Abstract
Cell-cell signaling regulated by retinoic acid (RA), Wnt/β-catenin, and fibroblast growth factor (FGF) is important during body axis extension, and interactions between these pathways have been suggested. At early somite stages, Wnt/β-catenin and FGF signaling domains exist both anterior and posterior to the developing trunk, whereas RA signaling occurs in between in the trunk under the control of the RA-synthesizing enzyme retinaldehyde dehydrogenase-2 (Raldh2). Previous studies demonstrated that vitamin A deficient quail embryos and Raldh2−/− mouse embryos lacking RA synthesis exhibit ectopic expression of Fgf8 and Wnt8a in the developing trunk. Here, we demonstrate that Raldh2−/− mouse embryos display an expansion of FGF signaling into the trunk monitored by Sprouty2 and Pea3 expression, and an expansion of Wnt/β-catenin signaling detected by expression of Axin2, Tbx6, Cdx2, and Cdx4. Following loss of RA signaling, the caudal expression domains of Fgf8, Wnt8a, and Wnt3a expand anteriorly into the trunk, but no change is observed in caudal expression of Fgf4 or Fgf17 plus caudal expression of Fgf18 and Cdx1 is reduced. These findings suggest that RA repression of Fgf8, Wnt8a, and Wnt3a in the developing trunk functions to down-regulate FGF signaling and Wnt/β-catenin signaling as the body axis extends.
Keywords: Retinoic acid signaling, Wnt/β-catenin signaling, FGF signaling, Spry2, Pea3, Axin2, Fgf8, Wnt8a, Wnt3a, Tbx6, Cdx, axis extension
1. Results and discussion
During vertebrate embryogenesis, the process of body axis extension begins when somitogenesis commences. As somites form, the body extends along the anteroposterior axis forming a new domain (the developing trunk) located between the headfolds and the epiblast/primitive streak. Several secreted cell-cell signaling molecules control body axis extension including Wnt (Grigoryan et al., 2008), fibroblast growth factor (FGF) (Del Corral and Storey, 2004), and retinoic acid (RA) (Duester, 2008). Some of the actions of Wnt ligands are transduced through stabilization of β-catenin which can then enter the nucleus and bind to the LEF/TCF family of transcription factors (Logan and Nusse, 2004). During the early phase of body axis extension, Wnt/β-catenin signaling domains are limited to regions on either end of the developing trunk in the headfold and epiblast/primitive streak (Nakaya et al., 2005). Likewise, FGF signaling domains during the early phase of body axis extension are limited to regions on either end of the developing trunk in cardiac mesoderm (Sirbu et al., 2008) and epiblast/primitive streak (Sirbu and Duester, 2006). Although FGF signaling and Wnt/β-catenin signaling pathways play important roles in body axis extension, the mechanisms by which individual FGFs or Wnts control the processes of body patterning are largely unknown.
Interactions between the Wnt/β-catenin, FGF, and RA signaling pathways have been reported in RA-deficient embryos generated either through vitamin A deficiency which removes the precursor of RA, or through genetic loss of retinaldehyde dehydrogenase-2 (Raldh2) which controls RA synthesis (Duester, 2008). Loss of RA signaling in vitamin A deficient quail embryos and Raldh2−/− mouse embryos up-regulates caudal Fgf8 expression and results in segmentation defects during body axis extension including a shortening of the body along the anteroposterior axis and somite left-right asymmetry (Del Corral et al., 2003; Vermot et al., 2005). Further studies with Raldh2−/− embryos demonstrated that loss of RA signaling results in an anterior expansion of Fgf8 expression from the epiblast into the posterior neuroectoderm (Sirbu and Duester, 2006), and a posterior expansion of Fgf8 mRNA and FGF signaling from cardiac mesoderm into trunk lateral plate mesoderm (Ryckebusch et al., 2008; Sirbu et al., 2008); together, these two events reduce the size of the Fgf8-free zone where the trunk initially develops. Loss of RA signaling in Raldh2−/− embryos and vitamin A deficient quail embryos also results in ectopic trunk expression of Wnt8a (avian ortholog is Wnt8c), suggesting that Wnt/β-catenin signaling may also be down-regulated by RA signaling in the developing trunk (Niederreither et al., 2000; Olivera-Martinez and Storey, 2007).
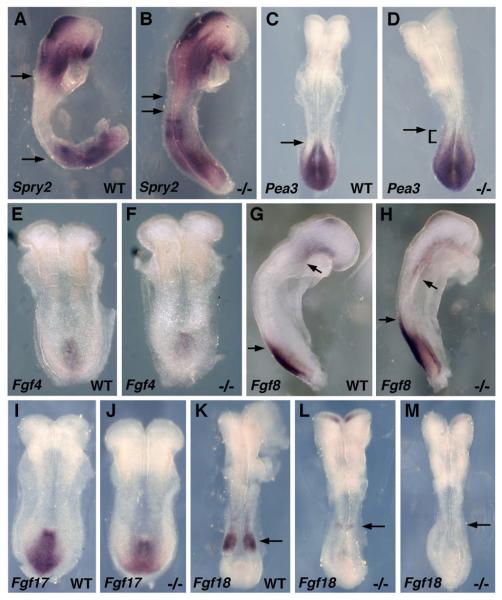
Here, we examine FGF and Wnt/β-catenin signaling in mouse Raldh2−/− embryos during the early phase of body axis extension. Raldh2−/− embryos are completely devoid of RA signaling from E7.5-E8.5 (0-10 somites) when body axis extension commences (Sirbu and Duester, 2006; Sirbu et al., 2005). As a marker of FGF signaling we examined expression of Sprouty2 (Spry2) which is induced by FGF signaling (Minowada et al., 1999) and acts as a negative-feedback regulator of the pathway (Hanafusa et al., 2002). Whereas Spry2 mRNA is normally expressed in two separate domains anterior and posterior to the developing trunk, Spry2 was greatly up-regulated in Raldh2−/− embryos such that the two domains are nearly joined in the developing trunk (Fig. 1A-B). We also examined another marker of FGF signaling, Pea3 encoding an Ets transcription factor induced by FGF (Raible and Brand, 2001), and observed an anterior extension of its caudal expression domain in Raldh2−/− embryos (Fig. 1C-D). These findings indicate that a loss of RA signaling in Raldh2−/− embryos results in an increase in FGF signaling.
Fig. 1.
FGF signaling following loss of RA synthesis. (A-B) Spry2 mRNA and (C-D) Pea3 mRNA at the 6-somite stage; arrows indicate that the Raldh2 mutant exhibits a large increase in Spry2 expression in the developing trunk, and an anterior extension of the caudal Pea3 expression domain. (E-F) Fgf4 mRNA at the 4-somite stage; no difference is observed between wild-type and Raldh2 mutant embryos. (G-H) Fgf8 mRNA at the 5-somite stage; arrows indicate that the anterior and posterior domains of Fgf8 expression extend further into the trunk in the Raldh2 mutant. (I-J) Fgf17 mRNA at the 4-somite stage showing no difference between wild-type and mutant. (K-M) Fgf18 mRNA at the 7-somite stage; arrows indicate that caudal Fgf18 expression is either lost or greatly reduced in Raldh2 mutants.
Previous studies have demonstrated that caudal expression of Fgf8 extends ectopically into posterior neuroectoderm following loss of RA at the 1-3 somite stages (Sirbu and Duester, 2006). Here, we show that a 5-somite Raldh2−/− embryo exhibits an anterior extension of the caudal Fgf8 expression domain plus a posterior extension of the cardiac Fgf8 expression domain (Fig. 1G-H). As Fgf4, Fgf17, and Fgf18 are also expressed caudally and may have overlapping functions with Fgf8 in body axis extension (Maruoka et al., 1998; Niswander and Martin, 1992), we examined these genes in Raldh2 mutants. Fgf4 and Fgf17 expression was not significantly changed in Raldh2−/− embryos compared to wild-type (Fig. 1E-F, I-J). Fgf18 mRNA was either lost or greatly down-regulated in Raldh2−/− embryos (Fig. 1K-M); a similar down-regulation of Fgf18 was previously reported (Vermot et al., 2005). Taken together with the findings on Spry2 and Pea3, these observations suggest that the large increase in FGF signaling observed after loss of RA signaling is due to a specific increase in Fgf8 expression but not expression of Fgf4, Fgf17 or Fgf18. As Fgf18 is induced by RA signaling, this gene appears to play a much different role in caudal development than Fgf8 which is repressed by RA.
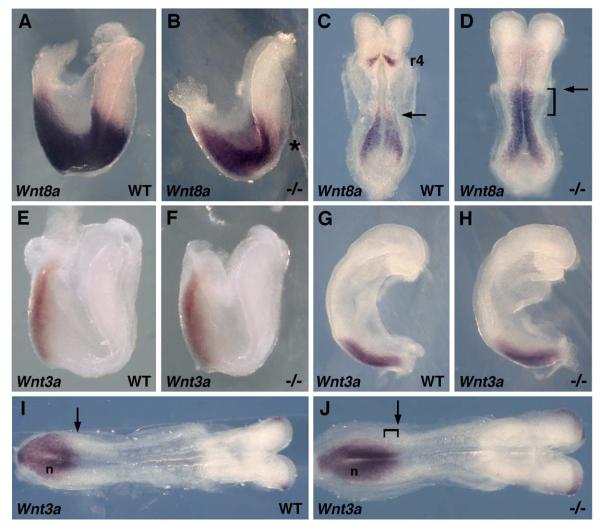
Two caudally-expressed Wnt genes most often associated with vertebrate Wnt/β-catenin signaling during body axis extension are Wnt3a in mouse (Nakaya et al., 2005; Takada et al., 1994) and Wnt8 in Xenopus and zebrafish (Lekven et al., 2001; Smith and Harland, 1991). Previous studies in vitamin A deficient quail embryos demonstrated a large up-regulation of Wnt8c expression in trunk neuroectoderm (Olivera-Martinez and Storey, 2007). We examined expression of Wnt8a, the mouse homolog of Wnt8c, in Raldh2−/− embryos. At the 1-somite stage, when Wnt8a expression is normally observed continuously along the anteroposterior axis from the posterior hindbrain to the epiblast, Wnt8a expression was down-regulated in the hindbrain of Raldh2−/− embryos compared to wild-type (Fig. 2A-B). At the 5-somite stage, when Wnt8a expression normally resolves into two domains, i.e. epiblast and hindbrain rhombomere 4 (Niederreither et al., 2000), we found that Raldh2−/− embryos had lost Wnt8a expression in rhombomere 4 but the caudal Wnt8a domain now extended much further anteriorly from the epiblast into the trunk neuroectoderm (Fig. 2C-D). We also examined expression of Wnt3a which plays an essential role during body axis extension in the mouse (Nakaya et al., 2005; Takada et al., 1994). At both the 1-somite and 4-somite stages, Wnt3a expression was unchanged in Raldh2−/− embryos compared to wild-type (Fig. 2E-H). However, at the 7-somite stage we observed an anterior extension of the caudal Wnt3a expression domain relative to the node (Fig. 2I-J). These findings indicate that RA-deficient mouse embryos exhibit an early increase in caudal Wnt8a expression, followed by a later increase in caudal Wnt3a expression.
Fig. 2.
Loss of RA signaling up-regulates caudal Wnt8a and Wnt3a expression. (A-B) Detection of Wnt8a mRNA in wild-type (WT) and Raldh2−/− embryos at the 1-somite stage; overall, Wnt8a expression is lower in the mutant, and an asterisk points out down-regulation specifically in the hindbrain. (C-D) At the 5-somite stage it can be seen that Wnt8a expression is up-regulated in the trunk (arrows indicate that the anterior border of Wnt8a expression in the trunk is shifted anteriorly in the mutant); also, expression in rhombomere 4 (r4) of the hindbrain is lost in the mutant at 5-somites. Detection of Wnt3a mRNA at the 1-somite stage (E-F), 4-somite stage (G-H), and 7-somite stage (I-J) shows that initially no difference exists between wild-type and Raldh2 mutant, but at 7-somites the mutant exhibits an anterior extension of the caudal Wnt3a expression domain relative to the node (n).
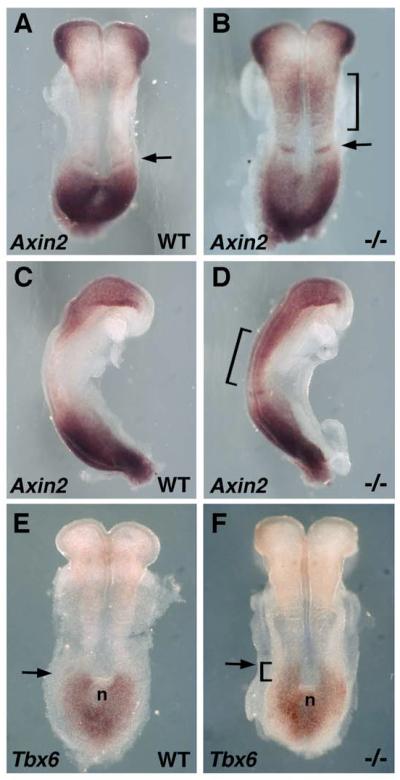
We examined Wnt/β-catenin signaling in wild-type and Raldh2−/− embryos. Axin2 encodes a negative feedback inhibitor of Wnt/β-catenin signaling that is induced by this signaling pathway in a wide range of tissues (Jho et al., 2002). In 4-somite Raldh2−/− embryos, an ectopic domain of Axin2 mRNA domain was observed in the developing trunk (Fig. 3A-D). The ectopic domain of Axin2 mRNA observed in Raldh2−/− embryos was in a region which overlaps the ectopic domain of Wnt8a expression (compare Fig. 2D and Fig. 3B); Axin2 up-regulation is most likely due to up-regulation of Wnt8a which occurs prior to up-regulation of Wnt3a in Raldh2−/− embryos. We also examined expression of Tbx6 which is induced by Wnt/β-catenin signaling during mouse body axis extension (Dunty et al., 2008). In 5-somite Raldh2−/− embryos we observed an anterior extension of the caudal Tbx6 expression domain relative to the node (Fig. 3E-F). From these findings it appears that a loss of RA does lead to a significant increase in trunk Wnt/β-catenin signaling as monitored by both Axin2 and Tbx6 expression.
Fig. 3.
Analysis of Wnt/β-catenin signaling in embryos lacking RA synthesis. (A-D) Detection of Axin2 mRNA at the 4-somite stage; brackets indicate a region in the developing trunk where the Raldh2 mutant exhibits a significant increase in Axin2 expression relative to wild-type; an arrow indicates that the mutant exhibits an increase in Axin2 expression at the somite determination front. (E-F) Tbx6 mRNA at the 5-somite stage shows that the Raldh2 mutant exhibits an anterior extension of the caudal Tbx6 expression domain relative to the node (n).
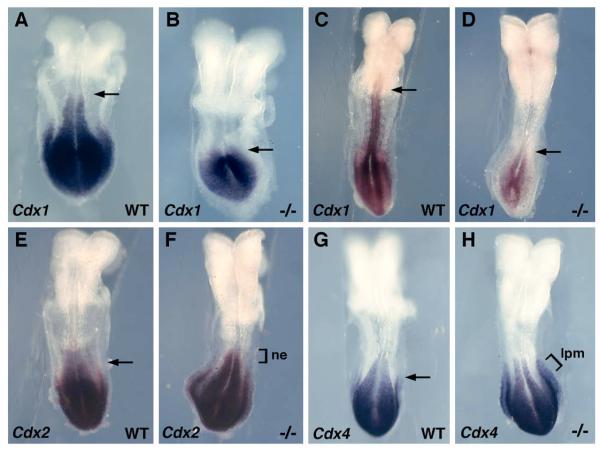
Cdx1, Cdx2, and Cdx4 play essential roles in vertebrate caudal development through their ability to regulate posterior Hox gene expression (Van den Akker et al., 2002; van Nes et al., 2006). In zebrafish, expression of Cdx1a and Cdx4 is strongly reduced in Wnt3a/Wnt8 double knock-down embryos, and both Wnt3a/Wnt8 and Cdx1a/Cdx4 knockdowns result in complete loss of somitogenesis during mid-segmentation, indicating that Cdx1a and Cdx4 mediate Wnt-dependent body axis extension (Shimizu et al., 2005). In mouse, the early caudal expression domains of Cdx1 and Cdx4 are directly induced by Wnt/β-catenin signaling (Pilon et al., 2006; Pilon et al., 2007), and the Cdx2 promoter contains binding sites for LEF/TCF factors regulating Wnt/β-catenin signaling (Wang and Shashikant, 2007). As another means of determining whether a loss of RA increases Wnt/β-catenin signaling, we examined expression of the Cdx gene family in Raldh2−/− embryos. Cdx1 expression was not increased following loss of RA signaling, but was instead significantly decreased in the posterior neuroectoderm and primitive streak mesoderm (Fig. 4A-D). Both Cdx2 and Cdx4 were upregulated in Raldh2−/− embryos, but in different tissues; Cdx2 expression exhibited an anterior extension in neuroectoderm (Fig. 4E-F) whereas Cdx4 exhibited an anterior extension in lateral plate mesoderm (Fig. 4G-H). Thus, our findings on Cdx2 and Cdx4 expression provide further evidence (along with our Axin2 and Tbx6 results), that caudal Wnt/β-catenin signaling increases following a loss of RA. Our observation that Cdx1 expression is greatly reduced in Raldh2−/− embryos can be explained by previous observations showing that Cdx1 is induced by RA as well as Wnt3a (Prinos et al., 2001). As the Cdx1 promoter contains a retinoic acid response element essential for high-level expression of Cdx1 caudally (Houle et al., 2003), a loss of RA signaling may prevent Cdx1 from being able to respond to an increase in Wnt/β-catenin signaling.
Fig. 4.
Regulation of Cdx genes by RA signaling. (A-B) Cdx1 mRNA at 4-somite stage and (C-D) 7-somite stage; Raldh2−/− embryos exhibit a loss of Cdx1 expression in the neuroectoderm (marked by arrows) and reduced expression in the primitive streak. (E-F) Cdx2 mRNA at 6-somite stage showing an Raldh2−/− embryo exhibiting an anterior extension of the caudal Cdx2 expression domain in neuroectoderm (ne). (G-H) Cdx4 mRNA at the 6-somite stage showing an Raldh2 mutant exhibiting an anterior extension of the caudal Cdx4 expression domain in lateral plate mesoderm (lpm) but not neuroectoderm or paraxial mesoderm.
In conclusion, inhibition of FGF signaling by RA signaling has been observed during body axis extension, and this appears to operate through the ability of RA to repress Fgf8 (Del Corral et al., 2003; Sirbu and Duester, 2006; Sirbu et al., 2008; Vermot et al., 2005). Other studies suggest that RA may directly repress Fgf8 since it was discovered that the Fgf8 promoter is controlled differentially by a retinoic acid response element, with expression of the major isoform Fgf8b being repressed when RA is present (Brondani et al., 2002). From the studies reported here on Raldh2−/− embryos, we show that loss of RA signaling results in the up-regulation of Spry2 and Pea3, markers for sites of FGF signaling. RA has also been suggested to inhibit Wnt/β-catenin signaling based on studies in vitamin A deficient quail embryos that display a large increase in Wnt8c expression (Olivera-Martinez and Storey, 2007); in those studies a feedback loop was proposed in which FGF signaling stimulates Wnt8c expression in posterior neuroectoderm leading to induction of Raldh2 in mesoderm which then results in release of RA that represses both Fgf8 and Wnt8c as the body axis extends. The FGF-Wnt portion of this feedback loop is supported by studies in Xenopus demonstrating that Fgf8a induces Wnt8 (Hong et al., 2008). In the developing trunk of mouse Raldh2−/− embryos, we observe increases in expression of Wnt8a (early) and Wnt3a (late), and a corresponding increase in Wnt/β-catenin signaling in the trunk monitored by Axin2, Tbx6, Cdx2, and Cdx4 expression. Wnt3a appears to be more important than Wnt8a for normal mouse development as Wnt3a knockout embryos exhibit arrested body axis extension at the 7-9 somite stage (Takada et al., 1994), whereas Wnt8a knockout mice have no obvious developmental phenotype (van Amerongen and Berns, 2006). Thus, Wnt3a expression appears to be much more efficient than Wnt8a in stimulating caudal Wnt/β-catenin signaling in mouse, but our studies demonstrate that Wnt8a over-expression in Raldh2−/− embryos can induce ectopic Wnt/β-catenin signaling. In addition to regulating Raldh2 as previously suggested (Olivera-Martinez and Storey, 2007), it will be interesting to determine whether Wnt8a regulates other genes involved in body axis extension.
2. Experimental procedures
2.1. Generation of Raldh2−/− Embryos
Generation of Raldh2+/− adult mice were previously described (Mic et al., 2002). Embryos from Raldh2+/− crosses were genotyped by PCR analysis of yolk sac DNA to identify Raldh2−/− embryos. Embryos were staged according to somite number. All mouse studies conformed to the regulatory standards adopted by the Animal Research Committee at the Burnham Institute for Medical Research.
2.2. In situ Hybridization
Detection of mRNA was performed by whole-mount in situ hybridization as previously described (Mic et al., 2002). Wild-type and Raldh2−/− embryos were treated under identical hybridization conditions and stained for the same length of time. For each gene analyzed, we collected data from at least three Raldh2−/− embryos and three wild-type embryos at a similar stage to draw conclusions.
Acknowledgements
We thank the following individuals for mouse cDNAs used to prepare in situ hybridization probes: F. Costantini (Axin2), P. Dolle (Wnt8a), B. Hogan (Pea3), N. Itoh (Fgf17, Fgf18), D. Lohnes (Cdx1, Cdx2, Cdx4), G. Martin (Fgf4, Fgf8, Spry2), R. Nusse (Wnt3a), and V. Papaioannou (Tbx6). This work was funded by the National Institutes of Health grant GM062848 (G.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brondani V, Klimkait T, Egly JM, Hamy F. Promoter of FGF8 reveals a unique regulation by unliganded RARa. J. Mol. Biol. 2002;319:715–728. doi: 10.1016/S0022-2836(02)00376-5. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Del Corral RD, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Del Corral RD, Storey KG. Opposing FGF and retinoid pathways: a signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. BioEssays. 2004;26:857–869. doi: 10.1002/bies.20080. [DOI] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–31. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunty WC, Jr., Biris KK, Chalamalasetty RB, Taketo MM, Lewandoski M, Yamaguchi TP. Wnt3a/ -catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development. 2008;135:85–94. doi: 10.1242/dev.009266. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–41. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signaling pathway. Nature Cell Biol. 2002;4:850–858. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- Hong CS, Park BY, Saint-Jeannet JP. Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development. 2008;135:3903–10. doi: 10.1242/dev.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle M, Sylvestre JR, Lohnes D. Retinoic acid regulates a subset of Cdx1 function in vivo. Development. 2003;130:6555–6567. doi: 10.1242/dev.00889. [DOI] [PubMed] [Google Scholar]
- Jho E, Zhang T, Domon C, Joo C-K, Freund J-N, Costantini F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu.Rev.Cell Dev.Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Maruoka Y, Ohbayashi N, Hoshikawa M, Itoh N, Hogan BLM, Furuta Y. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech.Dev. 1998;74:175–177. doi: 10.1016/s0925-4773(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129:2271–2282. doi: 10.1242/dev.129.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Nakaya MA, Biris K, Tsukiyama T, Jaime S, Rawls JA, Yamaguchi TP. Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development. 2005;132:5425–36. doi: 10.1242/dev.02149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- Niswander L, Martin GR. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114:755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Storey KG. Wnt signals provide a timing mechanism for the FGF-retinoid differentiation switch during vertebrate body axis extension. Development. 2007;134:2125–35. doi: 10.1242/dev.000216. [DOI] [PubMed] [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Bouchard N, Savory J, Lohnes D. Cdx4 is a direct target of the canonical Wnt pathway. Developmental Biology. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Savory JG, Lohnes D. Wnt signaling is a key mediator of Cdx1 expression in vivo. Development. 2007;134:2315–23. doi: 10.1242/dev.001206. [DOI] [PubMed] [Google Scholar]
- Prinos P, Joseph S, Oh K, Meyer BI, Gruss P, Lohnes D. Multiple pathways governing Cdx1 expression during murine development. Developmental Biology. 2001;239:257–269. doi: 10.1006/dbio.2001.0446. [DOI] [PubMed] [Google Scholar]
- Raible F, Brand M. Tight transcriptional control of the ETS domain factors Erm and Pea3 by Fgf signaling during early zebrafish development. Mech.Dev. 2001;107:105–117. doi: 10.1016/s0925-4773(01)00456-7. [DOI] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin S-C, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc. Natl. Acad. Sci. USA. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Developmental Biology. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Sirbu IO, Duester G. Retinoic acid signaling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol. 2006;8:271–277. doi: 10.1038/ncb1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Gresh L, Barra J, Duester G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132:2611–2622. doi: 10.1242/dev.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–766. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes and Development. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006;22:678–689. doi: 10.1016/j.tig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Van den Akker E, Forlani S, Chawengsaksophak K, De Graaff W, Beck F, Meyer BI, Deschamps J. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development. 2002;129:2181–2193. doi: 10.1242/dev.129.9.2181. [DOI] [PubMed] [Google Scholar]
- van Nes J, de Graaff W, Lebrin F, Gerhard M, Beck F, Deschamps J. The Cdx4 mutation affects axial development and reveals an essential role of Cdx genes in the ontogenesis of the placental labyrinth in mice. Development. 2006;133:419–28. doi: 10.1242/dev.02216. [DOI] [PubMed] [Google Scholar]
- Vermot J, Llamas JG, Fraulob V, Niederreither K, Chambon P, Dollé P. Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science. 2005;308:563–566. doi: 10.1126/science.1108363. [DOI] [PubMed] [Google Scholar]
- Wang WC, Shashikant CS. Evidence for positive and negative regulation of the mouse Cdx2 gene. Journal of Experimental Zoology Part B. Molecular & Developmental Evolution. 2007;308:308–21. doi: 10.1002/jez.b.21154. [DOI] [PubMed] [Google Scholar]