-Kb complexes were measured with specific Ab 25.D1.16 by flow cytometry. The percentage of GFP+ cells that were also 25.D1.16 positive is shown except for WT-CPXV showing total 25.D1.16-positive cells (*). (D) MHC-I downregulation by CPXV012 does not require Tapasin. WT and TPN−/− MEFs were infected with indicated viruses (MOI=5) in the presence of IFN-γ and surface H2-Kb was monitored by flow cytometry at 24 hpi. Kb-expression is shown for GFP+ cells.
Diminished TAP-transport could either be due to CPXV012 directly targeting TAP or an indirect consequence of interference with Tapasin function (Garbi et al., 2003). Therefore, we determined whether CPXV012 would interfere with TAP-independent peptide loading in TAP-deficient mouse embryonic fibroblasts (MEFs) (Van Kaer et al., 1992) infected with VACV-ES-OVA257–264 (Bacik et al., 1994) expressing ER-targeted SIINFEKL together with the various CPXV constructs. Both WT and Δ012 inhibited MHC-I surface expression measured with an antibody specific for the H2-Kb/SIINFEKL complex (Porgador et al., 1997) consistent with CPXV203 preventing intracellular transport of MHC-I independent of TAP (Fig. 7C, suppl. Fig. S3). In contrast, Kb/SIINFEKL complexes were observed at the surface of TAP−/− MEFs infected with Δ203 and Δ012Δ203 suggesting that CPXV012 was unable to prevent TAP-independent peptide loading and MHC-I transport. To further determine whether CPXV012 could inhibit TAP in the absence of Tapasin we infected Tapasin-deficient MEFs with Δ203 or Δ012Δ203 and monitored total H2-Kb expression. TPN−/− MEFs infected Δ012Δ203 expressed reduced levels of H2-Kb compared to WT-MEFs (Fig. 7D) since MHC-I molecules are less stable due to inefficient peptide loading in TPN−/− cells (Garbi et al., 2000; Grandea et al., 2000). However, MHC-I expression was further reduced when TPN−/− MEFs were infected with Δ203 (Fig. 7D) consistent with a lack of peptide supply upon CPXV012 expression. Taken together, these data indicate that CPXV012 targets TAP-dependent peptide transport directly and independent of Tapasin function.
DISCUSSION
Our data suggest that CPXV prevents antigen presentation to CD8+ T cells by expressing two unrelated and independently acting proteins. CPXV012 limits the TAP-dependent supply of peptides to the MHC-I peptide-loading complex. Since empty MHC-I molecules are retained in the ER by Tapasin (Ortmann et al., 1997; Schoenhals et al., 1999) the observed ER-retention by CPXV012 is thus an indirect consequence of inhibiting peptide loading. Also, the reduction of MHC-I steady state levels is likely the result of the ultimate degradation of empty MHC-I molecules as reported for cells expressing ICP47 (Hughes et al., 1997). In contrast, CPXV203 inhibits the intracellular transport of MHC-I independent of its peptide loading status via direct binding and KDEL-mediated retrieval (Byun et al., 2007). However, although each protein acts independently, CPXV expressing both ORFs was more efficient in preventing T cell stimulation than SKOs (Fig. 4). Moreover, both ORFs are co-expressed as immediate early genes (supplemental Fig. 2). A possible scenario is that MHC-I escaping CPXV012 (either due to incomplete TAP inhibition, or TAP-independent peptide loading or as empty molecules) are then retrieved by CPXV203. This sequential interference could conceivably take place in a complex consisting of CPXV012, Tap and MHC-I that also binds CPXV203 thus optimizing prevention of antigen presentation.
Remarkably, CPXV012 and CPXV203 prevent ER-exit of both human and mouse MHC-I. This is in stark contrast to the limited species specificity observed for most other viral MHC-I inhibitors, particularly TAP-inhibitory proteins (Ahn et al., 1996). In many instances, this restriction correlates with the limited host range of the corresponding viruses. In contrast, CPXV infects many mammalian species and this is reflected in its ability to inhibit both human and mouse MHC-I pathways. The availability of two independently acting MHC-I inhibitors might thus also increase the host range for CPXV. Furthermore, by independently targeting MHC-I and TAP, CPXV also counteracts the polymorphism of MHC-I. Proteins that interact directly with MHC-I often differentially affect polymorphic MHC-I alleles. Examples are Adenovirus E19 (Feuerbach et al., 1994), HCMV US2 and US11 (Barel et al., 2006; Machold et al., 1997), and MCMV m06 and m152 (Wagner et al., 2002). In contrast, TAP represents a conserved target.
So far, TAP-inhibitors were only described for the herpesvirus family. Although not every herpesvirus inhibits TAP, TAP-inhibitors were identified for members of each the α, β and γ subfamily. Interestingly, most herpesviral TAP-inhibitors are unrelated to each other suggesting that TAP inhibition has evolved independently multiple times. The repeated occurrence of TAP-inhibition within the herpesvirus family correlates with the ability of most herpesviruses to establish longterm persistent infections within their hosts. The acquisition of multiple TAP-inhibitors probably reflects the enormous immunological pressure exerted by CD8+ T cells during long-term infection by herpesviruses. In contrast, OPXVs are typically acute viruses that employ a “hit and run” strategy to disseminate within a host population. While OPXV-infection often causes significant morbidity, the host develops long-term protective immunity once the infection is cleared (Hammarlund et al., 2003). This protective immunity is considered to be mostly antibody mediated (Edghill-Smith et al., 2005). However, the fact that CPXV devotes two of its genes to counteract CD8+ T cells suggests that the cytolytic T cell immune response also plays a major role in controlling acute infection with CPXV.
Interestingly, CPXV012 of CPXV-BR seems to be derived by truncation and mutation from the D10L protein of other CPXV strains. D10L contains a C-type lectin domain homologous to host Clr-b, a ligand for the NK cell inhibitory receptor NKR-P1B (Voigt et al., 2007). While it is not known whether D10L activates this inhibitory receptor, NK cell inhibition was demonstrated for the related protein RCTL in rat CMV (Voigt et al., 2007). CPXV-BR thus seems to have modified an NK cell inhibitory protein to become a T cell evasion protein. This “exaptation” of unrelated viral proteins for TAP-inhibition is reminiscent of the UL49.5 protein which functions as a chaperone for the viral gM protein in all herpesviruses, but TAP-inhibition has been observed only for a few varicelloviruses (Koppers-Lalic et al., 2005). That TAP can be inhibited by several different proteins of different evolutionary origin and in entirely unrelated viral families is probably facilitated by several possible modes of TAP-inhibition. As ABC transporter, TAP pumps peptides across the ER-membrane by ATP-hydrolysis. Several of the multiple steps that are involved in this process can be experimentally distinguished such as peptide binding, ATP binding and ATP-hydrolysis. So far, TAP inhibitors can be classified as inhibitors of peptide binding (ICP47 (Ahn et al., 1996)), inhibitors of ATP-binding (US6, EHV UL49.5) (Hewitt et al., 2001; Koppers-Lalic et al., 2005) or both (BNLF2a) (Horst et al., 2009). Additionally, TAP-degradation has been reported for BHV-1 U49.5 (Koppers-Lalic et al., 2005). While further work will be required to determine the mechanism of TAP-inhibition by CPXV012 in more detail, its type II transmembrane topology together with the fact that the ER-luminal domain seems to be more sensitive to manipulation than the cytosolic domain renders it likely that CPXV012 will not prevent peptide-binding to TAP which occurs in the cytoplasm. In fact, both inhibitors of peptide binding, ICP47 and BNLF2a, represent short 60–70 amino-acid proteins that act on the cytosolic face of TAP. In contrast, EHV UL49.5 and HCMV US6 both inhibit ATP-binding, which is coupled to peptide translocation, and represent type I transmembrane proteins that act on the luminal side of TAP. It is thus possible that CPXV012 will similarly block ATP binding.
Truncated gene fragments similar to CPXV012 are widely found in poxviral genomes (www.poxvirus.org) and frequently represent loss-of-function phenotypes. Our finding suggests that such truncated ORFs might acquire a entirely new, unanticipated functions. It is thus important to consider this evolutionary “genetic debris” when considering poxviral proteomes and their associated function. Since TAP homologues are present in all vertebrate genomes and since all vertebrates are potential hosts of herpes and poxviruses, our data further suggest that TAP-inhibition might be more wide-spread than previously thought, particularly in large DNA viruses.
EXPERIMENTAL PROCEDURES
Cells and Viruses
HeLa, telomerized human fibroblasts (THF), 293T, HEK, MC57G mouse fibroblasts, and BHK-21 cells were maintained in DMEM (Mediatech, Manassas, VA) supplemented with 10% FBS (Hyclone, Logan, UT). Wildtype, TAP−/− and TPN−/− MEFs were obtained from Ann Hill, OHSU, and maintained in complete DMEM, as above. To induce MHC-I expression, MEFs were treated with 25U/ml recombinant mouse IFNγ (RND systems). A20 mouse B lymphoma cells and MJS human melanoma cells were grown in 10% FBS-RPMI 1640 medium (Hyclone). BSC40, African Green Monkey kidney cells were grown in MEM (Mediatech). VACV-ICP47, VACV-OVA, VACV-ES-OVA were obtained from Jon Yewdell, NIH. VACV, MVA and CPXV were propagated in BSC40 cells maintained in MEM, 5% FBS MEM. MVA was amplified in BHK-21 cells incubated in DMEM, 5%FBS. Viruses were purified using standard protocols. Briefly, infected cell lysates were precleared and layered onto 36% sucrose cushion. The virus was isolated by centrifugation at 40,000×g for 80 min, resuspended in 1mM Tris-HCl (pH8.0), and titered. For ICCS, virus was additionally purified by centrifugation (22,500×g, 40 min) through a 25% to 40% continuous sucrose gradient.
Human Subjects and Animals
VACV-immune subjects provided informed written consent before signing research authorization forms that complied with the US Health Insurance Portability and Accountability Act in addition to a medical history questionnaire. These studies were approved by the Institutional Review Board of OHSU. Female BALB/c mice at 5 months of age (The Jackson Laboratory) were infected intraperitoneally (i.p.) with 2 × 106 PFU/mouse of VACV-WR. All animal experiments were reviewed and approved by the OHSU institutional animal care and use committee.
Flow Cytometry
Cells were labeled with Phycoerythrin(PE)-conjugated monoclonal antibodies (mabs) specific for either HLA-A,B,C (W6/32, eBioscience, San Diego, CA) , CD44 (clone 515, BD Biosciences, San Jose, CA), or FLAG epitope (Invitrogen). Alternatively, biotinylated W6/32 was used followed by Allophycocyanin-conjugated streptavidin (eBioscience) as a secondary reagent. MC57G cells were labeled with primary mouse anti-Kb (Y3, ATCC) and secondary chicken anti-mouse antibodies conjugated to Alexa Fluor 647 (Invitrogen). MEF cells were labeled with Allophyocyanin-conjugated anti-(H2-Kb-SIINFEKL complex), clone 25.D1.16 (eBioscience). For intracellular staining the cells were permeabilized with Cytofix/Cytoperm (BD Biosciences) prior to antibody staining. Flow cytometry was performed using a FACSCalibur (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
In Vitro Translation
mRNA was transcribed with SP6 RNA polymerase (Promega Biosciences, San Luis Obispo, CA) and translated in rabbit reticulocyte lysate (Skach, 1998). Proteinase K digests were performed as indicated in the same reference. SP6 transcription vectors for CFP and prolactin were kindly provided by Bill Skach, OHSU. Samples were added to SDS-loading buffer and electrophoresed on a 16.5% Tris-tricine gel, followed by gel drying and autoradiography. Microsome pelleting was performed by loading 2 µl of the translation reaction onto a 100µl 0.5M sucrose cushion and centrifuging the sample for 10 min at 4°C at 70,000 rpm in a TLA 100 rotor in an Optima TL ultracentrifuge (Beckman Coulter, Fullerton, CA). Supernatants were removed, and pellets were resuspended in 10 µl of 1% SDS, 0.1 M Tris, pH 8.0, mixed with SDS loading buffer, and analyzed by SDS-PAGE and autoradiography.
Intracellular Cytokine Staining (ICCS)
(i) Human CD8+ T cells
PBMC were infected with virus at an MOI of 0.3 to 0.9 for 12 h, treated with Brefeldin A (BFA; ICN Biomedicals, Costa Mesa, CA) for an additional 6 h, and stained with anti-CD8β (2ST8.5H7, Beckman Coulter) and anti-CD4 (L200, BD Biosciences). Thereafter the cells were fixed, permeabilized, and stained intracellularly using antibodies to IFNγ (4S.B3, eBioscience) and TNFα (Mab11, eBioscience). Samples were acquired on an LSRII (BD Bioscience; ~106 events/sample) and analyzed using FloJo software. Non-viable cells were excluded using a live cell gate based on the viability stain Aqua (LIVE/DEAD® Fixable Dead Cell Stain, Invitrogen), followed by an optimized lymphocyte gate based on forward and side scatter characteristics. The number of virus-specific IFNγ+TNFα+ T cells was determined after gating on live CD4-CD8β+ T cells and subtracting the number of IFNγ+TNFα+ events from uninfected cultures. To determine efficiency of viral infection and measure MHC-I expression, the cells were stained with antibodies specific for CD14 (RMO52, Immunotech, Quebec, Canada) and HLA-A,B,C (W6/32, BioLegend). Thereafter, the cells were fixed, permeabilized, and stained with biotinylated rabbit polyclonal antibodies against OPXV antigens (ViroStat, Portland, ME) and streptavidin~PECy7. Samples were acquired on an LSRII (~3×105 events/sample) and analyzed using FloJo software.
(ii) Murine CD8+ T cells
Splenocytes were isolated from VACV-infected mice (2 × 106 PFU/mouse) at 8 dpi and analyzed as described (Dasgupta et al., 2007). A20 cells (5×105), uninfected or infected with virus at an MOI of 5 for16 h, were mixed with 1 ×106 splenocytes and incubated in the presence of BFA for 6 h. Following staining with anti-CD8 (5H10, Invitrogen), cells were fixed, permeabilized, and labeled intracellularly with antibodies to IFNγ (XMG1.2, BD Biosciences) and TNFα (MP6-XT22, BioLegend). The samples were acquired and analyzed as described above.
Pulse-Chase and Thermostability Assay
Metabolic labeling was carried out as described (Dasgupta et al. 2007). Briefly, HeLa and MC57G cells were labeled with EXPRE35S35S Protein Labeling Mix (PerkinElmer, Waltham, MA) at 100–300 µCi/106 cells for 20–30 min. Upon chase, cells were lysed in 1% NP-40 PBS (pH7.4) with protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany). Post-nuclear lysates were pre-cleared with Protein A/G PLUS - Agarose (Santa Cruz Biotechnology, Santa Cruz, CA) and incubated with antibodies to either HLA-A,B,C (W6/32;ATCC), HLA-A3 (GAP A3; ATCC), H2-Kb (Y3;ATCC), or FLAG epitope (Sigma-Aldrich) and captured with Protein A/G. The immunoprecipitates were treated with 10mU Endo H (Roche), separated by SDS-PAGE, and detected by autoradiography. Protein bands were quantified with ImageQuant (Amersham Biosciences). The half life and corresponding R2 value of CPXV012 were calculated with Prism (Graphpad, San Diego, CA).
The thermostability assay was done according to (Ljunggren et al. 1990). Briefly, labeled cell lysates were precleared with protein A~agarose beads at 4°C overnight in the presence of either 10µM SIINFEKL (GenScript, Piscataway, NJ) or 20µM of the Influenza-NP peptide ILRGSVAHK (GenScript)(Bakker et al., 2008). Thereafter the lysates were kept at 37°C for 1 h and then returned to 4°C for 1 h followed by immunoprecipitation.
Peptide Transport Assay
Cells were washed with ice-cold PBS (pH7.4), trypsinized, and permeabilized with streptolysin O (SLO; Murex Diagnostics, Dartford, UK). The assay was carried out in triplicates using 450 pmol of fluorescein isothiocyanate (FITC) conjugated CVNKTERAY peptide per 2×106 cells in the presence or absence of 10mM ATP as described (Koppers-Lalic et al., 2005). Fluorescence intensity, indicative of the amount of glycosylated peptides captured with Concanavalin A sepharose beads (GE Healthcare, Piscataway, NJ), was measured at the excitation wavelength of 490 nm and the emission wavelength of 525 nm.
Immunofluorescence Staining for Confocal Laser Scanning Microscopy
HeLa cells were transfected with plasmid constructs using Lipofectamine 2000 (Invitrogen). At 48hpt, cells were fixed/permeabilized with 100% methanol for 10 min and blocked in 2% bovine serum albumin (BSA)-PBS (P-BSA, pH 7.4). The cells were stained with primary mouse anti-FLAG (Sigma) and rabbit anti-calreticulin (Novus Biologicals, Lettleton, CO), anti-ERGIC57 (Sigma), and anti-Giantin (Abcam, Cambridge, MA) and secondary anti-mouse or anti-rabbit antibodies conjugated to Alexa Fluor 594 and Alexa Fluor 488 (Invitrogen), respectively. Coverslips were mounted on slides in ProLong Gold antifade reagent with 4,6-diamidino-2-phenylindole (DAPI; Invitrogen) and analyzed with a Leica TCS SP laser scanning microscope.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to our colleagues for providing the following reagents: Jon Yewdell for recombinant vaccine viruses, Ann Hill for MEFs, Wayne Yokoyama for CPXV-Δ203, Grant McFadden for pT7 E/L EGFP-GPT. We also thank Daniel Cawley for generating monoclonal antibodies and Bill Skach for advice and reagents for in vitro translation . The following funding sources are acknowledged: AI077048 and RR00163 (to KF and MKS), AO076506 (to MKS), Dutch Cancer Foundation (grant RUL 2005–3259) to EJW, Natural Sciences and Engineering Research Council of Canada Discovery Grant and NIAID grant HHSN266200400036C to CU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflict of interest.
References
- Ahn K, Meyer TH, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson PA, Früh K, Tampe R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. Embo J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- Bacik I, Cox JH, Anderson R, Yewdell JW, Bennink JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–387. [PubMed] [Google Scholar]
- Bakker AH, Hoppes R, Linnemann C, Toebes M, Rodenko B, Berkers CR, Hadrup SR, van Esch WJ, Heemskerk MH, Ovaa H, et al. Conditional MHC class I ligands and peptide exchange technology for the human MHC gene products HLA-A1, -A3, - A11, and -B7. Proc Natl Acad Sci U S A. 2008;105:3825–3830. doi: 10.1073/pnas.0709717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks TA, Jenkins FJ, Kanangat S, Nair S, Dasgupta S, Foster CM, Rouse BT. Vaccination with the immediate-early protein ICP47 of herpes simplex virus-type 1 (HSV-1) induces virus-specific lymphoproliferation, but fails to protect against lethal challenge. Virology. 1994;200:236–245. doi: 10.1006/viro.1994.1181. [DOI] [PubMed] [Google Scholar]
- Barel MT, Pizzato N, Le Bouteiller P, Wiertz EJ, Lenfant F. Subtle sequence variation among MHC class I locus products greatly influences sensitivity to HCMV US2- and US11-mediated degradation. International immunology. 2006;18:173–182. doi: 10.1093/intimm/dxh362. [DOI] [PubMed] [Google Scholar]
- Baxby D, Bennett M. Cowpox: a re-evaluation of the risks of human cowpox based on new epidemiological information. Arch Virol Suppl. 1997;13:1–12. doi: 10.1007/978-3-7091-6534-8_1. [DOI] [PubMed] [Google Scholar]
- Byun M, Wang X, Pak M, Hansen TH, Yokoyama WM. Cowpox virus exploits the endoplasmic reticulum retention pathway to inhibit MHC class I transport to the cell surface. Cell host & microbe. 2007;2:306–315. doi: 10.1016/j.chom.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Hammarlund E, Slifka MK, Früh K. Cowpox Virus Evades CTL Recognition and Inhibits the Intracellular Transport of MHC Class I Molecules. J Immunol. 2007;178:1654–1661. doi: 10.4049/jimmunol.178.3.1654. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, Nalca A, Hooper JW, Whitehouse CA, Schmitz JE, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Etteldorf S, Ebenau-Jehle C, Abastado JP, Madden D, Burgert HG. Identification of amino acids within the MHC molecule important for the interaction with the adenovirus protein E3/19K. J Immunol. 1994;153:1626–1636. [PubMed] [Google Scholar]
- Früh K, Ahn K, Djaballah H, Sempé P, van Endert PM, Tampé R, Peterson PA, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- Garbi N, Tan P, Diehl AD, Chambers BJ, Ljunggren HG, Momburg F, Hammerling GJ. Impaired immune responses and altered peptide repertoire in tapasin-deficient mice. Nat Immunol. 2000;1:234–238. doi: 10.1038/79775. [DOI] [PubMed] [Google Scholar]
- Garbi N, Tiwari N, Momburg F, Hammerling GJ. A major role for tapasin as a stabilizer of the TAP peptide transporter and consequences for MHC class I expression. Eur J Immunol. 2003;33:264–273. doi: 10.1002/immu.200390029. [DOI] [PubMed] [Google Scholar]
- Grandea AG, 3rd, Golovina TN, Hamilton SE, Sriram V, Spies T, Brutkiewicz RR, Harty JT, Eisenlohr LC, Van Kaer L. Impaired assembly yet normal trafficking of MHC class I molecules in Tapasin mutant mice. Immunity. 2000;13:213–222. doi: 10.1016/s1074-7613(00)00021-2. [DOI] [PubMed] [Google Scholar]
- Hammarlund E, Dasgupta A, Pinilla C, Norori P, Früh K, Slifka MK. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci U S A. 2008;105:14567–14572. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Hewitt EW, Gupta SS, Lehner PJ. The human cytomegalovirus gene product US6 inhibits ATP binding by TAP. Embo J. 2001;20:387–396. doi: 10.1093/emboj/20.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- Hislop AD, Ressing ME, van Leeuwen D, Pudney VA, Horst D, Koppers-Lalic D, Croft NP, Neefjes JJ, Rickinson AB, Wiertz EJ. A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J Exp Med. 2007;204:1863–1873. doi: 10.1084/jem.20070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst D, van Leeuwen D, Croft NP, Garstka MA, Hislop AD, Kremmer E, Rickinson AB, Wiertz EJ, Ressing ME. Specific targeting of the EBV lytic phase protein BNLF2a to the transporter associated with antigen processing results in impairment of HLA class I-restricted antigen presentation. J Immunol. 2009;182:2313–2324. doi: 10.4049/jimmunol.0803218. [DOI] [PubMed] [Google Scholar]
- Hughes EA, Hammond C, Cresswell P. Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Natl Acad Sci U S A. 1997;94:1896–1901. doi: 10.1073/pnas.94.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppers-Lalic D, Reits EA, Ressing ME, Lipinska AD, Abele R, Koch J, Marcondes Rezende M, Admiraal P, van Leeuwen D, Bienkowska-Szewczyk K, et al. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc Natl Acad Sci U S A. 2005;102:5144–5149. doi: 10.1073/pnas.0501463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Jones S. Zoonotic poxvirus infections in humans. Curr Opin Infect Dis. 2004;17:81–89. doi: 10.1097/00001432-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Ljunggren HG, Stam NJ, Ohlen C, Neefjes JJ, Hoglund P, Heemels MT, Bastin J, Schumacher TN, Townsend A, Karre K, et al. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Machold RP, Wiertz EJ, Jones TR, Ploegh HL. The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J Exp Med. 1997;185:363–366. doi: 10.1084/jem.185.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes JJ, Momburg F, Hammerling GJ. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993;261:769–771. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampe R, Spies T, Trowsdale J, et al. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277:1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- Raghavan M, Del Cid N, Rizvi SM, Peters LR. MHC class I assembly: out and about. Trends Immunol. 2008;29:436–443. doi: 10.1016/j.it.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhals GJ, Krishna RM, Grandea AG, III, Spies T, Peterson PA, Yang Y, Früh K. Retention of empty MHC class I molecules by Tapasin is essential to reconstitute antigen presentation in invertebrate cells. EMBO-J. 1999;18:743–753. doi: 10.1093/emboj/18.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skach WR. Topology of P-glycoproteins. Methods in enzymology. 1998;292:265–278. doi: 10.1016/s0076-6879(98)92021-3. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Voigt S, Mesci A, Ettinger J, Fine JH, Chen P, Chou W, Carlyle JR. Cytomegalovirus evasion of innate immunity by subversion of the NKR-P1B:Clr-b missing-self axis. Immunity. 2007;26:617–627. doi: 10.1016/j.immuni.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Wagner M, Gutermann A, Podlech J, Reddehase MJ, Koszinowski UH. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J Exp Med. 2002;196:805–816. doi: 10.1084/jem.20020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.