Abstract
This article summarizes the emergency department approach to diagnosing cerebellar infarction in the patient presenting with vertigo. Vertigo is defined and identification of a vertigo syndrome is discussed. The differentiation of common vertigo syndromes such as benign paroxysmal positional vertigo, Meniere’s disease, migrainous vertigo, and vestibular neuritis is summarized. Confirmation of a peripheral vertigo syndrome substantially lowers the likelihood of cerebellar infarction, as do indicators of a peripheral disorder such as an abnormal head impulse test. Approximately 10% of patients with cerebellar infarction present with vertigo and no localizing neurologic deficits. The majority of these may have other signs of central vertigo, specifically direction-changing nystagmus and severe ataxia.
INTRODUCTION
While most patients who present to emergency departments (ED) with isolated vertigo have benign disorders, approximately 0.7–3% have cerebellar infarction.1,2 Because the symptoms of cerebellar infarction overlap substantially with benign conditions it is commonly overlooked, with a misdiagnosis rate estimated at 35%2. Patients with missed cerebellar infarction in general are at higher risk for complications, with a mortality rate possibly as high as 40%.3
Physical diagnosis is the most important diagnostic modality for cerebellar infarction. Resorting to computed tomography (CT) is insufficient because it is only 26% sensitive for acute stroke.4 In contrast, important physical signs are present in the majority of patients with cerebellar infarction.
This review will first address differentiation of cerebellar infarction from the four most common vertigo syndromes: benign paroxysmal positional vertigo (BPPV), Meniere’s disease, migrainous vertigo, and vestibular neuritis.5 Then we will review the physical diagnosis of cerebellar infarction. Finally, we will propose indications for neuroimaging.
Definition of Vertigo
Vertigo is defined as a pathologic illusion of movement.6 Most commonly experienced as a spinning sensation, it arises from a pathologic imbalance in the peripheral or central vestibular system. Patients will often merely report feeling dizzy, and further questioning is required to identify vertigo.7 Caution is advised in classifying the dizzy patient. While differentiating vertigo from imbalance, presyncope and lightheadedness was traditionally taught, studies show that patients use overlapping terms to describe their experience and even change their minds during a single clinical encounter.8 The best way for a clinician to identify a vertigo syndrome is to realize that while most patients will report the classic rotational vertigo, approximately 17% will not.9 These patients may report episodic imbalance or dizziness that is made worse with head movement.
Benign Paroxysmal Positional Vertigo
Benign paroxysmal positional vertigo is a distinct condition not typically confused with cerebellar infarction. Central mimics of BPPV have been described, but they tend to be caused by tumors rather than strokes, and are recognized by association with other abnormalities.10 Patients present with brief episodes of intense vertigo, precipitated by a change in position. The paroxysms of intense symptoms lasting less than a minute are defining, as is positional provocation. Patients are well until a head movement, usually vertical, precipitates the paroxysm of symptoms. In most cases BPPV is idiopathic, although 10% follow a bout of vestibular neuritis and 20% follow an episode of head trauma.11
The most common form of BPPV, caused by an otolith in the posterior semicircular canal, is diagnosed by finding torsional nystagmus on the Dix-Hallpike test.12 The examiner holds the seated patient’s head 45° to the left or right. This aligns the posterior semicircular canal in the vertical plane. The patient should be asked to hold onto the examiner’s arm for stability. The examiner then drops the patient back to the supine position, with the head hanging down off the stretcher 10°–30°. This causes a large rotation of the posterior semicircular canal within its own plane, moving the loose otolith and reproducing the symptoms. The test is considered positive if it provokes the characteristic torsional and vertical nystagmus.13
The sensitivity of the Dix-Hallpike maneuver for BPPV has not been well-described in the ED setting. Even without a positive Dix-Hallpike test, patients with BPPV can be differentiated from those with cerebellar infarction by their episodic and positional symptoms. Those who do not fit this description require consideration of alternative diagnoses.
Meniere’s Disease
Meniere’s disease is suspected in the patient who presents with simultaneous vertigo and cochlear complaints. Cochlear complaints can be hearing loss, tinnitus, or aural fullness. Also called endolymphatic hydrops, Meniere’s disease is thought to be caused by a buildup of fluid in the endolymphatic compartment of the inner ear. Episodes commonly last a few hours, although they can range from 20 minutes to a few days. Formal diagnosis requires hearing loss documented on audiologic examination on at least one occasion.14 Patients may have normal audiologic examination between episodes.
It is not common for a stroke to present with isolated vertigo and hearing loss, although case reports do exist.15 It occurs in only 0.3% of all brainstem infarctions and tends to present with complete ipsilateral deafness.16 In general, vertigo with hearing loss, unless it is with total ipsilateral deafness, indicates a peripheral disorder. The clinician should perform a thorough neurologic and neuro-otologic examination on these patients, as discussed below. Patients with a negative work-up for stroke should be referred to an otolaryngologist for further testing and consideration of alternative diagnoses, such as labyrinthitis and schwannoma.6,17 Patients with Meniere’s disease tend to have recurring episodes, so if the patient has not had previous episodes, labyrinthitis should be suspected instead. Labyrinthitis is thought to be the same disease process as vestibular neuritis, with the additional involvement of the auditory system.
Migrainous Vertigo
Despite being the second most common cause of vertigo seen in clinical practice, migrainous vertigo remains under-recognized.5 Its clinical spectrum can be elusive. Half of patients present without headache.18 Its presenting features can vary, not only among different patients but in the same patient over time. Some have aura while others do not. Some have photophobia during attacks while others do not. For some, the migrainous vertigo appears to be like an aura that lasts for a few minutes (18%), but for others the vertigo lasts for longer than 24 hours (27%).19 Physical examination should reveal a normal neurologic examination, including coordination and gait. Video oculography has demonstrated findings of central nystagmus, but this has not yet been demonstrated on bedside physical examination.20
Formal diagnostic criteria for migrainous vertigo have been proposed.21 Strict criteria require: 1) recurrent episodes of vertigo; 2) a formal migraine diagnosis by International Headache Society (I.H.S.) criteria; 3) a migraine symptom during the attack (e.g. headache, photophobia, or aura); and 4) the exclusion of other causes. The category of “probable migrainous vertigo” is used for patients with some elements in the presentation to suggest migraine but no other identifiable cause.
Cerebellar infarction is not expected to present with migraine-associated symptoms, so most patients with criteria for migrainous vertigo and a normal neurologic examination can be treated for their migraine process without further work-up.
Vestibular Neuritis
Vestibular neuritis is characterized by the acute vestibular syndrome, caused by decreased vestibular tone on one side. Etiology is currently thought to be viral.22 Vestibular neuritis is recognized by characteristic findings on history and ocular examination. The history typically reveals a gradual onset, unlike for stroke where symptoms reach maximal intensity at onset. In vestibular neuritis, symptoms peak during the first day and begin to improve within a few days, although full recovery takes weeks to months.6 The associated vertigo is persistent and ongoing, although like all forms of vertigo, positional exacerbation is characteristic, as any head movement amplifies the disparity in bilateral vestibular tone. Associated autonomic symptoms of nausea and vomiting are prominent.
General neurologic examination is normal, including motor, sensory, reflexes, cranial nerves and mental status. Most importantly, limb coordination is preserved on finger-to-nose, heel-to-shin, and rapid-alternating movement testing. Although some mild incoordination is expected, the patient should retain the ability to ambulate. Their central nervous system continues to integrate its somatosensory, visual, and proprioceptive information, coordinating ambulation.
The vestibulopathic patient has decreased vestibular tone on the affected side. This imbalance in tone normally occurs when someone turns their head away from that side, so the vestibulopathic patient experiences the world as if they were continually turning their head away from the affected side. The vestibulo-ocular reflex causes a compensatory slow drift of the eyes back toward the hypoactive ear, which is the slow phase of nystagmus. The fast phase of nystagmus is the corrective beating opposite the hypoactive ear.1 Its direction is both horizontal and torsional, and it is considered unidirectional, meaning regardless of where the patient looks, the direction of nystagmus will not change.22 It is accentuated when looking away from the hypoactive ear (Alexander’s Law). The patient feels this and will often shut her eyes when looking away from the hypoactive side. It should be remembered that unidirectional nystagmus can also occur in 46% of patients with cerebellar infarction so it cannot be used to confirm a peripheral disorder.23 However, there is one physical finding in particular that may provide some help.
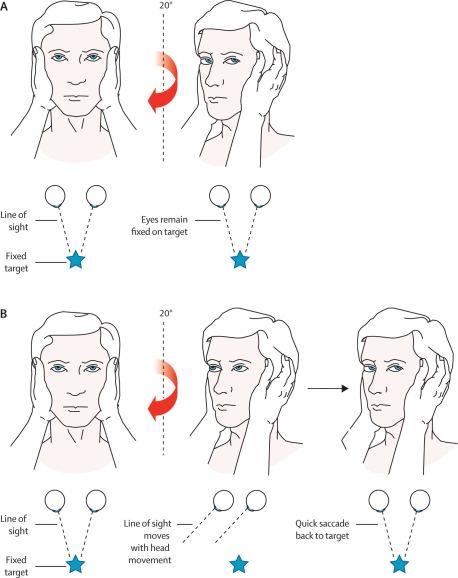
The vestibulo-ocular reflex (VOR) hinges eye movement to head movement. When a normal person looks to one side, the labyrinth on that side signals the turn, and the eyes automatically move opposite to maintain fixed gaze. For example, the reader can turn the head alternately to each side yet continue to read, because of the VOR. For the vestibulopathic patient, when the head is rapidly moved toward the pathologic side, the eyes move with the head and visual fixation is broken. A refixation (“catch-up”) saccade is seen as the patient looks back to the original object. This is tested by the head thrust test, also called the head impulse test.24 The patient’s head is placed midline, and the patient is instructed to maintain visual fixation on the examiner’s nose. The examiner thrusts the patient’s head quickly to one side, and if a refixation saccade back to the nose is seen, canal paresis is present on the side to which the head was turned (Figure 1). The test should be done on both sides. A video of the test being performed is available online.25, 26 In other settings it has been described as only 34–39% sensitive for vestibular hypofunction.27,28 When studied in more highly selected ED populations a positive head impulse test was 100% sensitive for a peripheral disorder.26 A negative head impulse test was found in 91 to 96% of patients with cerebellar infarction.23, 26 Further studies need to address the role of the head impulse test in undifferentiated ED patients with vertigo syndromes. Until that time it can only be said that a positive head impulse test is more consistent with acute peripheral vestibulopathy, such as vestibular neuritis, and a negative head impulse test raises the concern for cerebellar infarction.
Figure 1.
Head impulse test. A: The right ear has intact peripheral vestibular function. When the head is turned to the right, the vestibulo-ocular reflex moves the eyes to maintain visual fixation. B: The right ear now has impaired vestibular function. When the head is turned to the right, the eyes move with it, breaking visual fixation, and a refixation saccade is seen as the eyes dart back to the examiner’s face. This indicates a peripheral vestibular disorder on the right side. Reprinted from The Lancet Neurology, Vol. 7, Edlow JA, Newman-Toker DE, and Savitz SI, Diagnosis and initial management of cerebellar infarction”, Page No. 959, Copyright 2008, with permission from Elsevier.
Cerebellar Infarction
Cerebellar infarction represents approximately 2.3 % of acute strokes overall.29 These can result from occlusion of the superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), or the posterior inferior cerebellar artery (PICA). Larger cerebellar infarcts produce symptoms and signs localizing to the brainstem, such as diplopia, dysarthria, limb ataxia, dysphagia, and weakness or numbness. Approximately 10% of patients with cerebellar infarction can present with isolated vertigo, that is, vertigo with no localizing findings on motor, sensory, reflex, cranial nerve, or limb coordination examination. Most of these are infarcts of the medial branch of the PICA (96%).23
Patients with isolated vertigo due to cerebellar infarction pose a significant diagnostic challenge to the emergency physician (EP). It is known for being frequently misdiagnosed, often with consequent disability.3 While its infrequency precludes good ED studies on its presenting signs, the literature is sufficient to offer some historical and physical clues that may alert the clinician to the possibility of a cerebellar infarction.
First, stroke in general tends to present with the sudden and immediate onset of symptoms, usually reaching maximal intensity at once. Second, vascular risk factors raise the prior probability of disease. Hypertension and cardioaortic diseases are found in the majority of patients with cerebellar infarction, and an embolic source is found in 24–40%.23,29 Finally, two easily overlooked physical signs have been shown to indicate cerebellar infarction.
The first is severe ataxia, which has classically been considered a sign of central vertigo.6 Seventy-one percent of patients with cerebellar infarction and isolated vertigo will present with the inability to walk without support.23 Graded by two independent observers, this appears to be an objective and reproducible finding, although the observers were not blinded to the diagnosis. The other 29% have mild to moderate imbalance with ambulation, which would not in itself permit differentiation from vestibular neuritis.
The second important physical sign of cerebellar infarction is direction-changing nystagmus, also called multidirectional nystagmus, or gaze-evoked nystagmus. Such patients have nystagmus that changes directions according to the patient’s gaze. For example, if the patient looks to the right it beats to the right, and when the patient looks left it beats to the left. This was found to be 56% sensitive for cerebellar infarction, although the clinicians were not blinded to the diagnosis.23 The clinician should avoid the mistake of extreme lateral strain, as this produces end-point nystagmus, which is normal and reflects only muscle fatigue.30 Certain medications, especially anti-epileptics and alcohol, can cause nystagmus as well.
The inability to walk without support and direction-changing nystagmus are important signs because they are commonly present even when no other findings of brainstem ischemia are present. At least one of these two signs was seen in 84% (21 of 25) of the patients with cerebellar infarction and isolated vertigo.23
Indications for Neuroimaging
Evidence-based recommendations for neuroimaging in the vertiginous patient have not been established. Based upon currently available evidence, clear indications for neuroimaging include any focal neurologic deficit, the inability to walk without support, and direction-changing nystagmus.
When neuroimaging is indicated, diffusion-weighted magnetic resonance imaging (MRI) with magnetic resonance angiography is currently considered the optimal study. For hemorrhagic strokes, computed tomography (CT) and MRI are both excellent studies.31 However, for ischemic strokes, MRI is clearly superior with an 83% sensitivity compared to 26% for CT.4 Physicians should therefore not rely on CT scanning to rule out cerebellar infarction.
CONCLUSION
Cerebellar infarction is present in 3% of patients presenting with vertigo.1 Of these, only 10% lack focal neurologic deficits. Evidence cited in this review suggests that even when there are no neurologic deficits, most cases of cerebellar infarction will present with either the inability to walk without support or direction-changing nystagmus.23 In a patient with a low prior probability of stroke, the emergency physician will usually have good justification for discharging the patient who has isolated vertigo and a completely normal neurologic and neuro-otologic examination. One study of 15 cases of missed cerebellar infarction showed that all 15 lacked documented performance of standard neurologic examination and gait.3 Prompt evaluation by a neurologist or otolaryngologist is recommended for patients who have not received a definite diagnosis.
Table 1.
Red flags in vertigo
|
Footnotes
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
Supervising Section Editor: Kurt R. Denninghoff, MD
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
REFERENCES
- 1.Seemungal BM. Neuro-otological emergencies. Curr Opin Neurol. 2007;20:32–9. doi: 10.1097/WCO.0b013e3280122514. [DOI] [PubMed] [Google Scholar]
- 2.Kerber KA, Brown DL, Lisabeth LD, et al. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke. 2006;37:2484–7. doi: 10.1161/01.STR.0000240329.48263.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savitz SI, Caplan LR, Edlow JA. Pitfalls in the diagnosis of cerebellar infarction. Acad Emerg Med. 2007;14:63–8. doi: 10.1197/j.aem.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 4.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–8. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuhauser HK. Epidemiology of vertigo. Curr Opin Neurol. 2007;20:40–6. doi: 10.1097/WCO.0b013e328013f432. [DOI] [PubMed] [Google Scholar]
- 6.Hotson JR, Baloh RW. Acute vestibular syndrome. N Engl J Med. 1998;339:680–5. doi: 10.1056/NEJM199809033391007. [DOI] [PubMed] [Google Scholar]
- 7.Drachman DA, Hart CW. An approach to the dizzy patient. Neurology. 1972;22:323–34. doi: 10.1212/wnl.22.4.323. [DOI] [PubMed] [Google Scholar]
- 8.Newman-Toker DE, Cannon LM, Stofferahn ME, et al. Imprecision in patient reports of dizziness symptom quality: a cross-sectional study conducted in an acute care setting. Mayo Clin Proc. 2007;82:1329–40. doi: 10.4065/82.11.1329. [DOI] [PubMed] [Google Scholar]
- 9.Neuhauser HK, von Brevern M, Radtke A, et al. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurology. 2005;65:898–904. doi: 10.1212/01.wnl.0000175987.59991.3d. [DOI] [PubMed] [Google Scholar]
- 10.Baloh RW. Differentiating between peripheral and central cause of vertigo. Otolaryngol Head Neck Surg. 1998;119:55–59. doi: 10.1016/S0194-5998(98)70173-1. [DOI] [PubMed] [Google Scholar]
- 11.Furman JM, Cass SP. Benign paroxysmal positional vertigo. N Engl J Med. 1999;341:1590–6. doi: 10.1056/NEJM199911183412107. [DOI] [PubMed] [Google Scholar]
- 12.Dix MR, Hallpike CS. The pathology, symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med. 1952;45:341–354. doi: 10.1177/003591575204500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viirre E, Purcell I, Baloh RW. The Dix-Hallpike test and the canalith repositioning maneuver. Laryngoscope. 2005;115:184–7. doi: 10.1097/01.mlg.0000150707.66569.d4. [DOI] [PubMed] [Google Scholar]
- 14.Committee on Hearing and Equilibrium, American Academy of Otolaryngology-Head and Neck Foundation, Inc Guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. Otolaryngol Head Neck Surg. 1995;113:181–5. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 15.Son EJ, Bang JH, Kang JG. Anterior inferior cerebellar artery infarction presenting with sudden hearing loss and vertigo. Laryngoscope. 2007;117:556–8. doi: 10.1097/MLG.0b013e3180303ed0. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Baloh RW. Sudden deafness in vertebrobasilar ischemia: clinical features, vascular topographical patterns and long-term outcome. J Neurol Sci. 2005;228:99–104. doi: 10.1016/j.jns.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Kentala E. Characteristics of six otologic diseases involving vertigo. Am J Otol. 1996;17:883–92. [PubMed] [Google Scholar]
- 18.Brantberg K, Trees N, Baloh RW. Migraine-associated vertigo. Acta Otolaryngol. 2005;125:276–9. doi: 10.1080/00016480510003165. [DOI] [PubMed] [Google Scholar]
- 19.Neuhauser H, Lempert T. Vertigo and dizziness related to migraine: a diagnostic challenge. Cephalalgia. 2004;24:83–91. doi: 10.1111/j.1468-2982.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 20.von Brevern M, Zeise D, Neuhauser H, et al. Acute migrainous vertigo: clinical and oculographic findings. Brain. 2005;128:365–74. doi: 10.1093/brain/awh351. [DOI] [PubMed] [Google Scholar]
- 21.Olesen J. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8:9–96. [PubMed] [Google Scholar]
- 22.Baloh RW. Vestibular neuritis. N Engl J Med. 2003;348:1027–32. doi: 10.1056/NEJMcp021154. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Sohn SI, Cho YW, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. 2006;67:1178–83. doi: 10.1212/01.wnl.0000238500.02302.b4. [DOI] [PubMed] [Google Scholar]
- 24.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–9. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 25.Lewis RF, Carey JP. Abnormal eye movements associated with unilateral loss of vestibular function. N Engl J Med. 2006;355:e26. doi: 10.1056/NEJMicm031134. [DOI] [PubMed] [Google Scholar]
- 26.Newman-Toker DE, Kattah JC, Alvernia JE, et al. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. 2008;70:2378–85. doi: 10.1212/01.wnl.0000314685.01433.0d. [DOI] [PubMed] [Google Scholar]
- 27.Beynon GJ, Jani P, Baguley DM. A clinical evaluation of head impulse testing. Clin Otolaryngol. 1998;23:117–22. doi: 10.1046/j.1365-2273.1998.00112.x. [DOI] [PubMed] [Google Scholar]
- 28.Harvey SA, Wood DJ. The oculocephalic response in the evaluation of the dizzy patient. Laryngoscope. 1996;106:6–9. doi: 10.1097/00005537-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Tohgi H, Takahashi S, Chiba K, et al. Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients. The Tohoku Cerebellar Infarction Study Group. Stroke. 1993;24:1697–701. doi: 10.1161/01.str.24.11.1697. [DOI] [PubMed] [Google Scholar]
- 30.Leigh RJ, Rucker JC. Nystagmus and related ocular motility disorders. In: Miller NR, Newman NJ, editors. Walsh and Hoyt’s Clinical Neuro-Opthalmology. Baltimore, MD: Lippincott, Williams & Wilkins; 2004. [Google Scholar]
- 31.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–30. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]