Abstract
A general method for copper-catalyzed deprotonative dimerization of arenes by employing oxygen as the terminal oxidant has been developed. Electron-rich and electron-poor heterocycles as well as electron-poor arenes are reactive. The method is tolerant to functionalities such as nitro, cyano, dialkylamino, and ester groups.
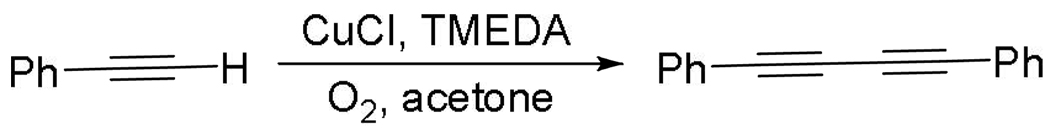
Direct functionalization of C-H bonds allows to shorten synthetic schemes by allowing transformations to be carried out in fewer steps compared with traditional cross-coupling methods.1 In most cases ruthenium, rhodium, platinum, iridium, or palladium catalysts are employed.1h It would be advantageous to employ less exotic metals such as copper or iron for C-H bond functionalization. Even though copper is one of the first transition metals shown to promote functionalization of C-H bonds,2 methods that utilize copper catalysis for conversion of C-H bonds to C-C bonds are rare.3 A notable example of such catalysis is the Glaser-Hay alkyne dimerization that has been known since 1869 (Scheme 1).4 Interestingly, oxygen is used as the terminal oxidant. Copper-catalyzed conversion of C-H to C-heteroatom bonds has been described, and in some cases oxygen was employed as the terminal oxidant.5 Copper-promoted 2-arylpyridine dimerization has been recently described.6
Scheme 1.
Glaser-Hay reaction
While palladium-catalyzed arene dimerization by employing oxygen as the terminal oxidant is known, the corresponding copper-catalyzed reaction has not been reported for substrates other than phenols.7 We report here a deprotonative, copper catalyzed arene dimerization by employing oxygen as the terminal oxidant.
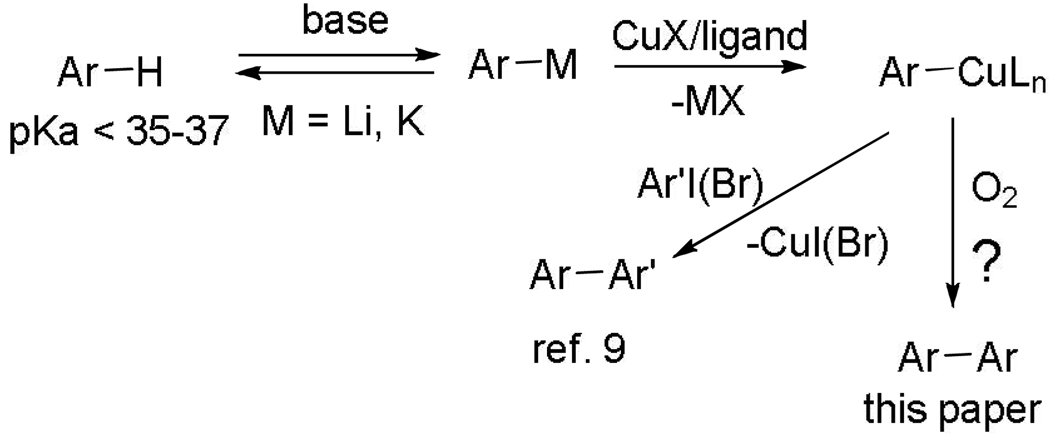
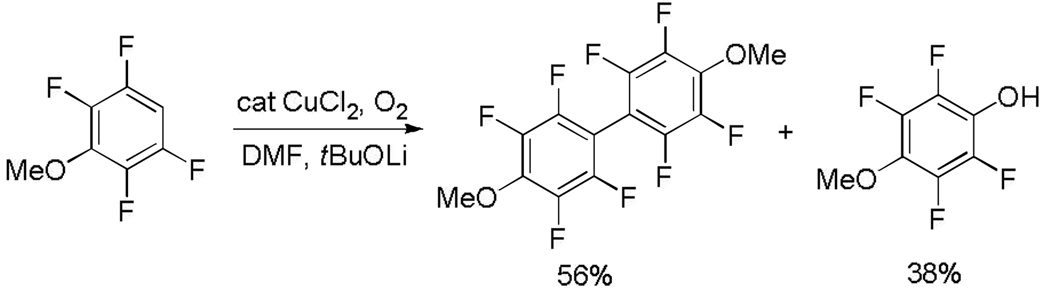
We have recently demonstrated that acidic sp2 C-H bonds can be arylated by aryl halides under copper catalysis (Scheme 2).8 The reaction involves generation of an organocopper intermediate followed by the reaction with aryl iodide affording a biaryl product. We speculated that under an oxygen atmosphere, the intermediate arylcopper species is expected to form biaryl and a low-valent copper complex. Regeneration of arylcopper by reaction of arylmetal with the low-valent Cu species would close the catalytic cycle. The initial experiments focused on the use of tBuOLi base that was earlier shown to be successful for copper-catalyzed deprotonative arylation. However, major amounts of phenol byproduct were formed upon reacting methoxytetrafluorobenzene with tBuOLi and catalytic CuCl2 under O2 atmosphere (Scheme 3). Formation of phenol byproducts has been reported in copper-catalyzed reaction of arylboronic acids with various nucleophiles under oxygen.9
Scheme 2.
Copper-catalyzed arylation of arene C-H bonds
Scheme 3.
Phenol byproduct formation
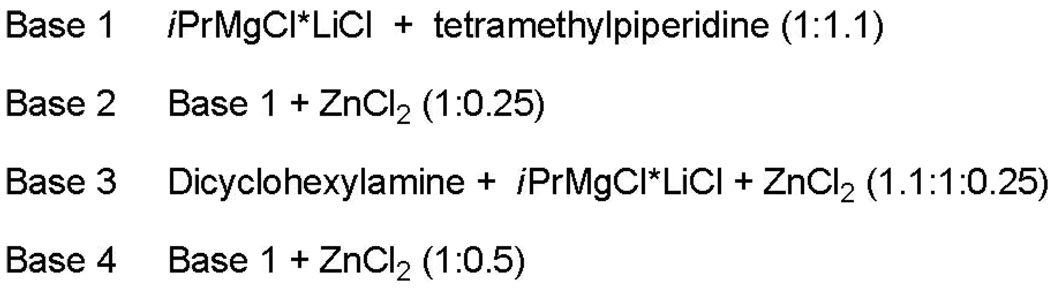
Phenol can be formed either by the direct reaction of ArLi intermediate with oxygen, or by reaction of a high-valent ArCu with hydroxide derived from water.9 A metal that binds hydroxide removing it from the reaction mixture is required to avoid phenol formation. Less polarized C-metal bond in the intermediate should also decrease the reactivity of arylmetal with oxygen. Magnesium t-butoxide was inefficient and a stronger base was required. Fortunately, hindered zinc and magnesium amide bases that have been extensively investigated by Knochel worked well.10 Exact composition of the base needs to be optimized for each substrate. Synthesis of bases used in dimerizations is presented in Scheme 4. Best results are obtained by employing tetramethylpiperidides.
Scheme 4.
Bases employed in dimerization
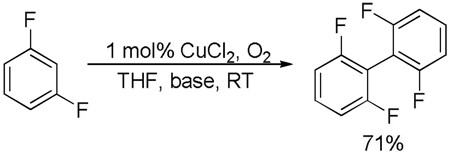
The results of the dimerization reactions are shown in Table 1. Reactions are run in THF solvent under oxygen atmosphere at 0–50 °C, typically at RT, and 1–3 mol % of CuCl2 catalyst is employed. Electron-rich heterocycles such as thiazole, benzofuran, 2-chlorothiophene, N-butylimidazole and triazole are dimerized in good yields (entries 1–5). Electron-poor heterocycles 2-methoxypyrazine and 3,5-dichloropyridine were also dimerized successfully (entries 6 and 7). Polyfluorinated arenes are reactive and tetrafluoroanisole was dimerized in 91% yield (entry 8). 1,3-Difluorobenzene afforded tetrafluorobiphenyl in 71% yield (entry 10). The reaction is tolerant to functional groups such as amino (entry 9), nitro (entry 11), cyano (entry 12), and ester (entry 13). For dimerization of 2-chlorothiophene and tetrafluoroanisole cheaper dicyclohexylamide bases can be employed. In other cases, tetramethylpiperidides afford better results.
Table 1.
Arene Dimerizationa
 | |||
|---|---|---|---|
| Entry | Ar-H, base | Product | Yield, % |
| 1 | Thiazole, base 4 | 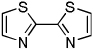 |
71 |
| 2 | Benzofuran, base 1 | 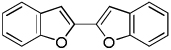 |
56 |
| 3 | 2-Chlorothiophene, base 3 | 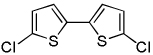 |
74 |
| 4 | N-Butylimidazole, base 1 | 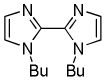 |
73 |
| 5 | N-Butyltriazole, base 1 | 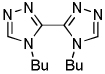 |
73 |
| 6 | Methoxypyrazine, base 1 + base 4 | 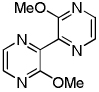 |
50 |
| 7 | 3,5-Dichloropyridine, base 2 | 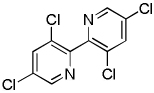 |
51 |
| 8 | Tetrafluoroanisole base 3 | 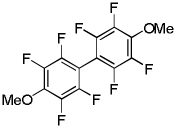 |
91 |
| 9 | Tetrafluoro-N,N-dimethylaniline, base 4 | 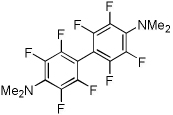 |
85 |
| 10 | 1,3-Difluorobenzene, base 2 | 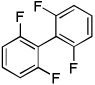 |
71 |
| 11 | 2,4-Difluoronitrobenzene, base 4 | 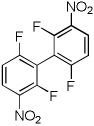 |
70 |
| 12 | 3,5-Difluorobenzonitrile, base 4 + base 1 | 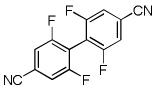 |
76 |
| 13 | Ethyl 3,4-difluorobenzoate, base 1 | 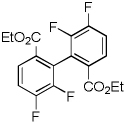 |
70 |
Substrate (1 equiv), base (1.2–1.5 equiv). Yields are isolated yields. See Supporting Information for details.
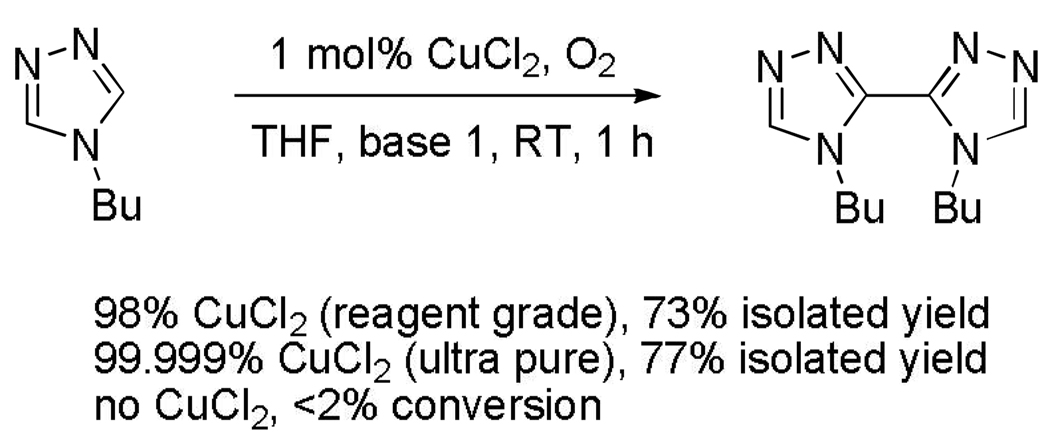
Control experiments were run to determine if trace of another transition metal catalyzes the dimerization (Scheme 5).11 With reagent grade or ultra-pure CuCl2 similar results were obtained showing that reactivity by contaminants is unlikely. If copper salt was omitted, no product was obtained.
Scheme 5.
Control Experiments
In conclusion, we have developed a general method for copper-catalyzed, deprotonative dimerization of arenes by employing oxygen as the terminal oxidant. Electron-rich and electron-poor heterocycles as well as electron-poor arenes are reactive. The method is tolerant to functionalities such as nitro, cyano, amino, and ester groups.
Supplementary Material
ACKNOWLEDGMENT
We thank the Welch Foundation (Grant No. E-1571), National Institute of General Medical Sciences (Grant No. R01GM077635), A. P. Sloan Foundation, Camille and Henry Dreyfus Foundation, and Norman Hackerman Advanced Research Program for supporting this research.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Lewis JC, Bergman RG, Ellman JA. Acc. Chem. Res. 2008;41:1013. doi: 10.1021/ar800042p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Campeau L-C, Fagnou K. Chem. Commun. 2006:1253. doi: 10.1039/b515481m. [DOI] [PubMed] [Google Scholar]; (c) Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem., Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/b606984n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Dick AR, Sanford MS. Tetrahedron. 2006;62:2439. [Google Scholar]; (f) Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]
- 2.Steinkopf W, Leitsmann R, Hofmann KH. Liebigs Ann. Chem. 1941;546:180. [Google Scholar]
- 3.(a) Phipps RJ, Gaunt MJ. Science. 2009;323:1593. doi: 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]; (b) Yoshizumi T, Tsurugi H, Satoh T, Miura M. Tetrahedron Lett. 2008;49:1598. [Google Scholar]; (c) Besselièvre F, Piguel S, Mahuteau-Betzer F, Grierson DS. Org. Lett. 2008;10:4029. doi: 10.1021/ol801512q. [DOI] [PubMed] [Google Scholar]; (d) Ackermann L, Potukuchi HK, Landsberg D, Vicente R. Org. Lett. 2008;10:3081. doi: 10.1021/ol801078r. [DOI] [PubMed] [Google Scholar]; (e) Li Z, Li C-J. J. Am. Chem. Soc. 2005;127:6968. doi: 10.1021/ja0516054. [DOI] [PubMed] [Google Scholar]; (f) Yotphan S, Bergman RG, Ellman JA. Org. Lett. 2009;11:1511. doi: 10.1021/ol900103a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Glaser C. Ber. Dtsch. Chem. Ges. 1869;2:422. [Google Scholar]; (b) Hay AS. J. Org. Chem. 1962;27:3320. [Google Scholar]
- 5.(a) Basle O, Li C-J. Chem. Commun. 2009:4124. doi: 10.1039/b905275e. [DOI] [PubMed] [Google Scholar]; (b) Chen X, Hao X-S, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:6790. doi: 10.1021/ja061715q. [DOI] [PubMed] [Google Scholar]; (c) Hamada T, Ye X, Stahl SS. J. Am. Chem. Soc. 2008;130:833. doi: 10.1021/ja077406x. [DOI] [PubMed] [Google Scholar]; (d) Monguchi D, Fujiwara T, Furukawa H, Mori A. Org. Lett. 2009;11:1607. doi: 10.1021/ol900298e. [DOI] [PubMed] [Google Scholar]; (e) Brasche G, Buchwald SL. Angew. Chem., Int. Ed. 2008;47:1932. doi: 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Dobereiner G, Hao X-S, Giri R, Maugel N, Yu J-Q. Tetrahedron. 2009;65:3085. [Google Scholar]
- 7.Pd-catalyzed arene intermolecular homo/heterodimerization: Okamoto M, Yamaji T. Chem. Lett. 2001:212. Brasche G, García-Fortanet J, Buchwald SL. Org. Lett. 2008;10:2207. doi: 10.1021/ol800619c. Hagelin H, Oslob JD, Åkermark B. Chem.-Eur. J. 1999;5:2413. Grignard homodimerization under O2: Cahiez G, Moyeux A, Buendia J, Duplais C. J. Am. Chem. Soc. 2007;129:13788. doi: 10.1021/ja075417k. Maji MS, Pfeifer T, Studer A. Angew. Chem., Int. Ed. 2008;47:9547. doi: 10.1002/anie.200804197. Phenol dimerization under Cu/O2: Sakamoto T, Yonehara H, Pac C. J. Org. Chem. 1994;59:6859. doi: 10.1021/jo961377j. Enolate homocoupling: DeMartino MP, Chen K, Baran PS. J. Am. Chem. Soc. 2008;130:11546. doi: 10.1021/ja804159y.
- 8.(a) Do H-Q, Daugulis O. J. Am. Chem. Soc. 2007;129:12404. doi: 10.1021/ja075802+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Do H-Q, Daugulis O. J. Am. Chem. Soc. 2008;130:1128. doi: 10.1021/ja077862l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Do H-Q, Khan RMK, Daugulis O. J. Am. Chem. Soc. 2008;130:15185. doi: 10.1021/ja805688p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) King AE, Brunold TC, Stahl SS. J. Am. Chem Soc. 2009;131:5044. doi: 10.1021/ja9006657. [DOI] [PubMed] [Google Scholar]; (b) Lam PYS, Bonne D, Vincent G, Clark CG, Combs AP. Tetrahedron Lett. 2003;44:1691. [Google Scholar]; (c) Demir AS, Reis Ö, Emruallahoglu M. J. Org. Chem. 2003;68:10130. doi: 10.1021/jo034680a. [DOI] [PubMed] [Google Scholar]
- 10.(a) Mosrin M, Knochel P. Org. Lett. 2009;11:1837. doi: 10.1021/ol900342a. [DOI] [PubMed] [Google Scholar]; (b) Dubbaka SR, Kienle M, Mayr H, Knochel P. Angew. Chem., Int. Ed. 2007;46:9093. doi: 10.1002/anie.200703097. [DOI] [PubMed] [Google Scholar]; (c) Wunderlich SH, Knochel P. Angew. Chem., Int. Ed. 2007;46:7685. doi: 10.1002/anie.200701984. [DOI] [PubMed] [Google Scholar]
- 11.Buchwald SL, Bolm C. Angew. Chem., Int. Ed. 2009;48:5586. doi: 10.1002/anie.200902237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.