Abstract
Synapses are asymmetric cellular adhesions that are critical for nervous system development and function, but the mechanisms that induce their formation are not well understood. We have previously identified thrombospondin as an astrocyte-secreted protein that promotes CNS synaptogenesis. Here we identify the neuronal thrombospondin receptor involved in CNS synapse formation as α2δ–1, the receptor for the anti-epileptic and analgesic drug gabapentin. We show that the VWF-A domain of α2δ–1 interacts with the epidermal growth factor-like repeats common to all thrombospondins. α2δ–1 overexpression increases synaptogenesis in vitro and in vivo and is required postsynaptically for thrombospondin and astrocyte-induced synapse formation in vitro. Gabapentin antagonizes thrombospondin binding to α2δ–1 and powerfully inhibits excitatory synapse formation in vitro and in vivo. These findings identify α2δ–1 as a receptor involved in excitatory synapse formation and suggest that gabapentin may function therapeutically by blocking new synapse formation.
INTRODUCTION
Central nervous system (CNS) synapses are complex cell-cell adhesions between neurons. Their establishment requires an interaction between axons and dendrites, accompanied by the appositional organization of pre and postsynaptic specializations. Several neuronal cell surface molecules and secreted signals have been shown to be involved in processes that lead to synaptic organization and maturation (Fox and Umemori, 2006), but molecules that regulate the formation of initial synaptic adhesions remain poorly understood. Accumulating evidence from our lab and others has shown that astrocytes play active roles in the formation of synapses (Eroglu et al., 2008). We have previously identified thrombospondins (TSP) as a necessary and sufficient synaptogenic signal secreted by astrocytes that increases synapse number (Christopherson et al., 2005). TSP is present in astrocyte-conditioned media (ACM), and is responsible for the ability of astrocytes to increase synapse number in vitro (Christopherson et al., 2005). TSPs are also important for synapse formation in vivo. TSP1/2 deficient mice have a significant decrease in the number of excitatory synapses. TSP1 and 2 are expressed during early postnatal ages when the majority of synapses are forming, and these proteins are absent from the adult brain when the amount of excitatory synaptogenesis is significantly reduced (Christopherson et al., 2005). Upon injury to the CNS TSP1/2 levels are upregulated, and lack of TSP1/2 impairs synaptic and functional recovery from stroke (Liauw et al., 2008).
TSP is able to promote synaptic adhesion and initiate the events that lead to the establishment of pre and postsynaptic specializations. Interestingly, these TSP-induced synapses are ultrastructurally identical to fully developed synapses and are presynaptically active but postsynaptically silent due to the lack of surface AMPA receptors. Astrocytes secrete a second unrelated signal that is able to convert these silent synapses into fully active ones ((Christopherson et al., 2005) and N. A. and B. A. B. unpublished data).
TSPs are large oligomeric, multidomain, extracellular matrix proteins that have been previously shown to play important roles in cell attachment, cell migration, cytoskeletal dynamics and angiogenesis (Bornstein et al., 2004). TSP mediates these functions via its interaction with various cell surface receptors through specific domains (Adams and Lawler, 2004). We hypothesized that TSPs induce synapse formation by interacting with a neuronal cell surface receptor. Here we show that TSPs mediate synaptogenesis through their epidermal growth factor (EGF) -like domains, common to all TSP isoforms. Using this domain information, we identified the gabapentin receptor α2δ-1 as the TSP receptor involved in synapse formation.
α2δ-1 (cacna2d1) was originally isolated as a non-essential subunit of the L-type calcium channel complex from skeletal muscle (Arikkath and Campbell, 2003) and also binds to other proteins (Kaltenbach et al., 2007). α2δ-1 is ubiquitously expressed in many tissues and is highly expressed by many CNS neurons (Cole et al., 2005) including retinal ganglion cells (RGCs). α2δ-1 is translated from a single gene product, which gets post-translationally cleaved into α2 and δ parts that remain associated via disulfide bridges. The α2 part of the protein (~950 amino acids) is entirely extracellular while the δ part has a small extracellular part that is attached to α2, and a transmembrane domain with a very short cytoplasmic tail that tethers the protein to the membrane (Davies et al., 2007).
Much research on α2δ-1 has focused on its role in the regulation of calcium channel function and trafficking. However, the presence of a large extracellular region containing a well-known protein-protein interaction fold, the Von Willebrand Factor A (VWF-A) domain, suggests that this protein could serve as a receptor for extracellular ligands. A recent study on skeletal muscle cells, which express high levels of α2δ-1, described such a role for α2δ-1 in myoblast attachment and extracellular signaling that is independent of calcium channel function (Garcia et al., 2007).
α2δ-1 is the high affinity receptor for two commonly prescribed anti-epileptic, anti-neuropathic pain medications gabapentin (GBP, Neurontin) and pregabalin (Lyrica) (Gee et al., 1996). GBP and pregabalin were initially designed as hydrophobic gamma amino butyric acid (GABA) analogs that could cross the blood brain barrier. Further studies have shown that even though they posses anti-convulsant properties, they do not bind to GABA receptors or transporters. A recent study using a knock-in mouse that expresses a mutant α2δ-1 which cannot bind GBP or pregabalin has shown that α2δ-1 is the in vivo target for these drugs, and that these drugs mediate their therapeutic action through binding to α2δ-1 (Field et al., 2006). GBP or pregabalin do not affect the single channel kinetics of calcium channels and has only modest effects on neurotransmission (Dooley et al., 2007). Thus the cellular mechanisms underlying the mode of action of these drugs are unclear.
In this study, we show that EGF-like domains of TSP directly bind to α2δ-1 and mediate its synapse-inducing activity via this receptor. These findings identify α2δ–1 as a neuronal TSP receptor that is required for CNS synapse formation. This function of α2δ-1 is independent of calcium channel function. We also show that GBP is a potent inhibitor of TSP/astrocyte-induced excitatory synapse formation in vitro and in vivo. This function of GBP may be a central part of its mechanism of action.
RESULTS
All TSP isoforms induce synapse formation
There are five TSP isoforms in mammals, which fall into two groups according to their domain structure and oligomerization states (Figure 1A). Trimeric subgroup A TSPs, TSP1 and 2, are synaptogenic (Christopherson et al., 2005). To determine whether pentameric subgroup B TSPs are also synaptogenic, RGCs were cultured in the presence of astrocytes, or with TSP 1, 3, 4 or 5. All subgroup B TSPs increased synapse number significantly to similar levels as TSP1 or astrocytes (Figures 1B–1D). These results suggest that the synaptogenic domain of TSP is located in the conserved C-terminal portion of TSP, which is common to all isoforms spanning the EGF-like repeats, the calcium binding repeats and C-terminal L-type lectin-like globular domain.
Figure 1. All thrombospondin isoforms are synaptogenic.
(A) TSPs are divided into two subgroups. N-terminal domain (black), the procollagen (red) and properdin-like repeats (orange), EGF-like repeats (blue), calcium binding repeats (grey) and C-terminal L-lectin like globular domain (green).
(B) Immunostaining of RGCs for synaptotagmin (red) and PSD-95 (green). White arrows point to co-localized synaptic puncta. Scale bar =30µm.
(C) Quantification of the effects of astrocytes, purified TSP1,4 and 5 (8nM each) and (D) conditioned media from COS7 cells overexpressing either TSP3 or empty vector, on synapse number. In all graphs n=20 cells, Error bars mean ± SEM, * p<0.05.
The synaptogenic activity of TSP maps to its EGF-like repeats
TSPs interact with a number of known cell surface receptors through specific domains (Adams and Lawler, 2004). To identify the synaptogenic domain of TSP, we treated RGCs with a panel of recombinant truncation constructs of TSP1 and 2. The TSP fragments that contained the EGF-like repeats mimicked the ability of full-length TSP to induce synapses (Figures 2B–2C). A fragment containing the third EGF-like repeat together with the C-terminal region of TSP2 also significantly increased synapse number, however, the third EGF like domain alone did not induce a significant increase in synapse number (Figure 2C).
Figure 2. EGF like repeats of TSPs are synaptogenic.
(A) The domain structure of TSP1 and 2. N-terminal domain (black), oligomerization domain and a procollagen repeat (red, PC), three properdin-like (TSP type 1, orange, P1-3), three EGF-like (TSP type 2, blue, E1-3), and 13 calcium binding (TSP type 3, grey) repeats (Ca(wire)) and a C-terminal L-type lectin like globular domain (green, C).
(B) Quantification of the effect of TSP1 and (C) TSP2 fragments on synapse number. RGCs were treated with astrocytes, full-length TSP1 or a panel of TSP1 or TSP2 fragments (8nM each).
(D) Location of epitopes targeted by TSP blocking antibodies (modified from (Carlson et al., 2008)). Inset shows magnified structure of EGF-like repeats and the Ca binding wire region and the C-terminal L-lectin like domain. Highlighted domains indicate putative synaptogenic domain of TSP.
(E) Quantification of the effect of monoclonal anti-TSP antibodies on TSP’s synaptogenic activity. In all graphs n=20 cells, Error bars mean ± SEM, * p<0.05.
We confirmed the importance of the EGF-like repeats in synapse formation by functionally blocking synaptogenic effect of TSP on cultured RGCs, using monoclonal antibodies directed against different epitopes of TSP (Figure 2D). Monoclonal antibodies against the second (HB8432 and C6.7 (Annis et al., 2007)) or third EGF-like repeats (A4.1 (Annis et al., 2006)) blocked the synaptogenic effect of TSP while an antibody against the N-terminal domain (mAb200-1) did not (Figure 2E).
To aid us in our efforts to identify the neuronal TSP receptor involved in synapse formation, we expressed and purified a myc and 6-Histidine tagged TSP2 fragment containing all three EGF-like repeats (Figures S1A–S1B). This TSP2 fragment (designated SD2 for synaptogenic domain 2) was strongly synaptogenic (Figures S1C–S1D). Collectively, these data suggest that TSP-induced synapse formation is mediated by an interaction involving EGF-like repeats of TSP.
α2δ-1 interacts with the synaptogenic domain of TSP
The EGF-like domains of TSP4 have been shown to bind to the VWF-A domain of integrin αM (Pluskota et al., 2005). Thus, we investigated whether integrin αM or other VWF-A domain containing integrins in RGCs were involved in TSP-induced synapse formation. None of the integrins that contained the VWF-A domain and were expressed by RGCs were crucial for the synaptogenic activity of TSP (not shown).
Another class of cell surface proteins that contains VWF-A domains is the alpha2 delta (α2δ) family. Among the α2δ proteins cloned to date (Klugbauer et al., 2003), RGCs express high levels of α2δ-1 (Figure S2A). We investigated whether α2δ-1 is localized to synapses using array tomography (Micheva and Smith, 2007). Ultra-thin sections from rat cortex or mouse LGN were immunolabeled with antibodies against α2δ-1 and the pre and postsynaptic markers synapsin and MAGUK. α2δ-1 gave a punctate staining pattern. Some α2δ-1 puncta localized to synapses identified as juxtapositioned pre and post-synaptic puncta while some co-localized exclusively with pre or post-synaptic puncta (Figure 3A and Figure S2B).
Figure 3. Thrombospondins interact with α2δ-1.
(A) Array tomography analysis of synaptic localization of α2δ–1 in cerebral cortex. RGCs were immunostained for Synapsin I (blue) and for MAGUK (green). α2δ-1 puncta (red) associate both with synapses (white circles) or with isolated presynaptic (diamonds) or post-synaptic (squares) puncta. Scale bar =2 µm.
(B) Western-blot analysis of α2δ-1 on the immunoprecipitation (IP) fractions performed using antibodies specific to TSPs1, 2 or 4 as well as calcium channel α1C (Cav1.2), or Agrin (positive and negative controls for IP, respectively).
(C) Western blot analysis of α2δ-1 interaction with the synaptogenic domain of TSP2 (SD2). Left panel, HEK293 cell lysates from non-transfected (1), α2δ-1-FLAG alone (2), SD2 alone (3), α2δ-1-FLAG and SD2 (4) and α1δ-1FLAG and Control-myc-His construct (5) transfected cells. SD2 and Control-his-myc protein are marked with red ●. Anti-his antibody cross-reacts with several histidine rich proteins in HEK293 cell lysates (marked with blue *). Anti-α2δ-1 antibody also weakly recognizes the human α2δ-1 expressed endogeneously in HEK293 cells at low levels (blue ♦) Right panel; anti-FLAG IP fractions from α2δ-1-FLAG alone (6), SD2 alone (7), α2δ-1-FLAG and SD2 (8) and α1δ-1FLAG and Control-myc-His construct (9) transfections.
(D) Domain structure of α2δ-1 protein and scheme of α2δ-1 protein C (PC) tagged constructs. SP=signal peptide, vWA_N and VGCC_a2=putative domains of unknown structure. Yellow boxes indicate prutative helical regions where no domain has yet been predicted. The red box shows the transmembrane (TM) region. Orange hexagons indicate predicted N-glycosylation sites, and purple bars indicate positions of cysteines.
(E) SD2 interacts with the VWF-A domain of α2δ-1. Lane 1 is non-transfected HEK293 cell lysate. SD2 was co-expressed with PC tagged full-length α2δ-1, α2 only or VWF-A only constructs (lanes 3, 4 and 5) as well as CXCR4. SD2 co-immunopurified with the α2 only (8) and VWF-A (9) only constructs of α2δ-1 as well as the full-length protein (7) using anti-PC beads, (red arrows). SD2 did not co-purify with CXCR4 (6).
To determine if α2δ-1 interacts with TSPs, we immunoprecipitated TSPs 1, 2 and 4 from P5 rat cerebral cortex lysate. α2δ-1 was detected in immunoprecipitation fractions performed using each of the three TSP antibodies (Figure 3B) suggesting α2δ-1 and TSPs interact in vivo.
To test whether there is a direct and specific binding interaction between the synaptogenic domain of TSP and α2δ-1, we co-expressed a FLAG-tagged α2δ-1 alone (Figure 3C, lane 2), with SD2 (lane 4) or with an unrelated secreted control protein that contained an EGF-like repeat (Control-myc-his, lane 5). Beads conjugated to an anti FLAG-tag antibody were used to immunoprecipitate α2δ-1-FLAG. SD2 co-immunoprecipitated with α2δ-1-FLAG but the Control-myc-his protein did not (Figure 3C, lanes 8 and 9, respectively), suggesting that α2δ-1 specifically interacts with the synaptogenic EGF-like domains of TSP.
We hypothesized that the EGF-like domains of TSP interact with the VWF-A domain of α2δ-1, which resides in the α2 region of the protein. To test this, three α2δ-1 constructs, the full-length α2δ-1, α2 or VWF-A domain, each with a C-terminal Protein C (PC) tag for purification (Figure 3D) were co-expressed with SD2. When we performed PC tag affinity purifications we could detect SD2 in all three purified fractions (Figure 3E, lanes 7,8 and 9). SD2 did not co-purify with an unrelated membrane protein that also contained the same PC tag (Figure 3E, lane 6). These data show that TSP and α2δ-1 interact through the synaptogenic EGF-like domains of TSP and the VWF-A domain of α2δ-1.
α2δ-1 is the neuronal TSP receptor involved in synapse formation
To determine whether α2δ-1 plays a role in TSP-induced synapse formation in vitro, we overexpressed α2δ-1 in RGCs and tested whether SD2-induced synapse formation was affected. RGCs that overexpressed α2δ-1 (identified with GFP co-expression) received twice as many synapses in response to SD2 as did RGCs transfected with empty vector (Figure 4A), indicating that α2δ-1 overexpression enhances TSP-induced synapse formation. α2δ-1 overexpression was not sufficient to induce synapse formation in the absence of SD2, suggesting that SD2 is required for the enhancement of synapse formation by α2δ-1.
Figure 4. α2δ-1 is the TSP receptor involved in synaptogenesis.
(A) RGCs were transfected with empty vector (pcDNA3, Invitrogen) or pcDNA3 constructs that express full length α2δ-1, δ-1 only or α2δ-1-Adh. The synapses received by transfected cells (marked by GFP co-expression) were then quantified. n=20 cells, Error bars mean ± SEM, * p<0.05.
(B) Quantification of the effects of monoclonal antibodies 5A5 and 3B4 (mouse monoclonals raised against the VWF-A domain of α2δ-1, Mazorx Inc.) and anti-Thy1 antibody OX7 in synapse formation. 5A5 and 3B7 mimic TSP’s synaptogenic function. n=30 cells, Error bars mean ± SEM, *p<0.05.
(C) Western blot analysis of cell lysates from HEK293 cells, which were co-transfected with an expression vector for rat α2δ-1 and siControl or siα2δ-1 pools, with a monoclonal antibody against α2δ-1 or against β-actin.
(D) Immunostaining of siRNA transfected RGCs (marked blue by GFP co-expression) for co-localization of synaptotagmin (red) and PSD-95 (green). RGCs that were transfected with siα2δ-1 did not form many synapses even in the presence of astrocytes (See inserts i versus ii). Scale bars =30µm.
(E) Quantification of the effects of siRNA pools on astrocyte and TSP-induced synapse formation in RGCs. n=20 cells, Error bars mean ± SEM, * p<0.05.
(F) Overexpression of human α2δ-1, which is resistant to siα2δ-1(9), rescues the inhibition of SD2-induced synapse formation by siα2δ-1(9). n=20 cells, Error bars mean ± SEM, * p<0.05.
To determine which region of the α2δ-1 protein was responsible for its enhancement of SD2-induced synapse formation we utilized two α2δ-1 constructs (schemed in Figure 4A). Overexpression of a “α2δ-1Adh” construct that contains the entire extracellular region of α2δ-1 followed by the transmembrane domain from an unrelated type 1 membrane protein adhalin (Gurnett et al., 1996) mimicked the effect of full-length α2δ-1 in enhancing SD2-induced synapse formation (Figure 4A) suggesting that the critical region of α2δ-1 maps to the extracellular part of the protein. We next overexpressed a “δ-1 only” construct (lacking the α2 region), which inhibited both SD2-induced synapse formation (Figure 4A), indicating that the VWF-A-containing α2 region is necessary for enhancing synaptogenesis, and that regions within δ-1 may be involved in regulating downstream interactions that are critical for TSP-induced synapse formation.
Since the VWF-A domain of α2δ-1 binds TSP, we investigated whether antibodies against the VWF-A domain of α2δ-1 would interfere with TSP-induced synapse formation. Two monoclonal antibodies directed against the VWF-A domain of α2δ-1, 5A5 and 3B4, recognized α2δ-1 in Western blots and stained the surface of HEK293 cells overexpressing α2δ-1 (Figure S3A–S3B). When RGCs were cultured with these antibodies in the presence or absence of TSP and analyzed synapse number, both 5A5 and 3B4 induced synapse formation similar to TSP. A control antibody (OX7) against another RGC surface receptor (Thy1) did not affect synapse formation. The synaptogenic effect of 5A5 or 3B4 was not additive with that of TSP (Figure 4B). These data show that antibody binding to the VWF-A domain can mimic TSPs synaptogenic function and suggest that the interaction of TSP with the VWF-A domain of α2δ-1 is important for the initiation of synapse formation. Such ligand-mimicking antibodies were also described for VWF-A domain containing integrins (Wilkins et al., 1996).
To determine whether α2δ-1 is required for TSP-induced synapse formation, we used a small interfering RNA (siRNA) knockdown approach. An siRNA pool specific for rat α2δ-1 significantly reduced the expression of rat α2δ-1 in transfected HEK293 cells (Figure 4C). Knockdown of α2δ-1 in RGCs with this siRNA pool inhibited astrocyte or TSP-induced synapse formation in vitro (Figures 4D–4E), whereas the non-targeting control siRNA pool (siControl) did not affect synapse formation (Figures 4C–4D).
To show that the reduction in synapse formation by the α2δ-1 siRNAs was due to the specific knockdown of α2δ-1, we tested whether the siRNA inhibition could be rescued by co-expressing an siRNA resistant α2δ-1 construct. One of the siRNAs against rat α2δ-1, siα2δ-1 Duplex 9, blocked overexpression of rat α2δ-1, but not human α2δ-1 in HEK293 cells (Figure S4). When we co-transfected RGCs with siα2δ-1(9) and the human α2δ-1 construct we rescued SD2 or astrocyte-induced synapse formation (Figure 4F), showing that siRNA knockdown of α2δ-1 blocks synaptogenesis via specific inhibition of rat α2δ-1 mRNA. Taken together these results demonstrate that α2δ-1 is necessary for TSP and astrocyte-induced synapse formation in vitro. Since we analyze the effect of α2δ-1 overexpression and knockdown in the postsynaptic cells receiving synapses, these data show a postsynaptic sufficiency and necessity for α2δ-1 in astrocyte/TSP-induced synapse formation.
α2δ-1-mediated synapse formation does not depend on calcium channel surface level or function
α2δ-1 is known to enhance calcium channel function and trafficking (Arikkath and Campbell, 2003). We therefore investigated whether the activity of α2δ-1 in synapse formation is linked to its role in increasing calcium currents or calcium channel levels. Gene expression analysis of RGCs show that these cells express predominantly post-synaptic L-type and pre-synaptic N and P/Q type voltage gated calcium channels (VGCCs). To directly test whether VGCC function was required for astrocyte-induced synapse formation, we added L-type calcium channel blockers to RGCs to block L-type channel function. These drugs had no effect on SD2-induced synapse formation (Figure S5A). Similarly pre-synaptic N and P/Q type channel blockers did not block TSP-induced synapse formation (not shown). We next investigated whether increasing postsynaptic L-type calcium channel expression in RGCs would enhance synapse formation. Overexpression of L-type α1C and β subunits in RGCs had no effects on astrocyte-induced synapse formation (Figure S5B). Finally we tested whether TSP treatment would lead to an increase in cytoplasmic calcium levels in RGCs. Neither acute nor long-term TSP treatment led to a noticeable rise in spontaneous calcium oscillations in RGCs (Figure S6). Taken together these results show that the role of α2δ-1 in synapse formation cannot be directly linked to calcium channel expression levels or function.
Overexpression of α2δ-1 in neurons enhances synapse formation in vivo
To determine whether α2δ-1 plays a role in synapse formation in vivo we examined synapse number and synaptic activity in transgenic mice that selectively overexpress α2δ-1 in CNS neurons, under the control of the Thy1 promoter (Li et al., 2006). Sagittal brain sections from 21-day-old (P21) transgenic (TG) and wildtype (WT) littermate mice were co-immunostained for PSD95 and either the presynaptic vesicular glutamate transporter 1 or 2 (VGlut1 and VGlut2). We quantified the number of co-localized pre and postsynaptic puncta to determine the synaptic density in the cortices of these mice. The TG mice had significantly higher numbers of VGlut2 positive excitatory synapses in the cortex when compared with the littermate WT controls (1.8 fold, Figures 5A–5B), however there was no difference in the number of VGlut1 positive synapses between WT and TG mice (S7A–S7B). The observation that α2δ-1 overexpression increases VGlut2 positive synapses provides evidence that excitatory synapse formation is enhanced in the TGs.
Figure 5. α2δ-1 overexpression in vivo increases excitatory synapse number.
(A) Immunolabeling of cortices from littermate wildtype (WT) and α2δ-1 overexpressing transgenic (TG) P21 mice for VGlut2 and PSD95. Number of co-localized VGlut2/PSD95 puncta (white arrows in inlays i and ii) was higher in the TGs then the WTs. Scale bars = 20µm.
(B) Quantification of VGlut2/PSD95 co-localization in brain sections from WT and TG mice. (*p<0.05).
(C) Representative raw data traces of mEPSCs from layer IV cortical pyramidal neurons recorded from a WT and α2δ-1 TG mouse. Top, condensed trace. Bottom, expanded trace.
(D) Summary of the frequency of mEPSCs in layer IV cortical pyramidal neurons of α2δ-1 TG and WT. TG = 3.5±0.3Hz (n=11 cells), WT = 2.1±0.2Hz (n=12 cells), p=0.002.
(E) Summary of the amplitude of mEPSCs in layer IV cortical pyramidal neurons of α2δ-1 TG and WT. TG = 11.9±0.1pA, WT = 11.6±0.1pA, p=0.1.
In the adult cortex thalamic neurons projecting onto layer IV neurons form VGlut2 positive synapses, while synapses made between cortical neurons contain VGlut1 (Fremeau et al., 2004). We confirmed that the increase in VGlut2 positive synapse number was not due to an increase in the number of neurons in the cortex or thalamus, as the number of cells and neurons in WT and TG brains were identical in these brain regions (Figures S8A–S8B).
Excitatory synapses in the cortex are initially formed as VGlut2 positive, and there is an isoform switch to VGlut1 that happens around the second week of postnatal development (Miyazaki et al., 2003). During this period some synapses can transiently be both VGlut1 and 2 positive (Nakamura et al., 2005). We determined that the increase in VGlut2 positive synapses was not due to a prolonged co-localization of VGlut1 and 2 at the same synapse, since these proteins seldom co-localized at P21, and there were no differences in the frequency of co-localization of these proteins between genotypes (Figures S9A–S9B). Taken together these results show that the increase in VGlut2 positive synapses associated with α2δ-1 overexpression is neither due to an increase in the number of cortical or thalamic neurons nor due to a delay in the isoform switch from VGlut2 to 1 in the cortex.
In addition to analyzing synapse number by immunohistochemisry, we performed whole-cell patch-clamp recordings in layer IV cortical pyramidal neurons and assayed the number of active synapses by analyzing the frequency and amplitude of miniature excitatory postsynaptic currents (mEPSCs). Recorded cells were dye filled and their identity was verified (Figures S10A–S10B). We targeted layer IV pyramidal neurons both because these cells receive VGlut2 positive synapses and because array tomography revealed an increase in α2δ-1 immunostaining in TG animals in this layer (Figure S10C). There was a highly significant increase in the frequency of mEPSCs in α2δ-1 TG mice compared with WT (1.63 fold), with no effect on the amplitude of mEPSCs (Figures 5C–5E). The increase in the frequency of mEPSCs in TG mice is very consistent with the increased excitatory synapse number found by the immunohistochemical analysis described above. Taken together, these data show that α2δ-1 plays a role in promoting excitatory synapse formation in the brain.
Gabapentin, the high affinity ligand for α2δ-1, strongly inhibits TSP-induced synapse formation
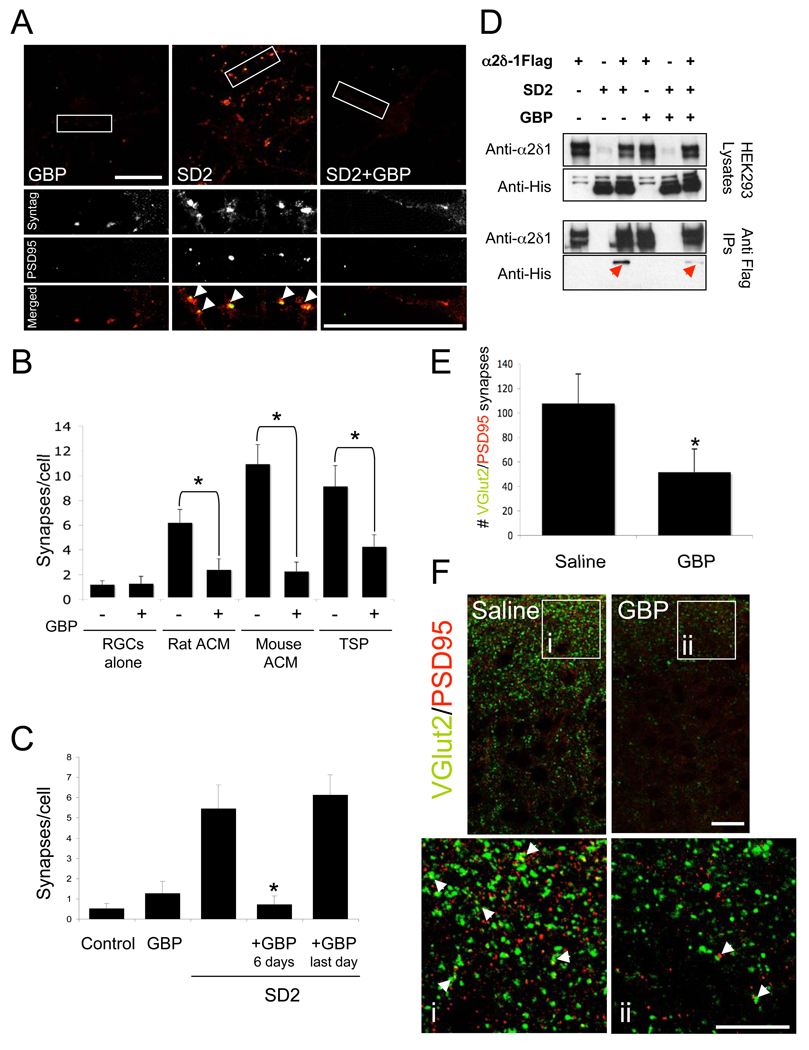
In order to determine whether GBP, the high affinity ligand for α2δ-1, affects TSP or astrocyte-induced synapse formation, we cultured RGCs with TSP, SD2 or ACM in the presence or absence of GBP (32µM). GBP strongly inhibited TSP, SD2 or astrocyte-induced synapse formation (Figure 6A–6C, Figure S1C). To determine whether GBP could dissolve already established synapses, we cultured RGCs with SD2 for 5 days to allow synapses to form and then added GBP for an additional day. GBP had no effect on synapse number when added after the synapses were formed (Figure 6C). Thus GBP blocks new synapse formation induced by TSP and astrocytes, but does not dissolve established synapses. Interestingly, GABA, an inhibitory neurotransmitter that binds to α2δ-1 with much lower affinity (IC50=650µM; (Suman-Chauhan et al., 1993)), also blocked SD2-induced synapse formation when used at high concentrations (Figure S11).
Figure 6. Gabapentin inhibits TSP/astrocyte induced synapse formation.
(A) Immunostaining for synaptotagmin (red) and PSD-95 (green) in RGCs treated with SD2 in the presence or absence of GBP Scale bars = 30µm.
(B) Quantification of the effect of GBP on astrocyte or TSP-induced synapse formation and (C) on SD2-induced synapse formation. GBP blocks SD2’s synaptogenic effect when added simultaneously with SD2 but not when added after synapses have formed. n=20 cells, Error bars mean ± SEM, * p<0.05.
(D) Western Blot analysis of effect of GBP on the SD2- α2δ-1 interaction. Red arrows point to SD2 protein co-immunoprecipitated with α2δ-1FLAG. Anti-α2δ-1 antibody also recognizes weakly expressed endogenous human α2δ-1 expressed by HEK293 cells (lanes 2 and 5, top blots).
(E) Quantification of co-localization of VGlut2 and PSD95 in brain sections from saline and GBP injected P7 mice (*p<0.05).
(F) Immunolabeling of saline and GBP injected P7 cortices for VGlut2 (green) and PSD95 (red). Half of GBP injected mice had a very strong reduction in the number, size and co-localization of synaptic puncta (white arrows, inlays i versus ii). Scale bars = 20µm.
To determine whether GBP blocks TSP-induced synapse formation by inhibiting the α2δ-1-TSP interaction, we co-cultured two populations of HEK293 cells, one expressing α2δ-1FLAG and the other expressing SD2, in the presence or absence of GBP. Immunoprecipitation with anti-FLAG antibodies revealed that the SD2-α2δ-1 interaction was diminished in the presence of GBP (Figure 6D) suggesting that GBP blocks TSP-induced synapse formation by interfering with the interaction between α2δ-1 and TSP.
To test whether GBP similarly blocks synapse formation in vivo, we injected neonatal mice with either GBP or saline for the first postnatal week, which coincides with the initiation of synapse formation in the brain. At this age glutamatergic synapses in the cortex are predominantly VGlut2 positive (Miyazaki et al., 2003). Therefore, we co-immunostained sagital brain sections from P7 saline or GBP-injected mice with antibodies against VGlut2 and PSD95, and quantified the number of co-localized pre and postsynaptic puncta in the cortex of these mice. There were significantly fewer excitatory synapses in the cerebral cortex of the GBP-injected mice relative to control mice (Figure 6E). This difference was mainly due to a severe decrease in synapse number in half of the GBP injected animals. In the mice that responded strongly to GBP the VGlut2/PSD95 synaptic densities went down profoundly, to less than 10% of the saline injected values, although the number of neurons did not change. GBP injection affected both VGlut2 and PSD95 puncta by reducing their number, size and co-localization (Figure 6E) similar to its effect on synaptic puncta in vitro. These findings show that GBP is a powerful inhibitor of new synapse formation both in vitro and in vivo.
Inhibition of TSP-induced synapse formation interferes with lesion-induced barrel cortex plasticity
To determine if astrocyte-induced synapse formation is involved in remodeling neural circuits during development, we utilized a well-established developmental plasticity paradigm, ‘the barrel cortex plasticity’ assay. The nerves that innervate the major whiskers on the snout of the mouse project to the brain as a topographically ordered “somatotopic” map (Erzurumlu et al., 2006). In the primary somatosensory cortex this map is organized as “barrels” (Figure 7A) that exhibit structural changes in response to peripheral whisker manipulations.
Figure 7. TSP induced synapse formation is involved in barrel cortex plasticity.
(A) Schematic presentation of the experimental paradigm: ablation of the C-row of whiskers at P1 causes corresponding reorganization of barrel representations at P7 in contralateral hemisphere.
(B Immunolabeling of thalamocortical afferents to the barrel cortex with an antibody against 5HT transporter. Left images show control barrel cortex. Right images are representative examples of lesion-induced plasticity following whisker follicle ablation in mice that were injected with saline (top), with GBP (middle). Bottom row are control (left) and lesioned (right) barrel cortices from a TSP1/2KO mouse. Arrows flank the C-row of barrels corresponding to lesioned whiskers. Brackets and dashed lines show the expansion of D-row barrels. Asterisks denote regions of abnormal lesion-induced plasticity.
(C) Hematoxylin staining of the whisker pads from mice whose barrels are shown in (B) showing selective ablation of C row follicles.
To test whether TSP-induced synapse formation is involved in mechanisms of experience-dependent plasticity, we injected two groups of neonatal mice either with GBP or saline daily starting at P0 until P7. On P1, five whiskers from the C-row on one side of each mouse were lesioned. The mice were sacrificed at P7 and barrel cortex organization in both the unlesioned “control” and the lesioned hemisphere was analyzed. Both saline and GBP injected mice had typical barrel organization formed on the control side (Figure 7B, top two left panels). On the lesioned side, while all saline injected mice displayed a normal barrel cortex plasticity pattern, 50% of the GBP injected mice displayed an atypical plasticity response (Figure 7B, right panels), where the A and B rows as well as the C row lost form and fused, even though the whisker follicles for these rows were undisturbed in all mice (Figure 7B–7C and Figure S12).
To more directly test the role of TSPs, we examined barrel cortex plasticity in TSP1/2 double null (KO) mice. A third of the TSP1/2KO mice we analyzed showed a very similar, aberrant barrel cortex plasticity phenotype (Figure 7B, bottom right), a pattern we never observed in any of the WT mice. These findings suggest that astrocyte-secreted TSPs are required for rewiring of the barrels post injury and that the main effect of GBP in barrel cortex plasticity is mediated by its inhibition of TSP-induced synapse formation.
DISCUSSION
α2δ-1 is the neuronal thrombospondin receptor responsible for synaptogenesis
The molecular interactions that regulate initiation of synapse formation are not well characterized. Our finding that α2δ-1 is the TSP receptor required for synaptogenesis provides molecular insight into the mechanism of synapse formation and raise the question of how TSP-α2δ-1 interaction leads to initiation of synaptogenesis.
Our findings lead us to the following working model: α2δ-1 is the extracellular ligand-binding portion of a postsynaptic “synaptogenic signaling complex” (Supplemental Figure S13) that regulates formation of an initial synaptic adhesion between a dendrite and an axon. TSP binding to the VWF-A domain of α2δ-1 causes a structural rearrangement in this molecule, which triggers subsequent conformational shifts in its binding partner(s) and switches this complex to an “active” state. α2δ-1 activation by TSP then leads to inter- and intra-cellular signaling events that trigger the recruitment of synaptic adhesion and scaffolding molecules to nascent synaptic sites. VWF-A domains are known protein-protein interaction domains that act as conformational switches and alter a protein’s structure upon binding to its ligand (Bork and Rohde, 1991; Whittaker and Hynes, 2002). The fact that antibodies directed against the VWF-A domain of α2δ-1 can mimic the synaptogenic function of TSP also suggests a binding-induced activation of α2δ-1 in synapse formation.
It is unlikely that α2δ-1 can induce intracellular signaling by itself since it has a very short cytoplasmic tail and the extracellular domain of α2δ-1 is able to mimic the full-length α2δ-1’s function in synapse formation. α2δ-1 may be linked to intracellular signaling mechanisms via other membrane proteins. A calcium channel α1 subunit can be a part of this complex. α1, after being recruited by α2δ-1 to dendrite-axon contact sites, could undergo conformational changes induced by TSP-α2δ-1 interaction and potentially serve as a platform for the nucleation of synaptic proteins at the new synaptic site (Supplemental Figure S13).
α2δ-1 has previously been shown to enhance calcium currents and surface trafficking of calcium channel α1 subunits (Arikkath and Campbell, 2003). Is α2δ-1’s role in synapse formation linked this function? We have several observations that suggest otherwise. First, overexpression of α2δ-1 in the absence of TSP enhances calcium channel surface expression (Gurnett et al., 1996), but does not lead to an increase in synapse number. Second, the α2δ-1Adh protein can mimic the effect of full-length α2δ-1 in enhancing synapse formation, but it does not induce the increase in calcium currents as the full-length protein (Gurnett et al., 1996). Third, neither the overexpression nor the pharmacological blocking of calcium channels interfered with TSP-induced synapse formation. Similarly, acute or long-term TSP treatment did not increase cytoplasmic calcium levels in RGCs, thus it is unlikely that TSP triggers activation of a homeostatic mechanism that can activate synapse formation. Taken together our results show that global changes in calcium channel numbers or currents are not involved in TSP-induced synapse formation. However, since α2δ-1Adh construct, which enhances synapse formation, can interact with the α1 subunit, and since the δ-1, which inhibits synapse formation, can interfere with the α2δ-1 and α1 interaction, a physical interaction between α2δ-1 and the calcium channel α1 subunits might be important for synapse formation. Future studies exploring whether knocking down expression of α1 subunits affect TSP-induced synapse formation are necessary to verify this possibility.
α2δ-1 might also interact with other proteins that are involved in organization of synaptic contacts. Such dual functions have been described for the γ subunits of VGCCs also known as stargazins. They were initially isolated as a component of the calcium channel complex but are now known to play primary roles in AMPA receptor regulation (Chen et al., 2000). Identification of the relevant α2δ-1 interacting molecules promises to provide new molecular insight into the process of synapse formation. In addition there could be other CNS molecules that share TSP’s and GBP’s abilities to bind to α2δ-1 and trigger or inhibit synapse formation.
Our findings have a number of important implications for future studies. First, TSP and α2δ-1 are also highly concentrated at neuromuscular junction, thus it is likely that these molecules are involved in formation of this synapse (Arber and Caroni, 1995; Arikkath and Campbell, 2003). Second, other α2δ family members might also regulate synapse formation. In fact, disruption of the α2δ-4 gene in mice, leads to a severe loss of ribbon synapses in the photoreceptor cells (Wycisk et al., 2006) and mutations in α2δ-3 cause defects in synaptic transmission and a morphological defect in presynaptic organization at the Drosophila neuromuscular junction ((Dickman et al., 2008); T. Schwarz personal communication). These observations suggest that the function of α2δ subunits in promoting synaptogenesis may be evolutionarily conserved and can be exerted presynaptically as well as postsynaptically.
Gabapentin is a powerful blocker of synapse formation
Our findings suggest that GBP blocks TSP-induced synapse formation by interfering with TSP-α2δ-1 interaction. GBP binding to α2δ-1 involves a region just upstream of the VWF-A domain in α2 (Wang et al., 1999). Therefore it is unlikely that TSP and GBP compete for the same binding site. It is known for integrins that conformational changes in VWF-A domains can be constrained by interactions made by regions flanking this domain (Bork and Rohde, 1991; Whittaker and Hynes, 2002). We propose that GBP binding to α2δ-1 restricts the conformation of the VWF-A domain and keeps α2δ-1 in its “inactive conformation”. This perturbs the TSP-α2δ-1 interaction and inhibits activation of the synaptogenic signaling complex (Supplemental Figure S13).
GABA, leucine and isoleucine can also bind to α2δ-1, albeit at lower affinity than GBP (Dooley et al., 2007), thus they can be physiological ligands for α2δ-1 and regulate excitatory synapse formation. In agreement with this we found that high concentrations of GABA inhibited synapse formation in culture. Such high concentrations of GABA are present in the CNS right next to a GABAergic axon. Dendritic filopodia in the developing brain actively seek for synaptic partners and establish exclusively glutamatergic contacts. Interestingly, dendritic filopodia that contact a GABAergic axon never stabilize the contact and retract (Lohmann and Bonhoeffer, 2008; Wierenga et al., 2008). In future studies, it will be interesting to explore whether α2δ-1 functions as a physiologically relevant GABA receptor that enables initial selectivity for the formation of excitatory synapses by dendritic filopodia.
α2δ-1-TSP interaction regulates synapse formation during development and after injury
The ability of GBP to strongly decrease synapse formation in wildtype mouse brains points to a critical role for TSP-α2δ-1 interaction and astrocytes in driving synaptogenesis in vivo. In addition, the correct execution of barrel cortex plasticity depends on TSP-induced synapse formation. Since the unlesioned barrel cortices are formed normally both in GBP injected and TSP1/2 KO mice, TSPs might specifically play a role in synaptic remodeling-plasticity upon injury in this system. These findings add to the growing data that astrocytes not only actively contribute to normal synaptogenesis but also mediate synaptic remodeling events after injury.
It is interesting that the effect of GBP in vivo is an “all or none” effect rather than a fractional decrease in synapse number, and only 50% of the mice responded strongly to GBP injections. It is possible that a critical threshold concentration of GBP in the cerebrospinal fluid is required to be effective in blocking synapse formation, which is only achieved in half of the mice. Gender could be critical in GBP responsiveness by effecting in GBP delivery to neural tissues and can explain the 50% penetrance we have observed. In fact a recent study demonstrated that intraperitoneal GBP injections were not as effective in blocking seizures in female mice than in males (Traa et al., 2008).
Since GBP strongly blocks TSP-induced synapse formation within its therapeutic concentration, it is possible that inhibition of excitatory synapse formation is an important mode of its therapeutic action in epilepsy and pain. Reactive astrocytosis is prominent both in epileptic lesions and in the spinal cord after peripheral nerve injury that leads to neuropathic pain (Liu et al., 2000; Ridet et al., 1997). Reactive astrocytes express high levels of TSPs 1 and 2 (Lin et al., 2003). Similarly, upon injury in the spinal nerve, both α2δ-1 and TSP4 genes are upregulated in the spinal cord (Valder et al., 2003; Wang et al., 2002). Increased α2δ-1 levels were shown to lead to enhanced excitatory synaptic transmission and elevated neuropathic pain states (Li et al., 2004; Li et al., 2006). Similarly, there is increased excitation in the epileptic brain (Prince, 1999). All these observations point to the possibility that aberrant excitatory synaptogenesis may contribute to the pathophysiology of neuropathic pain and epilepsy. Thus GBP may act by limiting these excess synapses from forming, a possibility which can now be directly tested in animal models of these diseases.
In conclusion, by identifying α2δ-1 as a receptor for TSP mediating glial-induced synapse formation, we have gained molecular understanding not only of astrocytes’ role in synapse formation in health and disease, but also of the process of synapse formation itself.
EXPERIMENTAL PROCEDURES
Materials and other experimental procedures are described in the Supplemental Data
Purification and culture of RGCs and astrocytes
RGCs were purified with greater than 99.5% purity from P5 Sprague-Dawley rats (Charles Rivers) and cultured in serum-free medium as previously described (Christopherson et al., 2005; Meyer-Franke et al., 1995; Ullian et al., 2001). Cortical astrocyte inserts and ACM were prepared as described in (Christopherson et al., 2005). RCGs were cultured for 3–4 days to allow robust process outgrowth and then cultured with astrocyte inserts, ACM or TSPs for an additional 6 days.
Mice
TSP1/2 double null mice on an FVB background were used (n=12) (Agah et al., 2004). WT FVB mice were purchased from Charles River Laboratories. Brains from P21, α2δ-1 overexpressing, TG mice and their littermate WT controls (n=8) were provided by Li and colleagues and are described in (Li et al., 2006).
Quantification of Synapse Numbers
For synapse quantification of RGCs we followed a previously developed immunohistochemistry (IHC) based method described and validated in (Christopherson et al., 2005; Ullian et al., 2001).
For quantification of excitatory synapse number in mouse brain, three sagital brain sections per animal were stained with pre- and post-synaptic markers and 5µm confocal scans were performed (optical section width 0.38µm, 14 optical sections each) at the cortex. The parameters for scanning were always setup for WT (or saline injected) brain sections and same imaging parameters were used for TG (or GBP injected) animals. Merged single optical section images at 1µm intervals were analyzed using the ImageJ-puncta analyzer option to count for number of co-localized pre- and post-synaptic puncta (5 optical sections/section, 15 images/brain). Average synaptic density per imaged area was calculated for each condition. Details on IHC conditions, image acquisition and quantification can be found in Supplemental Data.
Electrophysiological recordings
Experiments were carried out on littermate WT and α2δ-1 transgenic mice aged P21–P25, and recordings and analysis were both carried out blind to genotype. Whole-cell voltage-clamp recordings of layer IV pyramidal neurons in the visual cortex were carried out at room temperature in flowing isotonic saline containing 1 µM and 40 µM bicuculline to isolate mEPSCs. mEPSCs were recorded for one minute and analysed using Minianalysis software from Synaptosoft.
Saline and gabapentin injections
Mice were given daily intraperitoneal injections of either a single dose of 400mg/kg of GBP (Sigma-Aldrich) or a matching volume of saline solution (PBS). Pups were weighed just before injections to determine the dose administered and to follow weight gain and general health, which showed no differences between GBP and saline injected mice.
Whisker lesions and Barrel cortex immunohistochemistry
Neonatal mice were held on their left side under a dissecting scope and received two parallel incisions with a surgical blade flanking the C row of whiskers to be removed. The skin between the incisions was pulled back with forceps. Follicles were individually removed with forceps at the opening. The lesion site was then cauterized with silver nitrate using flexible caustic applicators (Tech-Med). Mice were allowed to recover in their home cage.
P7 mice were sacrificed and brains were harvested. Samples were blinded during rest of the analysis of the barrel cortex plasticity. Tangential cortical sections were stained with anti-serotonin (5-HT) transporter rabbit polyclonal antibody (Calbiochem, 1:400) Barrels were imaged using a Nikon Eclipse E800 fluorescent microscope, and images were digitally acquired using an SPOT camera (Diagnostic Instruments). The complete maps of the barrel cortex were reassembled from 5-HTT stained images of serial sections by reconstructing in Photoshop (Adobe Systems). Details on the immunohistochemistry conditions, image acquisition and data analysis can be found in Supplemental Data.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Maria L. Fabian, Navid Nouri and Mark Duquette for excellent technical assistance, and Drs Beth Stevens, Junryo Watanabe and Alissa Winzeler for critical reading of the manuscript. This work was supported by grants from the National Institute of Drug Addiction (DA15043 to B.A.B.), the National Heart, Lung and Blood Institute (HL49081 to J.L.), and the National Institute of Health (NS40135 and DE14545 to Z.D.L.). C.E. and N.J.A. were supported by the Human Frontiers Scientific Program long-term fellowships, and A.D.H by the Helen Hay Whitney postdoctoral fellowship. Arnon Rosenthal is founder and president of MAZORX Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams JC, Lawler J. The thrombospondins. The international journal of biochemistry & cell biology. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis DS, Gunderson KA, Mosher DF. Immunochemical analysis of the structure of the signature domains of thrombospondin-1 and thrombospondin-2 in low calcium concentrations. The Journal of biological chemistry. 2007;282:27067–27075. doi: 10.1074/jbc.M703804200. [DOI] [PubMed] [Google Scholar]
- Annis DS, Murphy-Ullrich JE, Mosher DF. Function-blocking antithrombospondin-1 monoclonal antibodies. J Thromb Haemost. 2006;4:459–468. doi: 10.1111/j.1538-7836.2006.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Caroni P. Thrombospondin-4, an extracellular matrix protein expressed in the developing and adult nervous system promotes neurite outgrowth. The Journal of cell biology. 1995;131:1083–1094. doi: 10.1083/jcb.131.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Current opinion in neurobiology. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Bork P, Rohde K. More von Willebrand factor type A domains? Sequence similarities with malaria thrombospondin-related anonymous protein, dihydropyridine-sensitive calcium channel and inter-alpha-trypsin inhibitor. The Biochemical journal. 1991;279(Pt 3):908–910. doi: 10.1042/bj2790908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. The international journal of biochemistry & cell biology. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci. 2008;65:672–686. doi: 10.1007/s00018-007-7484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA, Gu G. Differential distribution of voltage-gated calcium channel alpha-2 delta (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. The Journal of comparative neurology. 2005;491:246–269. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends in pharmacological sciences. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila alpha2delta voltage-gated calcium channel subunit reveal a crucial synaptic function. J Neurosci. 2008;28:31–38. doi: 10.1523/JNEUROSCI.4498-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends in pharmacological sciences. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA, Stevens B. Glia as active paricipants in the development and function of synapses. In: Hell J, Ehlers M, editors. Structural and Functional Organization of the Synapse. Springer; 2008. [Google Scholar]
- Erzurumlu RS, Chen ZF, Jacquin MF. Molecular determinants of the face map development in the trigeminal brainstem. The anatomical record. 2006;288:121–134. doi: 10.1002/ar.a.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Umemori H. Seeking long-term relationship: axon and target communicate to organize synaptic differentiation. Journal of neurochemistry. 2006;97:1215–1231. doi: 10.1111/j.1471-4159.2006.03834.x. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends in neurosciences. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Garcia K, Nabhani T, Garcia J. The calcium channel {alpha}2/{delta}1 subunit is involved in extracellular signaling. J Physiol. 2007 doi: 10.1113/jphysiol.2007.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. The Journal of biological chemistry. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel alpha 2 delta subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, Strand A, Torcassi C, Savage J, Hurlburt A, et al. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS genetics. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function, and drug binding. Journal of bioenergetics and biomembranes. 2003;35:639–647. doi: 10.1023/b:jobb.0000008028.41056.58. [DOI] [PubMed] [Google Scholar]
- Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, et al. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liauw J, Hoang S, Choi M, Eroglu C, Choi M, Sun GH, Percy M, Wildman-Tobriner B, Bliss T, Guzman RG, et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab. 2008;28:1722–1732. doi: 10.1038/jcbfm.2008.65. [DOI] [PubMed] [Google Scholar]
- Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke; a journal of cerebral circulation. 2003;34:177–186. doi: 10.1161/01.str.0000047100.84604.ba. [DOI] [PubMed] [Google Scholar]
- Liu L, Rudin M, Kozlova EN. Glial cell proliferation in the spinal cord after dorsal rhizotomy or sciatic nerve transection in the adult rat. Experimental brain research Experimentelle Hirnforschung. 2000;131:64–73. doi: 10.1007/s002219900273. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Fukaya M, Shimizu H, Watanabe M. Subtype switching of vesicular glutamate transporters at parallel fibre-Purkinje cell synapses in developing mouse cerebellum. The European journal of neuroscience. 2003;17:2563–2572. doi: 10.1046/j.1460-9568.2003.02698.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hioki H, Fujiyama F, Kaneko T. Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. The Journal of comparative neurology. 2005;492:263–288. doi: 10.1002/cne.20705. [DOI] [PubMed] [Google Scholar]
- Pluskota E, Stenina OI, Krukovets I, Szpak D, Topol EJ, Plow EF. Mechanism and effect of thrombospondin-4 polymorphisms on neutrophil function. Blood. 2005;106:3970–3978. doi: 10.1182/blood-2005-03-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA. Epileptogenic neurons and circuits. Advances in neurology. 1999;79:665–684. [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends in neurosciences. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Suman-Chauhan N, Webdale L, Hill DR, Woodruff GN. Characterisation of [3H]gabapentin binding to a novel site in rat brain: homogenate binding studies. European journal of pharmacology. 1993;244:293–301. doi: 10.1016/0922-4106(93)90155-3. [DOI] [PubMed] [Google Scholar]
- Traa BS, Mulholland JD, Kadam SD, Johnston MV, Comi AM. Gabapentin neuroprotection and seizure suppression in immature mouse brain ischemia. Pediatric research. 2008;64:81–85. doi: 10.1203/PDR.0b013e318174e70e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science (New York, NY. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. Journal of neurochemistry. 2003;87:560–573. doi: 10.1046/j.1471-4159.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, Holder DJ, Koblan KS. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114:529–546. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Wang M, Offord J, Oxender DL, Su TZ. Structural requirement of the calcium-channel subunit alpha2delta for gabapentin binding. The Biochemical journal. 1999;342(Pt 2):313–320. [PMC free article] [PubMed] [Google Scholar]
- Whittaker CA, Hynes RO. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Molecular biology of the cell. 2002;13:3369–3387. doi: 10.1091/mbc.E02-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nature neuroscience. 2008;11:1044–1052. doi: 10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]
- Wilkins JA, Li A, Ni H, Stupack DG, Shen C. Control of beta1 integrin function. Localization of stimulatory epitopes. The Journal of biological chemistry. 1996;271:3046–3051. [PubMed] [Google Scholar]
- Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nurnberg P, Ruether K, Berger W. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Investigative ophthalmology & visual science. 2006;47:3523–3530. doi: 10.1167/iovs.06-0271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.