Abstract
Evidence for signaling, communication, and conductivity in microtubules (MTs) has been shown through both direct and indirect means, and theoretical models predict their potential use in both classical and quantum information processing in neurons. The notion of quantum information processing within neurons has been implicated in the phenomena of consciousness, although controversies have arisen in regards to adverse physiological temperature effects on these capabilities. To investigate the possibility of quantum processes in relation to information processing in MTs, a biophysical MT model is used based on the electrostatic interior of the tubulin protein. The interior is taken to constitute a double-well potential structure within which a mobile electron is considered capable of occupying at least two distinct quantum states. These excitonic states together with MT lattice vibrations determine the state space of individual tubulin dimers within the MT lattice. Tubulin dimers are taken as quantum well structures containing an electron that can exist in either its ground state or first excited state. Following previous models involving the mechanisms of exciton energy propagation, we estimate the strength of exciton and phonon interactions and their effect on the formation and dynamics of coherent exciton domains within MTs. Also, estimates of energy and timescales for excitons, phonons, their interactions, and thermal effects are presented. Our conclusions cast doubt on the possibility of sufficiently long-lived coherent exciton/phonon structures existing at physiological temperatures in the absence of thermal isolation mechanisms. These results are discussed in comparison with previous models based on quantum effects in non-polar hydrophobic regions, which have yet to be disproved.
Keywords: Microtubules, Information processing, Coherent excitations, Quantum coherence, Phonons, Excitons
Introduction
It is currently assumed that information processing in the brain occurs solely at the level of the neuron. In this assumption, neurons act as Hodgkin–Huxley neurons, whereby incoming synaptic signals received by dendrites are passively integrated within the neuron cell body. If the total membrane potential exceeds some threshold value, an action potential spike is generated. However, it has been shown that passive membrane potential summation cannot account for the complexity of dendritic integration and that spike thresholds in a single neuron may vary from spike to spike [1]. This suggests that active integration is required. Thus far, the most basic subcellular structure proposed for information processing in relation to brain activity is the neuronal cytoskeleton [2, 3]. Microtubules (MTs), a major constituent of the cytoskeleton, have been a focus in this proposition, as they play an essential role within neurons through their role in axoplasmic transport and neuronal plasticity by their regulation of cell shape [4, 5]. The signaling capabilities of MTs have been shown [6, 7], and thus, due to their mesoscale size, they provide an ideal bridge between the classical and quantum regimes. MT-level classical information processing could provide an enormous increase in the brain’s computing power. Considering 1011 neurons in the brain with 104 synapses each switching at a rate of 103 Hz yields a computational power of 1018 operations per second on average. The currently accepted scientific model suggests that cognition and higher brain function arises from this computational complexity. Yet, this computational capability is dwarfed by the operational capabilities of the brain if neuronal MTs are actively involved. There are roughly 107 tubulin dimers, the MT-forming proteins, per neuron that may change their conformational states on the scale of 109 Hz, yielding 1016 operations per second per neuron or 1027 operations per second for an entire brain. If the tubulin dimer acts as a qubit, rather than a classical bit, the computational power is further magnified, becoming almost unimaginably vast.
A concise explanation of the role of MT computation in the overall function of a neuron is given by Priel et al. [8] in their hypothesis of the dendritic cytoskeleton as a computational device. In their model, electrical signals from a presynaptic neuron arrive at the postsynaptic density within a dendritic spine. These signals provoke ionic waves to travel along actin filaments to the connected MT network, serving as input signals. The MT network then evolves the input states via protein conformational changes, as suggested by Priel et al., or changes in the electromagnetic signal. These signals may be propagated along the MT network where they may be picked up by actin filaments and propagated to remote ion channels. These signals may then regulate the temporal gating state of voltage-sensitive ion channels by manipulating the distribution of open and closed channels through the neuron, and thus regulate membrane conductive properties.
Indirect evidence suggests that MTs are computationally relevant to cognitive processes. In addition to MTs propagating cellular signals, interactions between MTs and membrane activities have been clearly recognized [9–11]. Processes directly involved in the functioning of the brain, such as ion channels opening and closing, enzymes catalysis, motor proteins moving cargo inside cells, and the propagation of ionic waves along filaments, may also be inextricably linked to the function of MTs [8, 12]. During a critical period of visual development, neurons in the visual cortex produce massive amounts of tubulin [13]. MT degradation has been linked to the cognitive decline caused by Alzheimer’s disease [14]. As well, anesthetics, which induce unconsciousness, inhibit a number of neurotransmitter receptors, but differ from receptor inhibitors by having effects on the cytoskeleton [15]. While these suggest a possible relation between MTs and cognition, it is still far from trivial to reconcile the existence of quantum behavior with the persistent decoherence effects dominating quantum phenomena at physiological temperatures [16].
Tubulin’s biophysical properties
MTs are tubular polymers consisting of long fibers of the protein tubulin, called protofilaments, set alongside one another in a cylindrical fashion. In vitro studies have shown MTs to be dynamic assemblies [17], with MTs cross-linking themselves via microtubule-associated proteins (MAPs). Such connections form tracks along which organelles and other structures are transported around the cell via motor proteins, such as in the transport of neuronal vesicles from the cell body to the synapse in nerve cells.
The tubulin protein is a heterodimer composed of α-tubulin and β-tubulin monomers. The α-tubulin and β-tubulin monomers are highly homologous with an average weight of 50–55 kDa each. Each monomer is composed of approximately 450 amino acids, or about 7000 atoms [18]. The monomer structures, composed of a double β-sheet core encircled by several α-helices, can be separated into three functional domains: the amino-terminal domain containing a nucleotide-binding region, the intermediate domain containing the taxol and colchicine drug binding sites, and the carboxy-terminal domain containing the suggested motor protein binding region [18]. Both monomers can bind a molecule of GTP, but only the β-tubulin will hydrolyze it to GDP. Two different conformational states of the heterodimer are known to depend on this GTP hydrolysis [19] such that hydrolysis causes a small-angle distortion between the original center-to-center line joining the monomers, releasing 0.42 eV of energy per molecule in free tubulin and a few times less that amount when embedded in a MT [20]. On average, an αβ-heterodimer has dimensions of 46 ×80 ×65 Å and is a polar molecule with its positive end near the β-subunit [21]. Henceforth, unless it is otherwise stated, “tubulin” will be used to refer to the αβ-heterodimer.
The effect of electric fields on MTs is known to be of importance. For example, Vassilev et al. [22] observed aligned MTs assembled in vitro with pulsed electric fields on the order of 10 V/cm in strength, and Kirson et al. [23] have recently demonstrated that AC electric fields at ~2 V/cm can have pronounced mitotic effects in several cancerous cells lines linking them to electric field effects in MTs. As such, the electrical properties of tubulin are under active investigation. Based on the crystal structure of tubulin, computer simulations and experimental tests have yielded an electric dipole moment of 1,740 Debye, a refractive index of 2.90, a high frequency dielectric constant of 8.41, and a high frequency polarizability of 2.1 ×10 − 33 Cm2/V [24]. As well, an electrostatic potential map of the crystal structure has shown that the interior of the tubulin molecule contains a fairly symmetric double-well structure, providing a confining potential for a mobile electron (see [18] and Section 3).
Unfortunately, making direct conductance measurements on biopolymers is difficult due to the structural variety of polymers, the liquid state of samples, and the dependence of biological systems on environmental factors such as pH, temperature, and ion concentration. MT electric properties may involve both intrinsic effects of the protein and ionic contributions from counterions attracted and concentrated near the MT by its electric field. However, recent advances in nanoscale technologies are improving experimental conditions, allowing for serious investigations to take place. Thus, despite the inherent difficulties, a number of experiments have investigated either the intrinsic [25–28] or ionic [26, 29, 30] conductivities of MTs.
Fritzsche et al. [25] made electrical contacts to single dry MTs on a substrate containing gold microelectrodes and found results suggesting that the intrinsic resistance of dry MTs is much greater than the 40 MΩ/μm resistance of their wires. This same group measured MTs with a 30-nm-thick nickel coating [28] and found the resistance to be orders of magnitude lower with conduction due entirely to the metallic coating. Goddard and Whittier [27] reported measurements of samples containing only buffer, tubulin dimers in buffer, MTs in buffer, and MTs with MAPs in buffer, finding average resistances of 999, 424, 883, and 836 Ω, respectively. Assuming that all tubulin is polymerized in the MT cases and that there are uniform MT distributions with combinations of parallel and series networks of 10-μm-long MTs, a longitudinal MT resistance of 0.8 MΩ/μm can be estimated [31]. This compares favorably to an earlier theoretical estimate based on a Hubbard model with electrons hopping between tubulin monomers [32, 33] that predicts MT resistances to be 0.2 MΩ/μm. Additionally, this finding is two orders of magnitude smaller than dry MTs, which is to be expected.
Recently, Umnov et al. [26] attempted conductivity measurements of MTs in microchannels containing solution and determined a 90-S/m upper bound on the conductivity of a MT, corresponding to a minimum resistance of 24 MΩ/μm, close to the Fritsche et al. [28] result. As well, Minoura and Muto [30] made an attempt to measure the ionic conductivity of MTs using an electric field to orient MTs in solution and found a conductivity of 150 mS/m corresponding to a resistance of 27 GΩ/μm along the MT length. The overall picture of an induced dipole moment arising due to the polarizability of the MT counterion system suggested by Minoura and Muto is consistent with another study of ionic conductivity along electrically stimulated MTs in which isolated taxol-stabilized MTs were shown to conduct an electric pulse applied through a micropipette [29]. The current fraction collected by a second micropipette at the other end of the MT was 70% greater than the control current fraction between the same pipettes with no MT between them, indicating a higher conduction along MTs than in the buffer.
While it is difficult to separate the intrinsic and ionic contributions, the conduction properties of MTs seem to indicate the capability for electrical signal transduction and information processing, but the question remains how the delicate, weak, very small-scale quantum processes of electron movements within MTs can influence brain cell firing and communication.
A microscopic model of tubulin’s electronic degrees of freedom
A fair number of groups have published models of cytoskeletal information processing, including information processing in MTs, which relate cytoskeletal function to computer-related technologies [34]. Classical cellular automata models of MTs based on tubulin dipole transitions, represented as a discrete charge within the tubulin dimer, have demonstrated self-organizing patterns suggesting the potential for MTs to process information [35–37]. It has been shown that MT cellular automata networks may signal, adapt, recognize, and subserve neural-level learning [38]. Such models have also been used to simulate associative learning in MT networks as well as the dynamics of MT assembly and disassembly [36]. These models were highly speculative in nature with few parameters determined from experimental information. However, we are currently able to use atomic resolution information about the structure of tubulin in our computational models. Mershin et al. [24] have developed a model that treats MT as quantum mechanically isolated cavities exhibiting properties analogous to the electromagnetic cavities used in quantum optics and have suggested several experimental tests to further this idea.
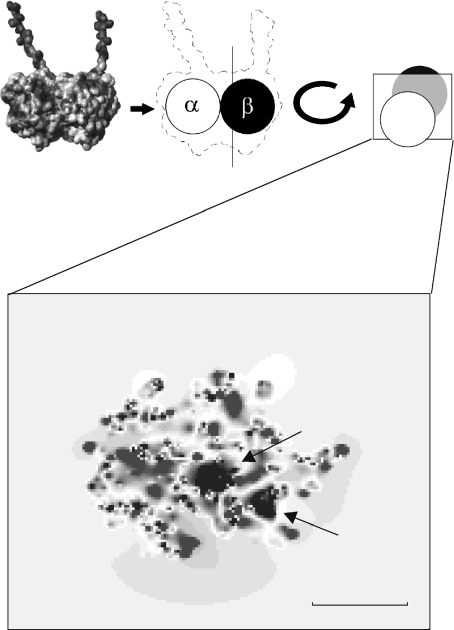
Recent studies using electron crystallography on zinc-induced tubulin sheets have yielded a refined structure of the tubulin molecule with a 3.5-Å resolution [39]. Solving the Poisson–Boltzmann equation for this structure via the adaptive Poisson–Boltzmann solver software [40] produced an electrostatic potential map of the protein. Two regions of positive potential surrounded by negative potential can be found by taking cross-sections of the resulting electrostatic map (see Fig. 1). The region is located near the separation between α-tubulin and β-tubulin, approximately 4.5 nm from the tip of the α-monomer, with the positive potential regions separated by 2 nm. This structure may provide a local double-well potential for a mobile electron to undergo an electron transfer process within the protein. As stated previously, making direct electrical measurements on biopolymers is difficult; as such, the electrostatic map is currently the only experimental evidence for such a structure.
Fig. 1.
Cross-section of an electrostatic map of the tubulin dimer (shown upper left) showing the protein’s interior. Black arrows indicate the regions of positive potential taken to constitute the double-well structure. Scale bar = 2 nm
It is common to have hydrophobic neutral residues in the interior of a protein, yet the double-well region seems decidedly polar: a negative electron with two positive holes. Examining the protein around the double-well region through the use of the program Visual Molecular Dynamics [41], it was found that the surrounding amino acids are mainly charged with only a few hydrophobic residues in the vicinity. Of particular interest is tryptophan (α-407) [39]. It is positioned approximately 8 Å away from the left well, as pictured in Fig. 1. This residue could be capable of supplying a mobile electron to the double-well structure for use in electron transfer processes. At physiological energies, long-range electron tunneling appears to be viable mechanism for standard electron transfer in proteins [42], with consecutive electron tunneling jumps occurring between specific redox sites or protein-bound cofactors [43, 44]. Typically, distances range between 10 and 15 Å [45, 46] with the interaction greatly diminished with distances greater than l0 Å [47]. Studies on tryptophan residues have yielded experimental evidence suggesting that electron transfer within and among tryptophan residues may mediate protein function [48, 49]. Additionally, Becker et al. [50] have shown non-radiative photon exchange between tryptophan and aromatics in adjacent tubulin dimers and between MTs and membranes. This suggests that tryptophan has a large electron resonance in comparison to other aromatics and is therefore a highly suited amino acid for transiting electrons and exchanging photons in MTs.
The double-well region was also observed to be approximately located <5 Å from the colchicine binding site as detailed in [51] and <8 Å from the non-hydrolyzable GTP on the α-tubulin monomer. This is of interest as colchicine is a MT inhibitor, and GTP must be bound to both the α- and β-monomers for a tubulin heterodimer to associate with other heterodimers to form a MT. The location of the double well within a short distance of these two cofactor-binding sites suggests that it may be related to the stability of the dimer and its ability to polymerize.
Furthermore, it can be found from the Coulomb interaction between an individual mobile electron interacting with mobile electrons of the surrounding nearest-neighbor electrons that the maximum energy of a bound electron is approximately 0.6 eV (with the distances given in Fig. 2, and a dielectric constant of 2). While the energy gap to the conduction band in tubulin is unknown, a typical gap value between 2.57 and 3.12 eV has been reported for a number of proteins, with 1.2 eV being the lowest value recorded for cytochrome C [52]. As such, it is reasonable to assume that the energies of the mobile valence electrons in the double-well structures are insufficient to move the electrons to the conduction band in tubulin. Thus, for the purpose of the model investigated here, the electrostatic interior of the tubulin dimer may be conveniently considered to be an infinite double-well potential defined as
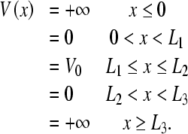 |
1 |
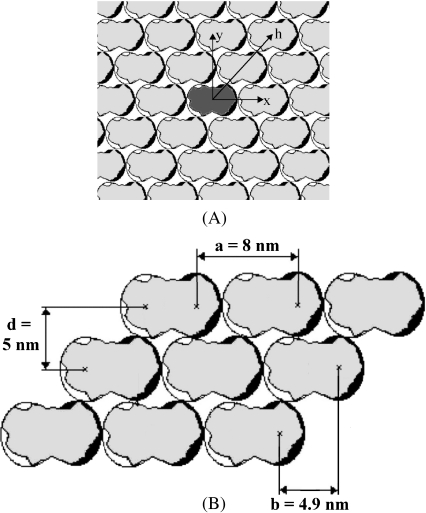
Fig. 2.
Diagram of tubulin neighborhood groups. a Symmetry around a central dimer, x protofilament direction, y MT circumference, and h dimer helix direction. b Distances between dimers
The wavefunction of the electron in the above potential is defined as the eigenfunction 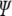 (x) of the one-dimensional time-independent Schrödinger equation
(x) of the one-dimensional time-independent Schrödinger equation
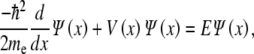 |
2 |
where the corresponding eigenvalues E determine the specific energy levels of the particle, and me is the mass of the electron. Following the work on infinite double square wells, the electron wavefunction bound in the potential described in (1) is constructed from a linear combination of solutions given by the discontinuities of the potential [53]. For electrons to be bound within the double-well structure, they must possess energies below the barrier height V0. The continuity of the wavefunctions and their spatial derivatives at the discontinuities in the potential is required. Enforcing these conditions and solving the equation yields three of the four wavefunction coefficients, with the fourth determined by the normalization condition, and a transcendental equation
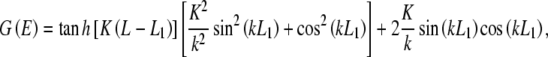 |
3 |
where
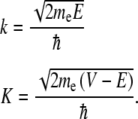 |
4 |
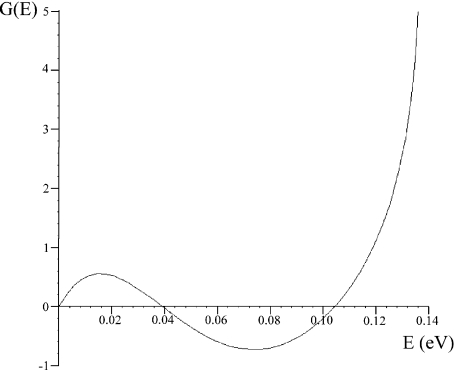
The solutions of (3) where G(E) = 0 yield the energy eigenvalue solutions to (2) and are very difficult to solve algebraically, but are readily obtained numerically. The double-well potential of the tubulin interior possesses a barrier width of approximately 0.2 nm, a separation between the two wells of approximately 2 nm, and an overall length of 3.6 nm. Taking these values and applying them to the double-well potential described in (1) yields L1 = 1.7 nm, 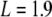 nm, and L3 = 3.6 nm. As indicated previously by Hameroff and Tuszynski [54], the potential well depth V0 in the tubulin dimer may range between 100 and 150 meV. Applying these values and plotting the transcendental equation G against energy E up to a value of the barrier height V0, the possible energy levels of an electron bound within the double-well structure were found. Figure 3 shows an example of such a plot.
nm, and L3 = 3.6 nm. As indicated previously by Hameroff and Tuszynski [54], the potential well depth V0 in the tubulin dimer may range between 100 and 150 meV. Applying these values and plotting the transcendental equation G against energy E up to a value of the barrier height V0, the possible energy levels of an electron bound within the double-well structure were found. Figure 3 shows an example of such a plot.
Fig. 3.
Plot of transcendental equation (3) over energies below a potential barrier height of 150 meV to solve for the energy levels of an electron bound in a symmetric double-well potential
Performing this analysis over the range of 100–150 meV showed that two bound states, a ground state and first excited state, exist for all given barrier heights 105 meV and above. For the minimum barrier height of 105 meV, the ground state and first excited state energy level values were found to be approximately 36 and 104 meV, respectively, with an energy difference between these levels of 68 meV. At the other extreme, with a barrier height of 150 meV, approximate values of 40 and 104 meV were found for the ground and excited states, yielding an energy gap of 64 meV. Thus, the estimated range of the gap between the lowest two energy levels is given as:
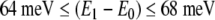 |
5 |
where E0 is the ground state energy and E1 is the energy of the first excited state. This result will be used below when we discuss excitonic degrees of freedom within a MT.
The ground and excited states discussed above are for an electron that is not localized to either well. In terms of information processing, the position of the electron in either of the double wells would alter the electronic conformation of the tubulin dimer, but not necessarily the physical conformation of the protein. For tubulin to act as a quantum bit, the mobile electron must be considered to be in superposition between the two wells. Communication between dimers is essential for information processing to be feasible. As such, the following analysis will consider the mobile electron in the double well to exist in spatial superposition between the two wells rather than being localized to one well with the possibility of tunneling to the other and will not consider any energetic superposition between the ground and excited states. In the next section, we discuss collective excitations and energy transfer within a MT lattice, which, in addition to excitons, include phonons, i.e., quantized lattice vibrations.
Collective excitations in MTs
The phonon system
As mentioned previously, MTs are aggregates of tubulin molecules that are polymerized into a cylindrical form. By considering the MT as an aggregate layer in two dimensions, the lattice vibrations in a sheet of tubulin dimers can be investigated. The second quantized Hamiltonian for a phonon system is given as:
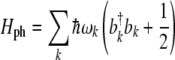 |
6 |
where 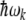 defines the phonon energy quantum. Elastic vibrations in seamless MTs considered as a two-dimensional planar lattice have been investigated previously [55]. It was found that for the canonical MT, a three-start 13-protofilament MT corresponding to the MT-13A-6 lattice type, the protofilament and dimer helical pathways possessed phonon frequencies of
defines the phonon energy quantum. Elastic vibrations in seamless MTs considered as a two-dimensional planar lattice have been investigated previously [55]. It was found that for the canonical MT, a three-start 13-protofilament MT corresponding to the MT-13A-6 lattice type, the protofilament and dimer helical pathways possessed phonon frequencies of 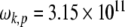 Hz, and
Hz, and 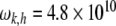 Hz, respectively. It can therefore be estimated that the protofilament and helical phonon energy quanta in the MT system are approximately 0.207 and 0.032meV, respectively. Taking the characteristic phonon times as
Hz, respectively. It can therefore be estimated that the protofilament and helical phonon energy quanta in the MT system are approximately 0.207 and 0.032meV, respectively. Taking the characteristic phonon times as
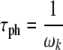 |
7 |
yields values of approximately 3.17 ×10 − 12 and 2.08 ×10 − 11 s for the protofilament and helical directions, respectively.
By simple manipulation of the definitions of lattice vibration properties, it can be shown that the root mean square values of the lattice fluctuations xrms in the high-temperature limit are given for phonons as [56]:
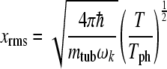 |
8 |
where mtub is the mass of a tubulin dimer taken as 110 kDa. The Debye temperature, Tph, is the characteristic temperature where all phonons become thermally active (i.e., above which they should be taken in the classical limit) and is defined as [57]:
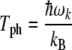 |
9 |
where kB is Boltzmann’s constant. The values obtained, 2.40 K and 0.37 K, for protofilament and helix, respectively, are exceedingly small, indicating an extreme classical limit for lattice vibrations. Moreover, at the physiologically relevant temperature of 310 K, this corresponds to lattice fluctuations of approximately 0.55 and 3.6 Å along the protofilament and dimer helix, respectively. The Debye temperature can be seen to be much less than the physiologically relevant temperature, implying that the thermal environment may easily excite macroscopic numbers of phonon quanta in the MT system. Thus, thermal vibrations will most likely drown out energy supplied to the phonon system via other sources, such as excitons or other phonons, unless the system is somehow shielded from the environment or strongly coupled to other degrees of freedom, such as excitons.
The exciton system
If there is only one electron bound within the double-well potential, then, when it is excited from the ground state to the first excited state, it will leave behind an empty state. This arrangement of an electron and hole is capable of forming an exciton. Thus, the second quantized Hamiltonian for a single exciton system is simply
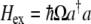 |
10 |
where 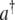 and a define the exciton creation and annihilation operators and
and a define the exciton creation and annihilation operators and 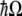 defines the energy difference between the ground and first excited states. As stated in the previous section, for the double-well system described by the tubulin dimer, values of
defines the energy difference between the ground and first excited states. As stated in the previous section, for the double-well system described by the tubulin dimer, values of 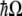 fall in the range 64–68 meV.
fall in the range 64–68 meV.
Mechanisms of excitonic energy transfer in single layers of organic molecules, known as Scheibe aggregates, have been previously examined [58], and we will be guided by these studies in our analysis of MTs. Scheibe aggregates are a class of molecular films that belong to the larger group of Langmuir–Blodgett films which are defined as one or more monolayers of an organic material composed of polar molecules deposited from the surface of a liquid onto a solid by continually submerging and removing the solid substrate in the liquid. In the case of Scheibe aggregates, the polar molecules are dye molecules composed of chromophores and fatty acids that form into a brick-layer-type arrangement. Exciton energy transfer in Scheibe aggregates can be understood in terms of an energy hopping mechanism, which is governed by the interaction of molecular dipoles between dye molecules. That is, assuming that the exciton energy can be transferred between two molecules via a hopping mechanism, the simplest second quantized Hamiltonian takes the form
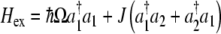 |
11 |
where J is the hopping constant and should correspond to the dipole–dipole interaction energy and the subscripts 1 and 2, on 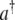 and a, denote a central molecule and a neighboring molecule, respectively. This will also be the approach we take for MTs.
and a, denote a central molecule and a neighboring molecule, respectively. This will also be the approach we take for MTs.
A transition dipole moment M is created when an electron makes a transition between its ground and first excited state [59] and may be given in one dimension by:
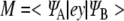 |
12 |
where e is the charge of an electron, y is the position operator, and 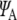 and
and 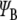 are the electron wavefunctions in the ground and first excited states, respectively. Taking the wavefunction for the electrons described for the tubulin dimer and the energies for the ground and excited states calculated earlier, the transition dipole moment M is found to have an approximate magnitude of 1.2 ×10 − 28 Cm, or 36 Debye, over all ranges with the direction restricted to the y-direction as defined in Fig. 2.
are the electron wavefunctions in the ground and first excited states, respectively. Taking the wavefunction for the electrons described for the tubulin dimer and the energies for the ground and excited states calculated earlier, the transition dipole moment M is found to have an approximate magnitude of 1.2 ×10 − 28 Cm, or 36 Debye, over all ranges with the direction restricted to the y-direction as defined in Fig. 2.
The interaction J of two point dipoles in a dielectric medium of tubulin can be obtained from [60] as:
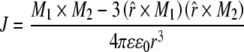 |
13 |
where M1 and M2 are the dipole moments of molecules 1 and 2, respectively, ε is the dielectric constant of the medium, 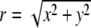 is the magnitude of the distance between molecules 1 and 2 with components along the x and y directions, and
is the magnitude of the distance between molecules 1 and 2 with components along the x and y directions, and  is a unit vector pointing in the direction of
is a unit vector pointing in the direction of 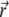 .
.
It can be seen from Fig. 2a that a given neighborhood group contains 6N dimers, where N determines the degree of separation from the central dimer. For nearest neighbors N = 1, thus containing six dimers in the normal tilted hexagonal lattice neighborhood. For the neighborhood of next nearest neighbors N = 2 yields 12 dimers, and so on for increasing neighbor groups. It can also be seen that the neighbors have symmetry about the central dimer. Due to this symmetry in the dimer neighborhoods, a purely excitonic model yields a dispersion relation as:
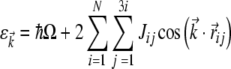 |
14 |
where i and j determine the neighborhood as mentioned above, Jij is the dipole–dipole interaction energy between the central dimer dipole and a given neighbor dipole, and 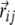 is the distance between the two dipoles.
is the distance between the two dipoles.
Unlike the Scheibe aggregate situation presented in [59] where the molecules of the monolayer are completely symmetrical around the x- and y-axes, the MT lattice structure does not provide a coordinate system in which the dipole contributions can be separated into two effective coupling constants in distinct directions. Due to the tilted hexagonal structure and the direction of the dipole moment, there is skewed symmetry in the dipole couplings; thus, the second term in (14) may not be written in terms of an orthogonal axis. Considering coupling only in the protofilament and dimer helix direction, the dispersion relations may be given as:
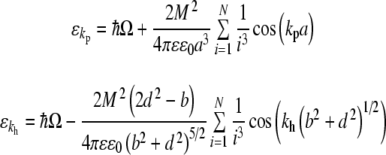 |
15 |
where the second term gives the dipole–dipole coupling to the Nth nearest neighbors and variables a, b, and d are defined in Fig. 2. As the coefficient is necessarily positive in both cases, the dispersion energy takes on a minimum when 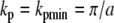 and for kh = khmin = 0 yielding values of
and for kh = khmin = 0 yielding values of
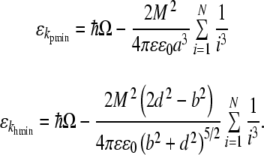 |
16 |
Expanding (15) around this minimum energy up to orders of k2 yields
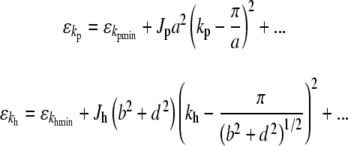 |
17 |
where
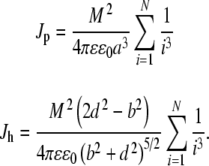 |
18 |
In the protofilament case, we may take the extreme case of an infinitely long MT, i.e., N→ ∞. The summation term in this case yields a value of approximately 1.202 [61, 62]. A typical lower end length of a MT is approximately 200 nm, corresponding to 12 dimers on either end of the central dimer. The sum of inverse cubes drops off rapidly with a sum to N = 12 yielding approximately 1.199, a value in good agreement with the infinite sum. Thus, since MTs range in length from the lower end value of approximately 200 nm to values on the order of many micrometers, the infinite sum is taken as an acceptable approximation. For the helical case, one turn around the MT corresponds to N = 6 dimers on both sides of the central dimer. The sum of inverse cubes up to N = 6 yields a value of approximately 1.179. As the dielectric constant of tubulin is unknown, a plausible range between 2 and 10 is taken, yielding a range of minimum energies and coupling values of:
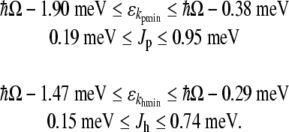 |
19 |
The characteristic exciton coupling time is defined as:
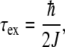 |
20 |
thus yielding a range of 0.35–1.73 and 0.45–2.19 ps for the protofilament and helix, respectively. The correlation length between two exciton sites is given by the expression [58]:
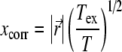 |
21 |
and the characteristic temperature for exciton coupling is defined as [58]:
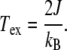 |
22 |
This gives a characteristic temperature range of 4.41–22.09 K and correlation length of 0.95–2.14 nm in the protofilament direction, while for the helical direction, ranges of 3.42–17.14 K and 0.74–1.65 nm are obtained. Thus, as in the phonon case, the temperature of the MT environment is well above this characteristic temperature, indicating that the thermal environment is likely to overwhelm the coupling between excitons, removing any transmission of energy.
The coupling between excitons and phonons comes about as a result of the distance dependence of the dipole–dipole interaction constant J in (13). One can expand this inverse cubic dependence on the equilibrium separation between monomers in terms of a small deviations from the equilibrium value, hence the connection to phonons. Lattice vibrations introduce periodic oscillations about the equilibrium lattice spacing. It can be readily found that the resultant exciton–phonon coupling constant, with respect to the dipole–dipole constant, is proportional to 3Δx/r0, where Δx is the amplitude of lattice oscillations roughly corresponding to the expressions in (8) and r0 is the equilibrium lattice spacing. Therefore, at 310 K, we estimate the relative value of the exciton–phonon constant as only 1% of the dipole–dipole energy. This allows us to determine the regime within which the MT exciton–phonon system is most likely to operate at physiological temperatures. In [58], a phase diagram is constructed in terms of the parameter space spanned by the coefficient γ which represents the ratio of the phonon energy to the exciton energy and the coefficient g which is one half of the product of the square of the ratio of the exciton–phonon energy to the dipole–dipole energy times the ratio of the exciton energy to the phonon energy. It is straightforward to estimate the range of the coefficient values as 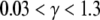 and 0 < g < 0.001. Based on the phase diagram in Fig. 5 of [58], this falls squarely into region III, i.e., a small polaron system. Therefore, the essential picture is that of a fairly well-localized exciton “dressed” with a cloud of lattice vibrations. In Table 1 of [58], one also finds characteristic timescales for the various processes involved. Most importantly, the radiative decay of the small polaron domain at 300 K is expected not to exceed 10 − 10 s, and the lifetime of an exciton–phonon coherent domain is estimated to be even shorter, namely, 10 − 13 s. Taken together with an exciton propagation velocity on the order of 104 m/s, one would not expect the coherent excitation of this type to advance more than 1 nm, i.e., to be localized to within the monomer.
and 0 < g < 0.001. Based on the phase diagram in Fig. 5 of [58], this falls squarely into region III, i.e., a small polaron system. Therefore, the essential picture is that of a fairly well-localized exciton “dressed” with a cloud of lattice vibrations. In Table 1 of [58], one also finds characteristic timescales for the various processes involved. Most importantly, the radiative decay of the small polaron domain at 300 K is expected not to exceed 10 − 10 s, and the lifetime of an exciton–phonon coherent domain is estimated to be even shorter, namely, 10 − 13 s. Taken together with an exciton propagation velocity on the order of 104 m/s, one would not expect the coherent excitation of this type to advance more than 1 nm, i.e., to be localized to within the monomer.
Discussion and conclusions
The collective excitation model, outlined above, is fundamentally different from previous models. It was developed to investigate coherent quantum excitations in MTs. The model was based on the dipole–dipole interaction of excitons formed from the transition of electrons between ground and excited states in a double-well structure based on the tubulin electrostatic map coupled to the MT lattice. Using previous studies of the elastic properties of MTs, phonon energies and characteristic times and temperatures in the protofilament and dimer helix direction were determined. The electron transition dipole and energy levels were determined by solving for the ground and first excited state wavefunctions for an electron in an infinite double-well structure. The energy difference between the ground and first excited state was taken as the exciton energy. With the dipole magnitude and direction, the dipole coupling values were estimated. The MT lattice was taken as an aggregate of tubulin dimers. Due to the tilted hexagonal structure and the transition dipole pointing in the direction of the MT circumference, one-dimensional coupling between dimers in the protofilament and dimer helix directions were considered. Expanding the energy dispersion relation around the minimum energy value yielded the dipole–dipole coupling constants. From these values, characteristic times and temperatures were determined. It was found that the exciton and phonon temperatures of activation were well below the temperature of the MT environment. All values were below 30 K, indicating that thermal vibrations of the environment are more than sufficient to excite phonons and excitons, removing any form of coherent collective excitation, giving credence to the statement that the inside of a cell is too “warm and wet” to support quantum coherence [16, 63, 64].
However, phonon and exciton energies and characteristic times were shown to be on comparable scales with energies in the range of millielectron volts and times on the order of 10 − 12–10 − 13 s, indicating a strong possibility for coupling between the two excitations in the absence of thermal effects. Replies to the “too warm and wet” line of reasoning state that mechanisms exist to shield a MT from the environment, allowing coherent quantum states to exist. Mechanisms to isolate MTs from their environment, such as layers of ordered water or counter ions and coherent pumping mechanisms, have been suggested; however they remain theoretical in nature, with no experimental evidence verifying their effect on MTs. One such suggested mode of isolation is from actin gel states that are purported to solidify around MTs, thus shielding them from thermal effects [65, 66]. Condensed ion clouds that are attracted to the MT due to its large negative surface charge, known as Debye screening, have been suggested as another form of shielding [65]. Similar to this is the idea of shielding due to the existence of ordered water surrounding the MT [24, 65]. Spectroscopic studies of resonant intermolecular transfer of vibrational energy in liquid water have shown that energy is transferred rapidly along water molecules before it dissipates [67], thus providing a mechanism for shielding the MT from thermal energy. The other suggested mechanism to avoid thermal decoherence is coherent pumping of the system via the environment. When a system is strongly coupled to its environment via given degrees of freedom, it may lock out other degrees of freedom, enabling coherent superpositions and entanglement to persist [68]. This locking out is described via a quantum Zeno effect in which an unstable state, if observed continuously, will never decay, since every measurement causes the wavefunction to reduce to a pure eigenstate, thus allowing unfavorable states to persist. This effect was not taken into account in the above analysis, yet since it has been shown that thermal vibrations of the environment easily excite phonons and excitons in the model, a coherent pumping mechanism in the environment can be understood to result in coherent collective excitations. However, to date, there is no known experimental evidence to show such a mechanism in relation to the MT. These anti-decoherence mechanisms were not accounted for in the above analysis, yet while the stated mechanisms seem generally plausible, the degree of isolation required for the possibilities discussed above is almost absolute in view of the results presented in this paper.
The notion of quantum information processing in MT has largely been fathered by the Penrose–Hameroff ‘Orch-OR’ model [3]. This model is based on London forces (between induced dipoles) in non-polar electron clouds in hydrophobic regions rather than the double-well electrons/excitons discussed above. The number of mobile electrons per tubulin involved in these dipole forces is much greater than the single electron proposed above and thus may be more resistant to decoherence. The exact mechanisms of electron transfer are still currently debated, but two major mechanisms are accepted [69]. The first is a bridge-assisted superexchange in which the conjugated bridge between donors and acceptors acts as a medium for the electron to pass through. The second is an electon-hopping mechanism where the electron resides for a short time on the bridge during its movement from one redox centre to another. Thus, electron transfer from tryptophan may either result from electrostatic influence of an ionic species, initiating a hopping charge transfer mechanism, as discussed above, or due to a “through-bond” electron transfer process which is the result of polarization effects. In the second case, polarizing London forces in tryptophan may affect electronic transitions in the double well such that the double well amplifies the aromatic ring’s quantum effects. Admittedly, such a mechanism has not been investigated in this report, but is a further alternative for future analysis which may result in room temperature coherence without the need for an isolation mechanism. Recent evidence indicates that room temperature quantum processes are supported by the structures in graphene [70] and conjugated polymers [71], structures that are not that dissimilar to aromatic rings. Additionally, quantum coherence at relatively warm temperatures has been shown to occur in the protein structures required for photosynthesis [72]. These proteins also show a similarity with the microtubule cytoskeleton; hence, we believe that if there are quantum effects present in microtubules, they must occur via a mechanism similar to the one manifested in photosynthesis. We conclude that three distinct mechanisms can be identified offering alternative explanations for the behavior of microtubules in neuronal signaling: (a) the London forces within non-polar regions, (b) electron or exciton transfer with or without phonon participation, and (c) the yet incompletely elucidated combination of the two. Most importantly, hard empirical evidence is still required to validate any of these models.
Acknowledgements
This research was supported by grants from NSERC, Alberta Cancer Foundation and the Allard Foundation.
References
- 1.Naundorf, B., Wolf, F., Volgushev, M.: Unique features of action potential initiation in cortical neurons. Nature 440, 1060 (2006). doi:10.1038/nature04610 [DOI] [PubMed]
- 2.Penrose, R.: Shadows of the Mind. Oxford University Press, New York (1994)
- 3.Hameroff, S.: Quantum computation in brain microtubules? The Penrose–Hameroff ‘Orch-OR’ model of consciousness (and discussion). Philos. Trans. R. Soc. Lond. A 365, 1869–1896 (1998). doi:10.1098/rsta.1998.0254 [DOI]
- 4.Dustin, P.: MTs. Springer, New York (1978)
- 5.Roberts, K., Hyams, J.S.: Microtubules. Academic, New York (1979)
- 6.Tuszynski, J., Hameroff, S., Sataric, M.V., Trpisova, B., Nip, M.L.A.: Ferroelectric behavior in MT dipole lattices: implications for information processing, signaling and assembly/disassembly. J. Theor. Biol. 174, 371–380 (1995). doi:10.1006/jtbi.1995.0105 [DOI]
- 7.Brown, J.A., Tuszynski, J.A.: Dipole interactions in axonal microtubules as a mechanism of signal propagation. Phys. Rev. E 56, 5834–5840 (1997). doi:10.1103/PhysRevE.56.5834 [DOI]
- 8.Priel, A., Tuszynski, J.A., Cantiello, H.F.: The dendritic cytoskeleton as a computational device: an hypothesis. In: Tuszynski, J.A. (ed.) The Emerging Physics of Consciousness, pp. 293–325. Springer, Berlin (2006)
- 9.Gundersen, G.G., Cook, T.A.: Microtubules and signal transduction. Curr. Opin. Cell Biol. 11, 81–94 (1999). doi:10.1016/S0955-0674(99)80010-6 [DOI] [PubMed]
- 10.Glanz, J.: Cell biology: force-carrying web pervades living cell. Science 276, 678–679 (1997) [DOI] [PubMed]
- 11.Manitois, A.J., Chen, C.S., Ingber, D.E.: Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. U. S. A. 94, 849–854 (1997). doi:10.1073/pnas.94.3.849 [DOI] [PMC free article] [PubMed]
- 12.Priel, A., Tuszynski, J.A., Woolf, N.J.: Transitions in microtubule C-termini conformations as possible dendritic signaling phenomenon. Eur. Biophys. J. 35, 40–52 (2005). doi:10.1007/s00249-005-0003-0 [DOI] [PubMed]
- 13.Cronly-Dillon, J., Perry, G.W.: Effect of visual experience on tubulin during a critical period of visual cortex development in the hooded rat. J. Physiol. 293, 469–484 (1979) [DOI] [PMC free article] [PubMed]
- 14.Lee, V.M.: Disruption of the cytoskeleton in Alzheimer’s disease. Curr. Opin. Neurobiol. 5, 663–668 (1995). doi:10.1016/0959-4388(95)80073-5 [DOI] [PubMed]
- 15.Bjornstrom, K., Eintrei, C.: The difference between sleep and anaesthesia is in the intracellular signal. Acta Anaesthesiol. Scand. 47, 157–164 (2003). doi:10.1034/j.1399-6576.2003.00007.x [DOI] [PubMed]
- 16.Tegmark, M.: Importance of quantum decoherence in brain processes. Phys. Rev. E 61, 4194–4206 (2000). doi:10.1103/PhysRevE.61.4194 [DOI] [PubMed]
- 17.Tuszynski, J.A., Kurzynski, M.: Introduction to Molecular Biophysics. CRC, Florida (2003)
- 18.Tuszynski, J.A., Brown, J.A., Crawford, E., Carpenter, E.J., Nip, M.L.A., Dixon, J.M., Sataric, M.V.: Molecular dynamics simulations of tubulin structure and calculations of electrostatic properties of microtubules. Math. Comput. Model. 41, 1055–1070 (2005). doi:10.1016/j.mcm.2005.05.002 [DOI]
- 19.Howard, W.D., Timasheff, S.N.: GDP state of tubulin: stabilization of double rings. Biochemistry 25, 8292–8300 (1986) [DOI] [PubMed]
- 20.Melki, R., Carlier, M.F., Pantaloni, D., Timasheff, S.N.: Cold depolymerization of microtubules to double rings: geometric stabilization of assemblies. Biochemistry 28, 9143–9152 (1989) [DOI] [PubMed]
- 21.McKean, P.G., Vaughan, S., Gull, K.: The extended tubulin superfamily. J. Cell Sci. 114, 2723–2733 (2001) [DOI] [PubMed]
- 22.Vassilev, P.M., Dronzine, R.T., Vassileva, M.P., Georgiev, G.A.: Parallel arrays of microtubules in electric and magnetic fields. Biosci. Rep. 2, 1025–1029 (1982). doi:10.1007/BF01122171 [DOI] [PubMed]
- 23.Kirson, E.D., Gurvich, Z., Schneiderman, R., Dekel, E., Itzhaki, A., Wasserman, Y., Schatzberger, R., Palti, Y.: Disruption of cancer cell replication by alternating electric fields. Cancer Res. 64, 3288–3295 (2004). doi:10.1158/0008-5472.CAN-04-0083 [DOI] [PubMed]
- 24.Mershin, A., Sanabria, H., Miller, J.H., Nawarathna, D., Skoulakis, E.M.C., Mavromatos, N.E., Kolomenski, A.A., Schuessler, H.A., Luduena, R.F., Nanopoulos, D.V.: Towards experimental tests of quantum effects in cytoskeletal proteins. In: Tuszynski, J.A. (ed.) The Emerging Physics of Consciousness, pp. 95–170. Springer, Berlin (2006)
- 25.Fritsche, W., Böhm, K., Unger, E., Köhler, J.M.: Making electrical contact to single moleclules. Nanotechnology 9, 177–183 (1998). doi:10.1088/0957-4484/9/3/006 [DOI]
- 26.Umnov, M., Paulsinski, O.A., Deymier, P.A., Guzman, R., Hoying, J., Barnaby, H., Yang, Y., Raghavan, S.: Experimental evaluation of electrical conductivity of microtubules. J. Mater. Sci. 42, 373–378 (2007). doi:10.1007/s10853-006-1075-7 [DOI]
- 27.Goddard, G., Whittier, J.E.: Biomolecules as nanomaterials: interface characterization for sensor development. Proc. SPIE 6172, 617206 (2006). doi:10.1117/12.658771 [DOI]
- 28.Fritzsche, W., Köhler, J.M., Böhm, K.J., Unger, E., Wagner, T., Kirsch, R., Mertig, M., Pompe, W.: Wiring of metallized microtubules by electron beam-induced structuring. Nanotechnology 10, 331–335 (1999). doi:10.1088/0957-4484/10/3/317 [DOI]
- 29.Priel, A., Ramos, A.J., Tuszynski, J.A., Cantiello, H.F.: A biopolmer transistor: electrical amplification by microtubules. Biophys. J. 90, 4639–4643 (2006). doi:10.1529/biophysj.105.078915 [DOI] [PMC free article] [PubMed]
- 30.Minoura, I., Muto, E.: Dielectric measurement of individual microtubules using the electroorientation method. Biophys. J. 90, 3739–3748 (2006). doi:10.1529/biophysj.105.071324 [DOI] [PMC free article] [PubMed]
- 31.Tuszynski, J.A., Priel, A., Brown, J.A., Cantiello, H.F., Dixon, J.M.: Electronic and ionic conductivities of microtubules and actin filaments, their consequences for cell signaling and applications to bioelectronics. In: Lyshevski, E. (ed.) Nano and Molecular Electronics Handbook Vol. 9, Ch. 18. CRC, London (2007)
- 32.Tuszynski, J.A., Brown, J.A., Hawrylak, P.: Dielectric polarization, electrical conduction, information processing and quantum computation in microtubules. Are they plausible? Philos. Trans. R. Soc. Lond. A 356, 1897–1926 (1998). doi:10.1098/rsta.1998.0255 [DOI]
- 33.Brown, J.A.: A Study of the interactions between electromagnetic fields and microtubules: ferroelectric effects, signal transduction and electronic conduction. Ph.D. thesis, University of Alberta (1999)
- 34.Hameroff, S.: Ultimate Computing. North Holland, Amsterdam (1987)
- 35.Smith, S.A., Watt, R.C., Hameroff, S.R.: Cellular automata in cytoskeletal lattices. Physica D 10, 168–174 (1984). doi:10.1016/0167-2789(84)90259-8 [DOI]
- 36.Rasmussen, S., Karampurwala, H., Vaidyanath, R., Jensen, K.S., Hameroff, S.: Computational connectionism within neurons: a model of cytoskeletal automata subserving neural networks. Physica D 42, 428–449 (1990). doi:10.1016/0167-2789(90)90093-5 [DOI]
- 37.Campbell, R.D.J.: Information processing in microtubules. Ph.D. thesis, Queensland University of Technology (2002)
- 38.Hameroff, S.R., Dayhoff, J.E., Lahoz-Beltra, R., Samsonovich, A.V., Rasmussen, S.: Models for molecular computation: conformational automata in the cytoskeleton. Computer 25, 30–39 (1992). doi:10.1109/2.166406 [DOI]
- 39.Lowe, J., Li, H., Downing, K.H., Nogales, E.: Refined structure of αβ-tubulin at 3.5 Å resolution. J. Mol. Biol. 313, 1045–1057 (2001). doi:10.1006/jmbi.2001.5077 [DOI] [PubMed]
- 40.Baker, N.A., Sept, D., Joseph, S., Holst, M.J., McCammon, J.A.: Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U. S. A. 98, 10037–10041 (2001). doi:10.1073/pnas.181342398 [DOI] [PMC free article] [PubMed]
- 41.Humphrey, W., Dalke, A., Schulten, K.: VMD—visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996). doi:10.1016/0263-7855(96)00018-5 [DOI] [PubMed]
- 42.Gray, H.B., Winkler, J.R.: Electron tunneling through proteins. Q. Rev. Biophys. 36, 341–372 (2003). doi:10.1017/S0033583503003913 [DOI] [PubMed]
- 43.Dreyer, J.L.: Electron transfer in biological systems: an overview. Experientia 40, 653–675 (1984). doi:10.1007/BF01949719 [DOI] [PubMed]
- 44.Marcus, R.A., Sutin, N.: Electron transfers in chemistry and biology. Biochim. Biophys. Acta 811, 265–322 (1985)
- 45.Canters, G.W., Dennison, C.: Biological electron transfer: structural and mechanistic studies. Biochimie 77, 506–515 (1995). doi:10.1016/0300-9084(96)88167-3 [DOI] [PubMed]
- 46.Baum, R.M.: Views on biological, long-range electron transfer stir debate. Chem. Eng. News 22, 20–23 (1993)
- 47.Gray, H.B., Winkler, J.R.: Electron transfer in proteins. Annu. Rev. Biochem. 65, 537–561 (1996). doi:10.1146/annurev.bi.65.070196.002541 [DOI] [PubMed]
- 48.Aubert, C., Vos, M.H., Mathis, P., Eker, A.P.M., Bretel, K.: Intraprotein radical transfer during photoactivation of DNA photolyase. Nature 405, 586–590 (2000). doi:10.1038/35014644 [DOI] [PubMed]
- 49.Wagenknecht, H.A., Stemp, E.D.A., Barton, J.K.: Evidence of electron transfer from peptides to DNA: oxidation of DNA-bound tryptophan using the flash-quench technique. J. Am. Chem. Soc. 122, 1–7 (2000). doi:10.1021/ja991855i [DOI]
- 50.Becker, J.S., Oliver, J.M., Berlin, R.D.: Fluorescence techniques for following interactions of microtubule subunits and membranes. Nature 254, 152–154 (1975). doi:10.1038/254152a0 [DOI] [PubMed]
- 51.Ravelli, R.B., Gigant, B., Curmi, P.A., Jourdain, I., Lachkar, S., Sobel, A., Knossow, M.: Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428, 198–202 (2004). doi:10.1038/nature02393 [DOI] [PubMed]
- 52.Eley, D.D.: Studies of organic semiconductors for 40 years—I. The mobile π-electron—40 years on. Mol. Cryst. Liq. Cryst. (Phila. Pa.) 171, 1–21 (1989). doi:10.1080/00268948908065783 [DOI]
- 53.Johnson, E.A., Williams, H.T.: Quantum solutions for a symmetric double square well. Am. J. Phys. 50, 239–243 (1982). doi:10.1119/1.13046 [DOI]
- 54.Hameroff, S.R., Tuszynski, J.A.: Search for quantum and classical modes of information processing in microtubules: implications for “the living state”. In: Musmeci, F., Ho, M. (eds.) Bioenergetic Organization in Living Systems. World Scientific, Singapore (2003)
- 55.Portet, S., Tuszynski, J.A., Hogue, C.W.V., Dixon, J.M.: Elastic vibrations in seamless microtubules. Eur. Biophys. J. 34, 912–920 (2005). doi:10.1007/s00249-005-0461-4 [DOI] [PubMed]
- 56.Bartnik, E.A., Blinowska, K.J.: Stability of quantum capture in Langmuir–Blodgett monolayers against positional disorder. Phys. Lett. A 169, 46–50 (1992). doi:10.1016/0375-9601(92)90803-T [DOI]
- 57.Marder, M.P.: Condensed Matter Physics. Wiley, New York (2000)
- 58.Tuszynski, J.A., Jørgensen, M.F., Möbius, D.: Mechanisms of exciton energy transfer in Scheibe aggregates. Phys. Rev. E 59, 4374–4382 (1999). doi:10.1103/PhysRevE.59.4374 [DOI]
- 59.Czikklely, V., Forsterling, H.D., Kuhn, H.: Extended dipole model for aggregates of dye molecules. Chem. Phys. Lett. 6, 207–210 (1970). doi:10.1016/0009-2614(70)80220-2 [DOI]
- 60.Jackson, J.D.: Classical Electrodynamics, 3rd edn, p. 151. Wiley, New York (1999)
- 61.Schoutens, J.: Dipole–dipole interactions in microtubules. J. Biol. Phys. 31, 35–55 (2005). doi:10.1007/s10867-005-3886-1 [DOI] [PMC free article] [PubMed]
- 62.Jolley, L.B.W.: Summation of Series, 2nd revision. Dover, New York (1961)
- 63.Seife, C.: Cold numbers unmake the quantum mind. Science 287, 791 (2000). doi:10.1126/science.287.5454.791 [DOI] [PubMed]
- 64.Koch, C., Hepp, K.: Quantum mechanics in the brain. Nature 440, 611 (2006). doi:10.1038/440611a [DOI] [PubMed]
- 65.Hameroff, S.: Consciousness, neurobiology and quantum mechanics: the case for a connection. In: Tuszynski, J.A. (ed.) The Emerging Physics of Consciousness, pp. 193–253. Springer, Berlin (2006)
- 66.Hameroff, S., Nip, A., Porter, M., Tuszynski, J.: Conduction pathways in microtubules, biological quantum computation, and consciousness. Biosystems 64, 149–168 (2002). doi:10.1016/S0303-2647(01)00183-6 [DOI] [PubMed]
- 67.Woutersen, S., Bakker, H.J.: Resonant intermolecular transfer of vibrational energy in liquid water. Nature 402, 507–509 (1999). doi:10.1038/990058 [DOI]
- 68.Nielson, M., Chuang, I.L.: Quantum Computation and Quantum Information. Cambridge University Press, Cambridge (2001)
- 69.Long, Y., Abu-Irhayem, E., Kratz, H.-B.: Peptide electron transfer: more questions than answers. Chem. Eur. J. 11, 5186 (2005). doi:10.1002/chem.200500143 [DOI] [PubMed]
- 70.Novoselov, K.S., Jiang, Z., Zhang, Y., Morozov, S.V., Stormer, H.L., Zeitler, U., Maan, J.C., Boebinger, G.S., Kim, P., Geim, A.K.: Room-temperature quantum Hall effect in graphene. Science 315, 1379 (2007). doi:10.1126/science.1137201 [DOI] [PubMed]
- 71.Collini, E., Scholes, G.D.: Coherent intrachain energy migration in a conjugated polymer at room temperature. Science 323, 369 (2009). doi:10.1126/science.1164016 [DOI] [PubMed]
- 72.Engel, G.S., Calhoun, T.R., Read, E.L., Ahn, T.-K., Mancal, T., Cheng, Y.-C., Blankenship, R.E., Fleming, G.R.: Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 446, 782 (2007). doi:10.1038/nature05678 [DOI] [PubMed]