Abstract
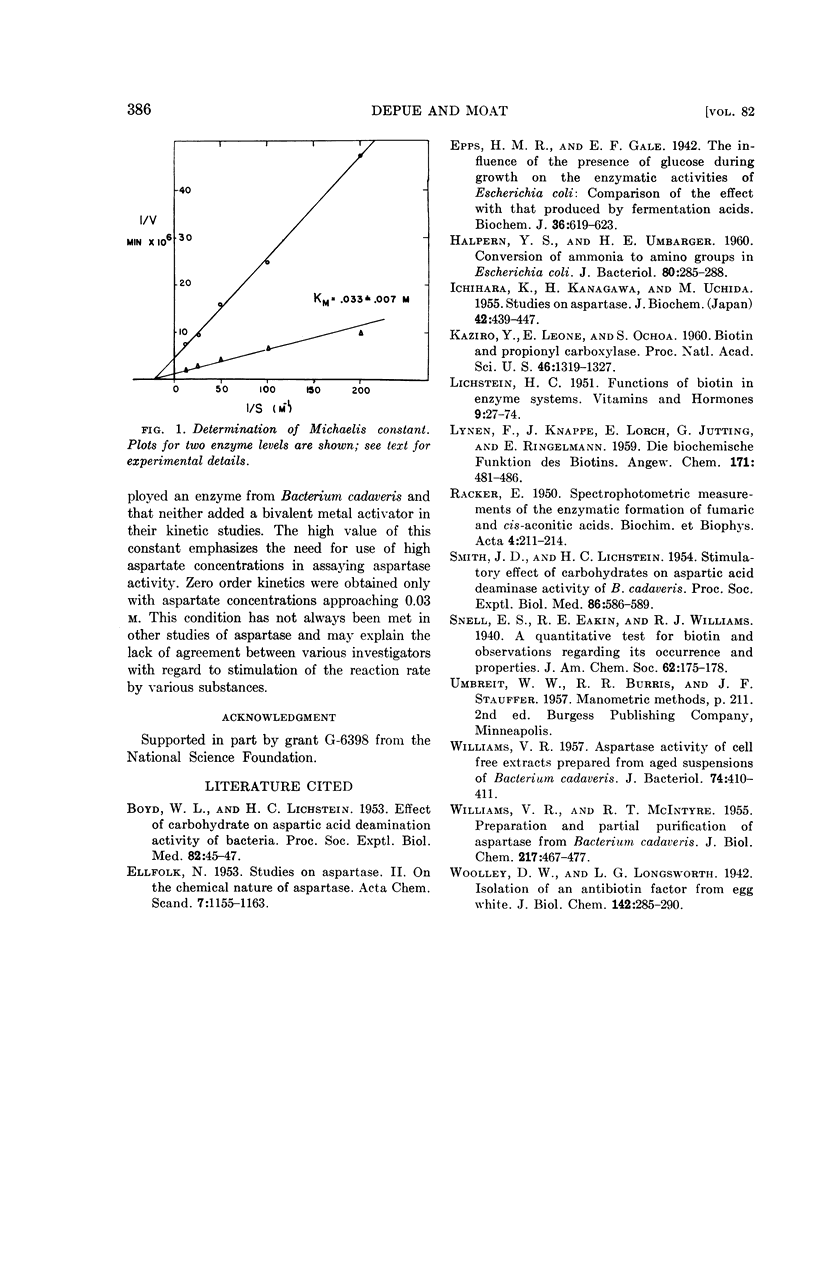
Depue, Robert H. (Hahnemann Medical College, Philadelphia), and Albert G. Moat. Factors affecting aspartase activity. J. Bacteriol. 82:383–386. 1961.—Cells of Escherichia coli grown in a glucose-mineral salts medium contain about one-fifth the amount of aspartase activity observed in cells grown in a yeast extract-peptone medium. The aspartase activity of the cells grown in glucose-salts medium would appear to be too low to provide a mechanism for synthesis of amino groups. Aspartase was purified approximately eightfold by ammonium sulfate precipitation and column chromatography of cell-free extracts. The purified preparation was specific for l-aspartic acid and contained no fumarase activity. A divalent metal ion requirement was demonstrated, this requirement being satisfied by cobaltous or manganous ions. The enzyme activity was found to be dependent upon free sulfhydryl groups. Biotin did not appear to be directly involved in the aspartase reaction since high concentrations of avidin did not alter the reaction rate. The Michaelis constant for aspartase with aspartic acid as substrate was determined to be 0.033 m.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
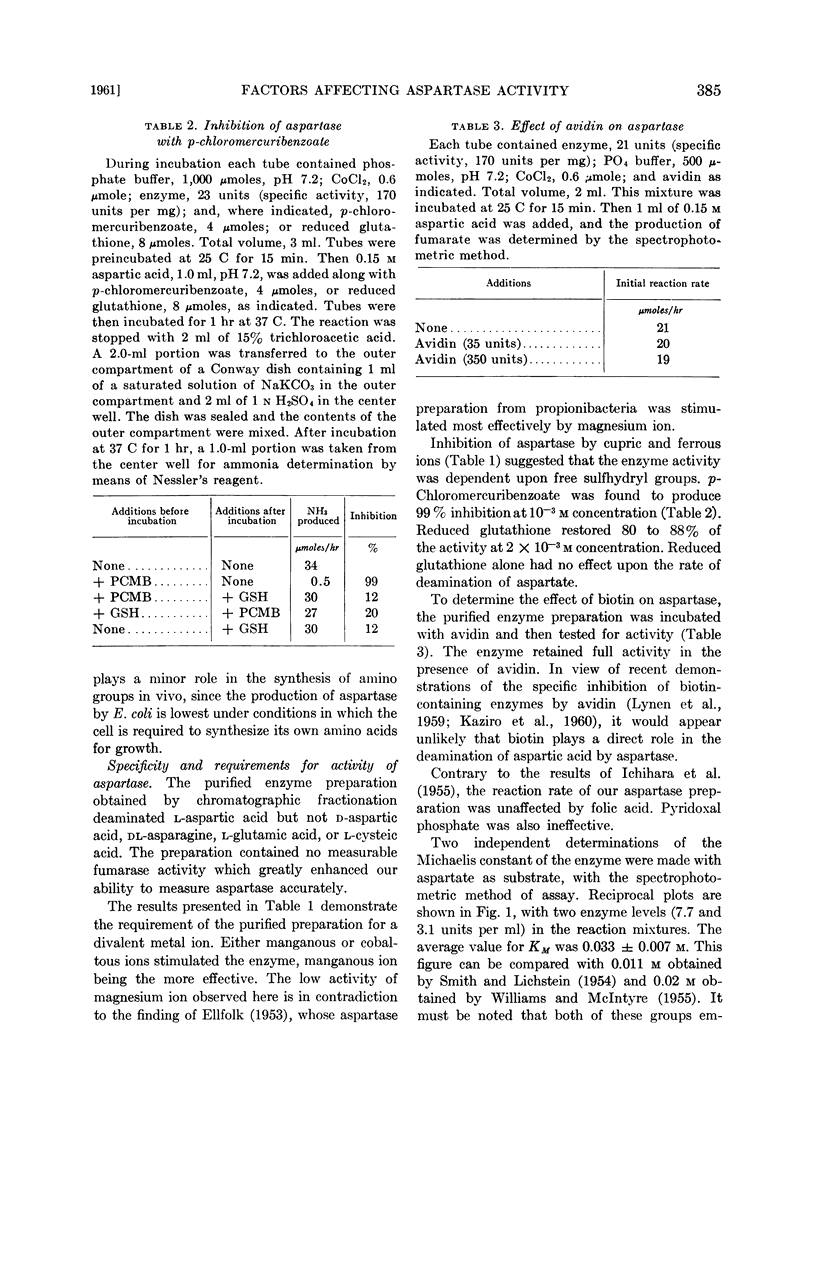
- BOYD W. L., LICHSTEIN H. C. Effect of carbohydrates on aspartic acid deaminase activity of bacteria. Proc Soc Exp Biol Med. 1953 Jan;82(1):45–47. doi: 10.3181/00379727-82-20018. [DOI] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Conversion of ammonia to amino groups in Escherichia coli. J Bacteriol. 1960 Sep;80:285–288. doi: 10.1128/jb.80.3.285-288.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaziro Y., Leone E., Ochoa S. BIOTIN AND PROPIONYL CARBOXYLASE. Proc Natl Acad Sci U S A. 1960 Oct;46(10):1319–1327. doi: 10.1073/pnas.46.10.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHSTEIN H. C. Functions of biotin in enzyme systems. Vitam Horm. 1951;9:27–74. doi: 10.1016/s0083-6729(08)60469-0. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- SMITH J. D., LICHSTEIN H. C. Stimulatory effect of carbohydrates on aspartic acid deaminase activity of Bacterium cadaveris. Proc Soc Exp Biol Med. 1954 Jul;86(3):586–589. doi: 10.3181/00379727-86-21172. [DOI] [PubMed] [Google Scholar]
- WILLIAMS V. R. Aspartase activity of cell free extracts prepared from aged cell suspensions of Bacterium cadaveris. J Bacteriol. 1957 Sep;74(3):410–411. doi: 10.1128/jb.74.3.410-411.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS V. R., MCINTYRE R. T. Preparation and partial purification of the aspartase of Bacterium cadaveris. J Biol Chem. 1955 Nov;217(1):467–477. [PubMed] [Google Scholar]


