Abstract
Children with single ventricle physiology have increased ventricular work and are at greater risk for developing heart failure than other children with congenital heart disease. However, diagnosis of heart failure is difficult because few objective measures have been validated in this cohort. Plasma proteins have been identified as biomarkers of heart failure in adults with structurally normal hearts. However, whether these similarly correlate with heart failure in children with single ventricle physiology is unknown, as the etiology of adult heart failure is typically ischemic heart disease, whereas heart failure in these children is presumed to be due to primary myocardial dysfunction. We conducted a single site cross-sectional observational study of young single ventricle patients. Clinical heart failure was defined as a Ross score >2. The association of several candidate biomarkers with heart failure was assessed using logistic regression and Receiver Operator Characteristic (ROC) curves. Nine of 29 included children (31%) were in clinical heart failure. A doubling of plasma B-type natriuretic peptide was associated with an odds ratio for heart failure of 2.17. The area under the ROC curve was 80.3%. A threshold value of ≥30 pg/mL showed both sensitivity and specificity for heart failure. Three other candidate biomarkers were not found to be associated with clinical heart failure in this sample. In conclusion, plasma B-type natriuretic peptide is a sensitive biomarker for clinical heart failure in young children with single ventricle heart disease. Use of this plasma biomarker may facilitate detection of heart failure in these complex patients.
Keywords: Single ventricle, heart failure, children, B-type natriuretic peptide
Introduction
While many biomarkers have been studied extensively in adults with structurally normal hearts, whether these correlate with heart failure (HF) in infants and young children with single ventricle physiology is not known, as the etiology of adult HF is typically coronary heart disease, whereas HF in these children is presumed to be due to primary myocardial dysfunction. Furthermore, in the single ventricle patient, ventricular “cross talk” 1 may be compromised or even absent. These important pathophysiological differences render it difficult to extrapolate adult data to pediatric single ventricle patients. We proposed that B-type natriuretic peptide (BNP), endothelin-1 (ET1), high sensitivity C-reactive protein (hsCRP) and/or cardiac troponin I (cTnI) plasma levels in children with single ventricle heart disease could serve as biomarkers for the presence of HF. To test this hypothesis, we measured the association of elevations in these plasma proteins with HF in a cohort of single ventricle children.
Methods
A single site cross-sectional observational study utilizing a secondary study base was conducted. All children 1 month – 7 years old with single ventricle physiology presenting to the UCSF Pediatric Heart Center between February 2007 and June 2008 were eligible for the study. Patients were excluded if they had trisomy 21, an acute intercurrent illness, a congenital defect that interfered with feeding (e.g., cleft palate, esophageal atresia), or had participated in an investigational drug or device study in the last 6 months. This study was approved by the UCSF Institutional Review Board, and written consent was obtained from the guardians of all subjects.
Each child was assigned a Ross score 2–4 by one of two study authors to determine the presence of clinical HF immediately prior to phlebotomy. The Ross score is a 12-point HF score based on historical data, vital signs, and physical exam findings that was developed for infants 2 and has been adapted for children 3,4. For this study, HF scoring was recorded independent of presumed mechanism, since the goal was to identify biomarkers in single ventricle patients that would be clinically useful across etiologies. The primary outcome was HF (Ross score 3–12) versus no HF (Ross score 0–2). Predictor measurement occurred subsequent to outcome determination, allowing assessors to be blinded to plasma protein levels.
At the time of cardiac catheterization, pre-operative evaluation for cardiac surgery, or medical admission, 6 mL of whole blood were obtained in conjunction with clinically indicated phlebotomy. Plasma BNP, cTnI, ET1 and hsCRP were measured following assignment of the Ross score. For cTnI and hsCRP, 2 mL of whole blood were sent to the UCSF Clinical Laboratory for assay by fluorescent enzyme immunoassay and rate turbidimetry, respectively. For BNP and ET1, plasma was collected from 4 mL of whole blood within 2 hours and stored at −80°C prior to protein assay. BNP was assayed using the Biosite Triage kit (Biosite Diagnostic, San Diego, CA) as previously described 5. Plasma ET1 was assayed as modified from published methods 6. Inter-assay and intra-assay variabilities were 10% and 4% respectively. Each sample was assayed in duplicate. Additional data collected included patient name, medical record number, sex, age, weight, single ventricle morphology, diagnosis, surgical history, and current medications.
The primary outcome was the presence of clinical HF. The relationship between raw Ross score (0–12) and plasma proteins was summarized using scatter plots, and analyzed using simple linear regression. Model checking was performed using component-plus-residual plots, DFBETA statistics, and residual plots. Single-predictor logistic regression was used to assess the crude association between each of the four cardiac-related plasma proteins and clinical HF. A p-value <0.05 was considered statistically significant. Receiver Operator Characteristic (ROC) curves were created to evaluate these proteins as potential biomarkers. Assays were deemed potentially useful if they carried a c-statistic ≥0.75 (corresponding to ≥75% of the graph area encompassed by the curve). For a potentially useful test, a clinically relevant cut point was identified that would distinguish single ventricle children in HF from those free of HF at the time of evaluation. All statistical analyses were performed using Stata 10 (StataCorp LP, College Station, TX).
Results
We approached 39 single ventricle children meeting the inclusion criteria for this study and presenting to the UCSF Pediatric Heart Center between February 2007 and June 2008 for enrollment. Four (10%) declined, and of the 35 remaining subjects, 6 (15%) were subsequently excluded from analysis because of missing data. Thus, 29 children were studied (Table). We obtained blood samples at the time of cardiac catheterization in 26 of 29 (90%). Of the remaining 3, 2 were drawn immediately prior to cardiac surgery, and 1 was drawn in the course of medical admission for HF.
Table.
Patient data
| Medications |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Dominant Ventricle | Ross Score | Age (mo) | Wt (kg) | Sex | Surgical Palliation* | Diuretics | Angiotensin converting enzyme inhibitor | Digoxin | Intravenous inotropic agents |
| Heart Failure (Ross Score ≥3) | ||||||||||
| Aortic and mitral atresia | Right | 6 | 5 | 6.3 | M | Stage I | X | X | ||
| Aortic and mitral atresia | Right | 3 | 6 | 5.6 | M | Stage I | X | X | ||
| Aortic and mitral atresia | Right | 9 | 24 | 11.0 | M | Stage II | X | X | X | X |
| Double outlet right ventricle | Right | 4 | 73 | 15.7 | M | Stage II | X | X | ||
| Unbalanced atrioventricular canal | Right | 3 | 6 | 5.3 | M | Stage II | ||||
| Unbalanced atrioventricular canal | Right | 8 | 42 | 17.0 | M | Stage II | X | X | X | X |
| Double inlet left ventricle | Left | 3 | 24 | 9.5 | M | Stage II | X | |||
| Double inlet left ventricle | Left | 6 | 54 | 12.4 | M | Stage II | X | |||
| Primitive ventricle | Indeterminate | 4 | 16 | 8.7 | M | Stage II | X | X | X | |
| No Heart Failure (Ross Score ≤2) | ||||||||||
| Aortic and mitral atresia | Right | 0 | 4 | 5.0 | M | Stage I | X | |||
| Aortic and mitral atresia | Right | 0 | 71 | 16.7 | M | Stage III | X | |||
| Aortic and mitral stenosis | Right | 1 | 4 | 6.0 | M | Stage I | X | |||
| Aortic and mitral stenosis | Right | 1 | 4 | 6.1 | M | Stage I | X | |||
| Aortic and mitral stenosis | Right | 1 | 4 | 5.6 | F | Stage I | X | X | ||
| Aortic and mitral stenosis | Right | 2 | 42 | 13.6 | M | Stage II | X | X | X | |
| Interrupted aortic arch | Right | 2 | 4 | 5.2 | F | Stage I | X | X | ||
| Double outlet right ventricle | Right | 1 | 29 | 19.0 | F | Stage II | ||||
| Interrupted aortic arch | Right | 2 | 4 | 5.2 | F | Stage I | X | X | ||
| Unbalanced atrioventricular canal | Right | 1 | 60 | 17.0 | M | Stage II | X | X | ||
| Unbalanced atrioventricular canal | Right | 2 | 5 | 5.2 | F | Stage I | X | X | X | |
| Unbalanced atrioventricular canal | Right | 2 | 40 | 15.4 | M | Stage II | ||||
| Interrupted aortic arch | Left | 2 | 5 | 5.7 | F | Stage I | X | X | ||
| Pulmonary atresia/intact ventricular septum | Left | 2 | 69 | 16.0 | M | Stage II | ||||
| Pulmonary atresia/ventricular septal defect | Left | 1 | 54 | 15.0 | M | Stage II | X | |||
| Tricuspid atresia | Left | 0 | 3 | 5.0 | M | Stage I | X | |||
| Tricuspid atresia | Left | 0 | 4 | 5.3 | F | Stage I | X | |||
| Tricuspid atresia | Left | 0 | 6 | 7.7 | M | Stage II | X | |||
| Tricuspid atresia | Left | 2 | 42 | 19.4 | F | Stage II | X | |||
| Primitive ventricle | Indeterminate | 2 | 66 | 17.0 | M | Stage II | X | X | ||
Stage I: stabilization of aortic and pulmonary blood flow, e.g., Norwood, pulmonary artery band, Blalock-Taussig shunt, central shunt; Stage II: establishment of partial cavopulmonary circulation between the superior vena cava and pulmonary arteries, e.g., Glenn shunt, Kawashima; Stage III: completion of cavopulmonary circulation, e.g., Fontan.
Of 29 children, 22 (76%) had systemic non-left ventricles. The median Ross score in the overall sample was 2, with a standard deviation of 2.3 Ross units. Among the 20 children free of clinical HF, the median Ross score was 1, with a standard deviation of 0.8 Ross units. Among the 9 children with clinical HF (Ross score >2), the median score was 4, with a standard deviation of 2.3 Ross units and a range of 3 to 9. The overall prevalence of HF in our included sample was 31%.
Only 4 of the 29 children (14%) were taking no cardiac medications at the time of the study. Diuretics were the most common cardiac medications (69%), followed by angiotensin converting enzyme inhibitors (55%). Two children were on inotropic therapy at the time of the study. Not surprisingly, these children were both in HF. Digoxin use was more prevalent in children free of HF.
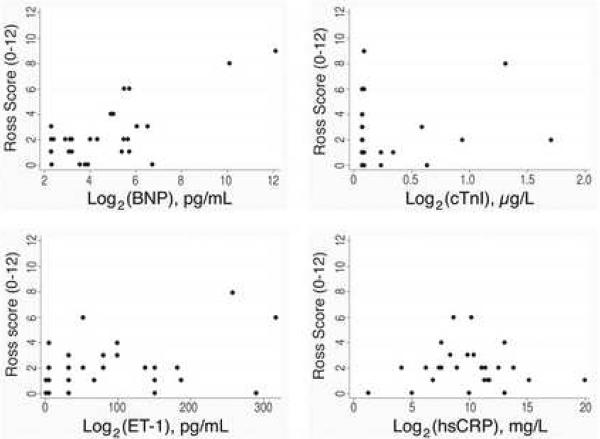
Figure 1 shows scatter plots of our candidate biomarkers versus the Ross HF score. The scatter plots provide a visual appraisal of the linearity of the relationship between the candidate biomarkers and the Ross HF score. Notably, after log2 transformation, BNP demonstrated a roughly linear relationship with the Ross score. A one unit increase on this scale corresponds to a doubling on the linear scale. Simple linear regression analysis on the transformed variable revealed that a doubling of plasma BNP was associated with a 0.7 unit increase in Ross score (95% CI 0.4, 1.0; p<0.001). Component-plus-residual plots demonstrated that the assumption of linearity had not been violated, and the residuals were normally distributed, suggesting that the linear model is a good fit for these data. Although DFBETA statistics revealed two potentially influential points, exclusion of these points preserved the linear association: a doubling of plasma BNP was still associated with a 0.4 unit increase in Ross score, with the 95% CI encompassing zero (95% CI −0.05, 0.8; p=0.082).
Figure 1. Scatter plots of biomarkers versus Ross scores.
Scatter plots of plasma protein concentrations versus raw Ross scores in 29 single ventricle patients. Log2 transformation of BNP revealed a roughly linear relationship with raw Ross score, while this was not the case for the other plasma proteins studied.
For hsCRP, a 0.2 mg/L increase was associated with a 0.8 unit increase in Ross score (95% CI −0.03, 1.6; p=0.058), however, DFBETA statistics and component-plus-residual plots revealed three highly influential points. Exclusion of these points abolished the association. Neither ET1 (20 unit increase associated with 0.07 decrease in Ross score, 95% CI (−0.3, 0.15), p=0.50) nor cTnI (0.1 microgram/L increase associated with a 0.07 unit increase in Ross score, 95% CI (−0.2, 0.3), p=0.61) was associated with increased Ross score in our sample. Exclusion of influential data points for these assays did not alter the results.
Using single-predictor logistic regression, a doubling of plasma BNP was associated with an odds ratio for HF of 2.17 (95% CI 1.10, 4.3; p=0.026). A 0.2 mg/L increase in hsCRP was associated with an odds ratio for HF of 1.76 (95% CI 0.69, 4.5; p=0.23), suggesting that elevations in hsCRP may be associated with increased odds of HF, but this result had poor precision (31–350% increase in odds). Likewise, neither cTnI (OR=0.72, 95% CI 0.05, 10; p=0.81) nor ET1 (OR=0.86, 95% CI 0.54, 1.36; p=0.52) was associated with clinically relevant alterations in the odds of HF.
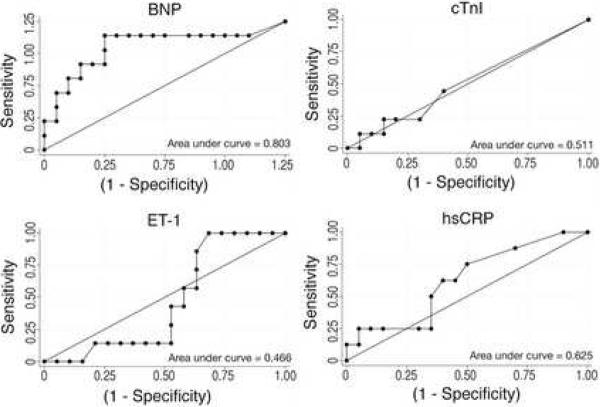
We generated Receiver Operator Characteristic (ROC) curves, which plot a test's true positives against its false positives, for each of the four potential biomarkers (Figure 2). Only one protein, BNP, exceeded our pre-specified threshold of ≥75% area contained by the ROC curve. The area under the curve for the plot of BNP against HF was 80.3%. cTnI failed to distinguish children in HF from those free of failure. Similarly, hsCRP and ET1 demonstrated relatively poor sensitivities and specificities for HF throughout their curves.
Figure 2. Receiver Operator Characteristic curves for BNP, cTnI, hsCRP and ET1.
False positives (1-Specificity) were plotted against true positives (Sensitivity). A test for which the area under the curve (c-statistic) is ≥0.75 was defined as a useful test.
The test characteristics for various plasma levels of BNP as markers of current clinical HF were determined. A cut point of ≥30 pg/mL showed both sensitivity (88.9%; 95% CI 51.8% – 99.7%) and specificity (75%; 95% CI 50.9% – 91.3%). The wide 95% confidence intervals demonstrate that our results are consistent with sensitivities as low as 51.8%. However, our sample consists of predominantly mild HF, and as such, we would expect this to be a relatively difficult population to detect. Given the roughly linear relationship between BNP doubling and raw Ross score, we expect BNP values to be higher in moderate than in mild failure. Thus, lowering the cut point further would increase the false positive rate without increasing detection of moderate HF. As such, we believe a cut point of ≥30 pg/mL presents the best threshold for identifying HF in young children with single ventricle physiology.
Discussion
The present study is the only one reported to date that shows a direct relation between clinical HF scores and BNP values in young children with single ventricle physiology (see Figure 2). We have suggested a cut point of ≥30 pg/mL for distinguishing between HF and non-failure in this population, which is distinct from the cut point value of 100 pg/mL established for adults 7. However, 30 pg/mL is also substantially higher than previously published normal values observed in similarly aged children with normal hearts (5.1–12.1 pg/mL) 8. We believe that this value should form the basis of future validation research and longitudinal studies needed to determine the value of BNP in monitoring severity of HF.
Despite published literature describing increased ET1 levels in adult patients with heart failure 9 and in the setting of pulmonary overcirculation, we did not find an association with clinical HF in patients with single ventricle physiology. In keeping with this, the ratio of pulmonary to systemic blood flow (Qp:Qs) assessed at the time of cardiac catheterization was not predictive of Ross score (data not shown).
hsCRP had been identified previously as a biomarker of both acute and chronic HF in adults, and is proposed to be elevated due to the general inflammatory response that accompanies these syndromes 10. We found no such association in our study, possibly because such elevations reflect inflammatory states that predispose to atherosclerotic plaque formation 11. Since atherogenesis is not a typical mechanism of HF in children with single ventricle heart disease, it is perhaps not surprising that hsCRP did not correlate with HF in this population.
cTnI also failed to distinguish single ventricle patients with HF from those without. Notably, despite the stresses of both pre- and post-Glenn physiology, cTnI levels were below the threshold of detection in most of our cohort. Since cTnI has been best described as a marker of ischemia 12, we wondered whether single ventricle patients with aortic atresia, who may have an increased risk of developing HF from ischemia, would demonstrate higher cTnI levels, but the present study lacked the power to examine this subgroup.
Our study had several limitations. First, the Ross score has not been specifically validated in the single ventricle population. However, it has been utilized in several previous studies of HF, including a recent multi-center trial of carvedilol therapy for pediatric HF that included single ventricle patients 13–15. As such, we considered it the most relevant outcome measure for this study.
Fifteen percent (6/39) of the children approached for the study—14% (5/35) of the consenting study subjects—did not have plasma drawn despite providing consent. This was typically due to logistical issues related to maintaining intravenous access in hospitalized children and laboratory hours. While this missing data could be a source of bias in our study, those 5 children with missing data were similar to the overall sample with regard to age, gender, weight, morphology of systemic ventricle, and medication usage. Because our ability to obtain samples was not related to a child's HF status, these missed data degraded our power, but should not have led to spurious associations. Rather, we may have failed to detect an association between hsCRP or ET1 and HF.
Finally, our sample was predominantly composed of children with systemic right ventricles, which limits our ability to comment on the accuracy of BNP for detecting HF of the single left ventricle. However, because single right ventricles are more difficult to evaluate functionally using current methods such as echocardiography, BNP has the potential to be particularly useful in children with a single right ventricle.
Acknowledgments
This work was supported by funds from the Lorraine Newman Fund of the UCSF Division of Pediatric Cardiology and a Strategic Opportunity Award from the UCSF Clinical & Translational Science Institute (NIH/NCRR UL RR024131) to H.S.B., and from Biosite Diagnostic to J.R.F. A.S. was supported by a postdoctoral fellowship from NHLBI (HL007544). A.M.F. was supported by a UCSF PACCTR Fellowship through the UCSF Clinical & Translational Science Institute (NIH TL1 RR024129).
The authors thank the UCSF pediatric cardiology fellows, the UCSF Congenital Cardiac Catheterization Laboratory staff, Kevin Swiryn, and the UCSF CTSI Biostatistics, Research Ethics, and Design Program for their invaluable assistance with the study. We also thank Julien I. E. Hoffman for biostatistical review, and J. Eduardo Rame and David F. Teitel for helpful discussions and critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamaguchi S, Harasawa H, Li KS, Zhu D, Santamore WP. Comparative significance in systolic ventricular interaction. Cardiovasc Res. 1991;25:774–783. doi: 10.1093/cvr/25.9.774. [DOI] [PubMed] [Google Scholar]
- 2.Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol. 1992;13:72–75. doi: 10.1007/BF00798207. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone DE, Abdulla A, Arnold JM, Bernstein V, Bourassa M, Brophy J, Davies R, Gardner M, Hoeschen R, Mickleborough L, et al. Diagnosis and management of heart failure. Canadian Cardiovascular Society. Can J Cardiol. 1994;10:613–631. 635–654. [PubMed] [Google Scholar]
- 4.Rosenthal D, Chrisant MR, Edens E, Mahony L, Canter C, Colan S, Dubin A, Lamour J, Ross R, Shaddy R, Addonizio L, Beerman L, Berger S, Bernstein D, Blume E, Boucek M, Checchia P, Dipchand A, Drummond-Webb J, Fricker J, Friedman R, Hallowell S, Jaquiss R, Mital S, Pahl E, Pearce B, Rhodes L, Rotondo K, Rusconi P, Scheel J, Pal Singh T, Towbin J. International Society for Heart and Lung Transplantation: Practice guidelines for management of heart failure in children. J Heart Lung Transplant. 2004;23:1313–1333. doi: 10.1016/j.healun.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Hsu JH, Oishi PE, Keller RL, Chikovani O, Karl TR, Azakie A, Adatia I, Fineman JR. Perioperative B-type natriuretic peptide levels predict outcome after bidirectional cavopulmonary anastomosis and total cavopulmonary connection. J Thorac Cardiovasc Surg. 2008;135:746–753. doi: 10.1016/j.jtcvs.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Xuan YT, Whorton AR, Shearer-Poor E, Boyd J, Watkins WD. Determination of immunoreactive endothelin in medium from cultured endothelial cells and human plasma. Biochem Biophys Res Commun. 1989;164:326–332. doi: 10.1016/0006-291x(89)91721-x. [DOI] [PubMed] [Google Scholar]
- 7.Maisel A. B-type natriuretic peptide measurements in diagnosing congestive heart failure in the dyspneic emergency department patient. Rev Cardiovasc Med. 2002;3(Suppl 4):S10–17. [PubMed] [Google Scholar]
- 8.Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–878. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinugawa T, Kato M, Ogino K, Osaki S, Igawa O, Hisatome I, Shigemasa C. Plasma endothelin-1 levels and clinical correlates in patients with chronic heart failure. J Card Fail. 2003;9:318–324. doi: 10.1054/jcaf.2003.39. [DOI] [PubMed] [Google Scholar]
- 10.Windram JD, Loh PH, Rigby AS, Hanning I, Clark AL, Cleland JG. Relationship of high-sensitivity C-reactive protein to prognosis and other prognostic markers in outpatients with heart failure. Am Heart J. 2007;153:1048–1055. doi: 10.1016/j.ahj.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 11.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavoinne A, Cauliez B. Cardiac troponin I and T: specific biomarkers of cardiomyocyte. Rev Med Interne. 2004;25:115–123. doi: 10.1016/s0248-8663(03)00218-2. [DOI] [PubMed] [Google Scholar]
- 13.Yeh JL, Hsu JH, Dai ZK, Liou SF, Chen IJ, Wu JR. Increased circulating big endothelin-1, endothelin-1 and atrial natriuretic peptide in infants and children with heart failure secondary to congenital heart disease. Int J Cardiol. 2005;104:15–20. doi: 10.1016/j.ijcard.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Ratnasamy C, Kinnamon DD, Lipshultz SE, Rusconi P. Associations between neurohormonal and inflammatory activation and heart failure in children. Am Heart J. 2008;155:527–533. doi: 10.1016/j.ahj.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]