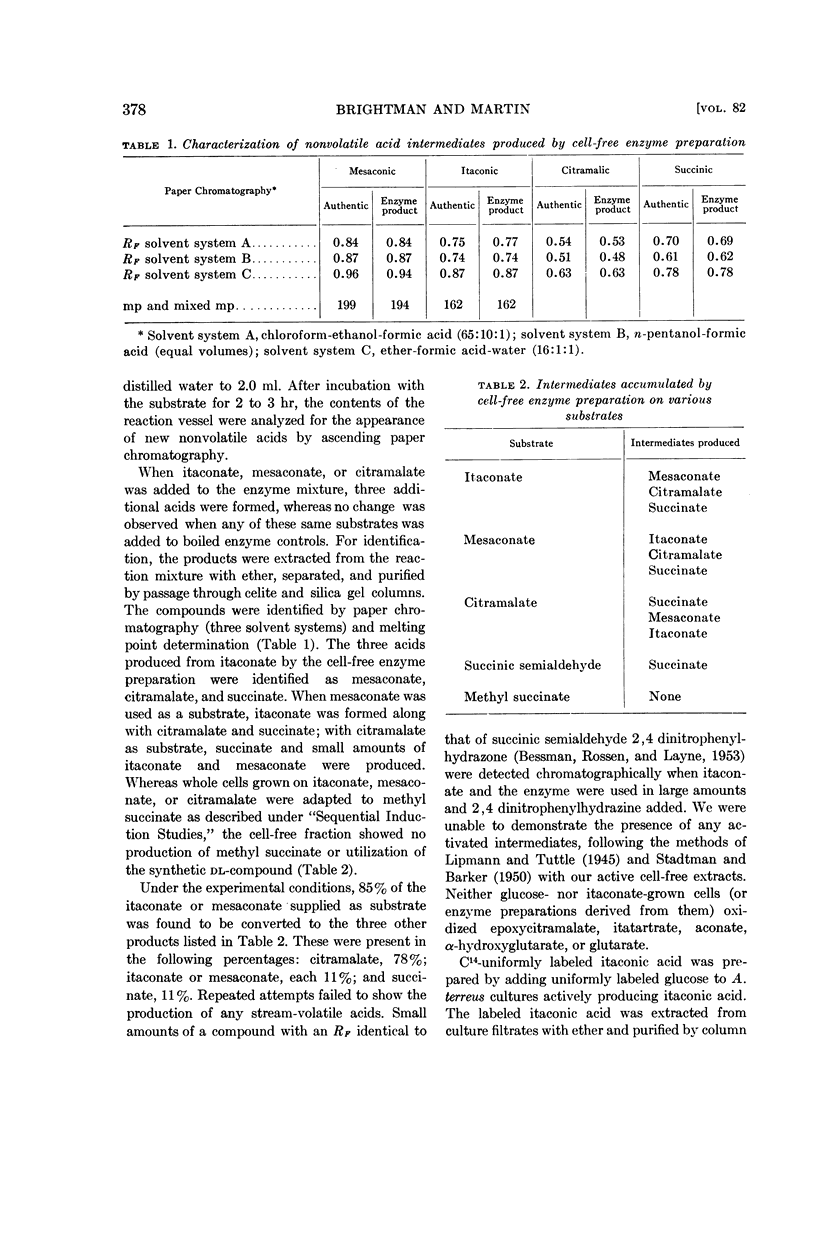
Abstract
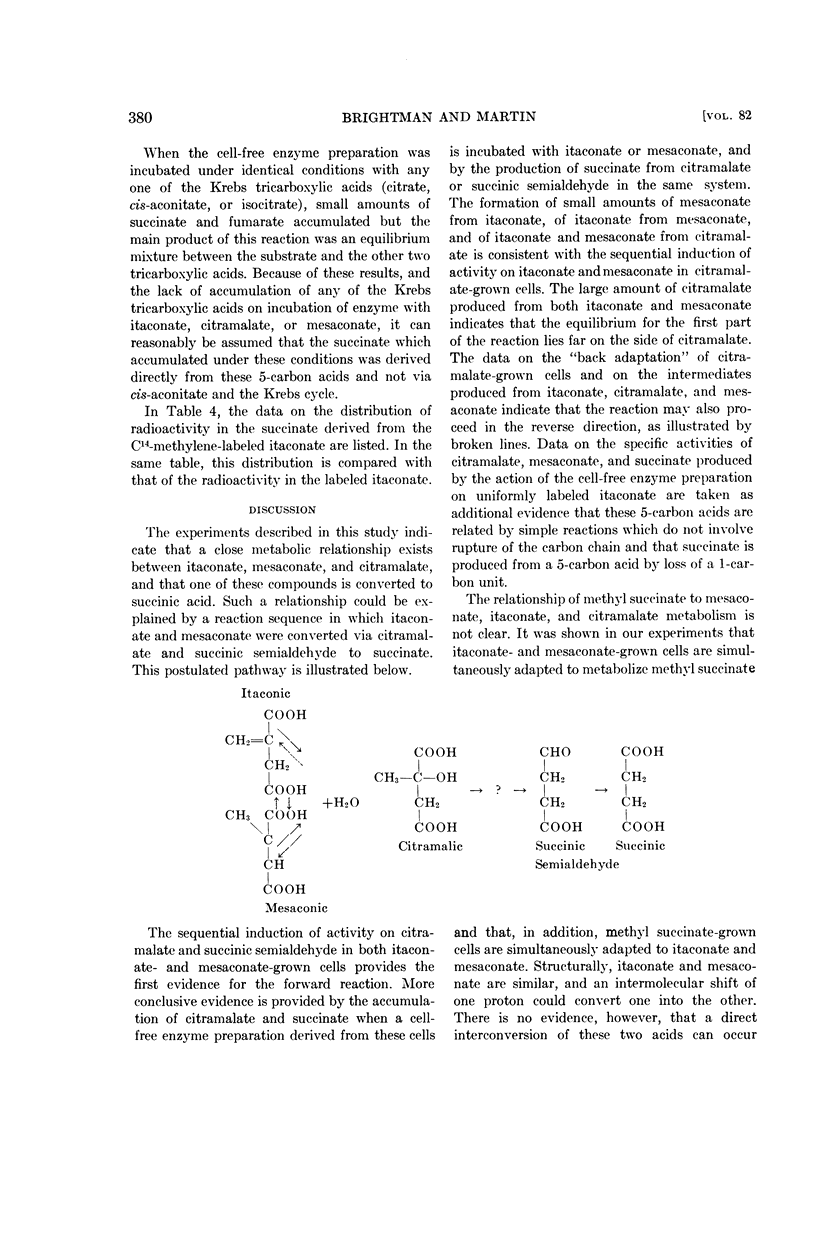
Brightman, Vernon (The University of Chicago, Chicago), and William R. Martin. Pathway for the dissimilation of itaconic and mesaconic acids. J. Bacteriol. 82:376–382. 1961.—Studies on the oxidation of itaconic and mesaconic acids by a Pseudomonas sp., adapted to utilize either of these acids as a sole carbon source, have provided evidence for a pathway converting both itaconate and mesaconate to succinate. A metabolic interconversion of itaconate, mesaconate, and citramalate has also been demonstrated by whole cell and cell-free enzyme studies.
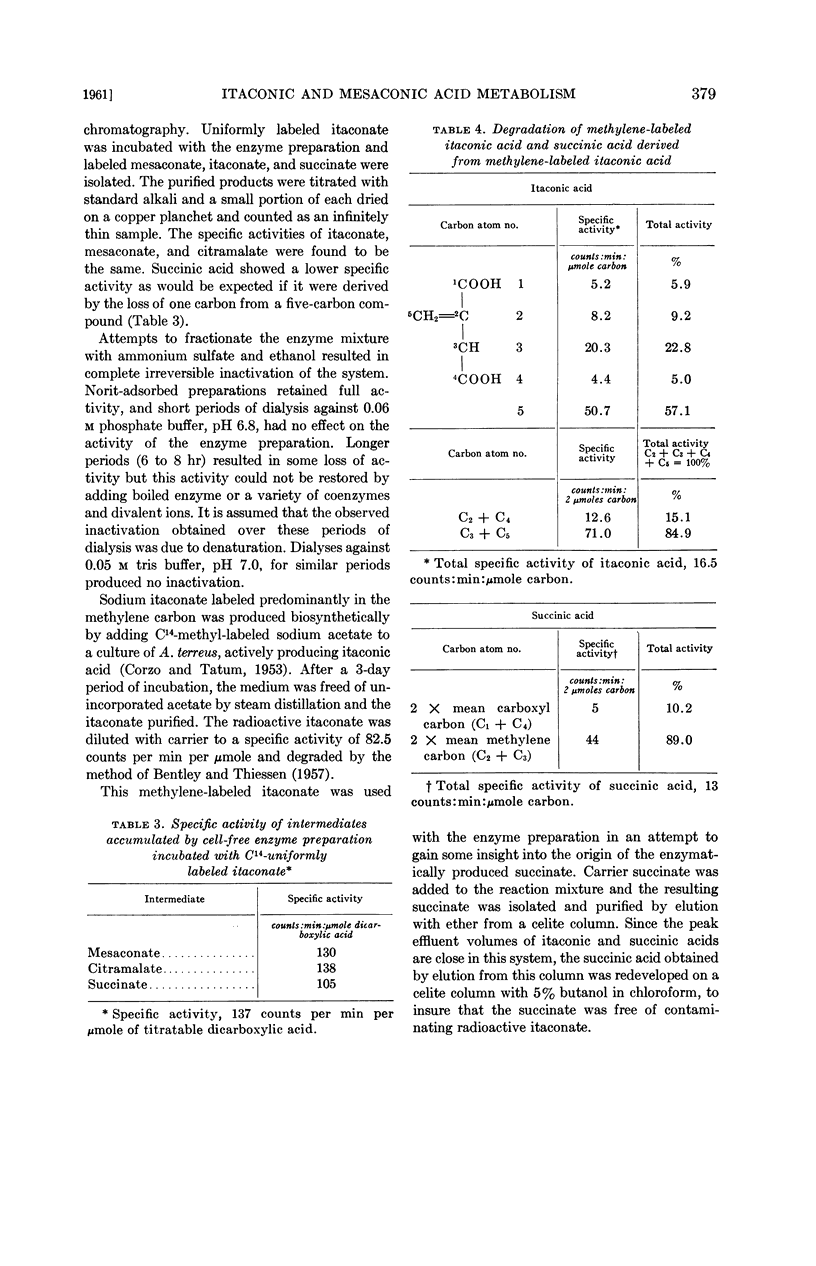
Succinate derived from methylene-labeled itaconate was found to be labeled in the inside carbon atoms, a fact which indicates that the branched chain compound was converted into a straight chain molecule by a shift of the methylene carbon (C-5) from the side chain of itaconate to a position between C-2 and C-3 in an, as yet, unknown straight chain intermediate prior to its conversion to succinate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER J., WANG S. F., LARDY H. A. The metabolism of itaconic acid by liver mitochondria. J Biol Chem. 1957 Dec;229(2):865–879. [PubMed] [Google Scholar]
- ARPAI J. Itaconicoxidase: an enzyme from an ultraviolet-induced mutant of Aspergillus terreus. Nature. 1958 Sep 6;182(4636):661–662. doi: 10.1038/182661a0. [DOI] [PubMed] [Google Scholar]
- BENTLEY R., THIESSEN C. P. Biosynthesis of itaconic acid in Aspergillus terreus. I. Tracer studies with C14-labeled substrates. J Biol Chem. 1957 Jun;226(2):673–687. [PubMed] [Google Scholar]
- BESSMAN S. P., ROSSEN J., LAYNE E. C. Gamma-Aminobutyric acid-glutamic acid transamination in brain. J Biol Chem. 1953 Mar;201(1):385–391. [PubMed] [Google Scholar]
- Barker H. A., Weissbach H., Smyth R. D. A COENZYME CONTAINING PSEUDOVITAMIN B(12). Proc Natl Acad Sci U S A. 1958 Nov 15;44(11):1093–1097. doi: 10.1073/pnas.44.11.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buston H. W. Note on the isolation of mesaconic acid from cabbage leaves. Biochem J. 1928;22(6):1523–1525. doi: 10.1042/bj0221523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASKINS R. H., THORN J. A., BOOTHROYD B. Biochemistry of the Ustilaginales. XI. Metabolic products of Ustilago zeae in submerged culture. Can J Microbiol. 1955 Dec;1(9):749–756. doi: 10.1139/m55-089. [DOI] [PubMed] [Google Scholar]
- ISENBERG H. D., SEIFTER E., BERKMAN J. I. The interrelationships of vitamin B12, beta-methylaspartate and thymine. Biochim Biophys Acta. 1960 Mar 25;39:187–189. doi: 10.1016/0006-3002(60)90150-5. [DOI] [PubMed] [Google Scholar]
- MUNCH-PETERSEN A., BARKER H. A. The origin of the methyl group in mesaconate formed from glutamate by extracts of Clostridium tetanomorphum. J Biol Chem. 1958 Feb;230(2):649–653. [PubMed] [Google Scholar]
- STADTMAN E. R., BARKER H. A. Fatty acid synthesis by enzyme preparations of Clostridium kluyveri. VI. Reactions of acyl phosphates. J Biol Chem. 1950 Jun;184(2):769–793. [PubMed] [Google Scholar]
- WANG S. F., ADLER J., LARDY H. A. The pathway of itaconate metabolism by liver mitochondria. J Biol Chem. 1961 Jan;236:26–30. [PubMed] [Google Scholar]


