Abstract
Since the discovery of the presence of biogenic magnetites in living organisms, there have been speculations on the role that these biomagnetites play in cellular processes. It seems that the formation of biomagnetite crystals is a universal phenomenon and not an exception in living cells. Many experimental facts show that features of organic and inorganic processes could be indistinguishable at nanoscale levels. Living cells are quantum “devices” rather than simple electronic devices utilizing only the charge of conduction electrons. In our opinion, due to their unusual biophysical properties, special biomagnetites must have a biological function in living cells in general and in the brain in particular. In this paper, we advance a hypothesis that while biomagnetites are developed jointly with organic molecules and cellular electromagnetic fields in cells, they can record information about the Earth’s magnetic vector potential of the entire flight in migratory birds.
Keywords: Biomagnetite formation by biological control, Aharonov–Bohm effect
Introduction
Usually when we hear about crystals in relation to living organisms or cells, we first think of pathological cases such as kidney stones, gallstones, etc. Deficient biochemical processes or microorganisms can produce different kinds of pathological biocrystal formations [1, 2]. However, some kinds of biocrystals can have functional roles in living cells.
Many scientists treat biomagnetites in a very cursory fashion while others use biological self-assembling techniques to create inorganic biomagnetites within living bacterial cells, because these biomagnetites have such special features that make them capable of functioning in computers as information storage components [3]. If biomagnetites can work in computers, why cannot they work in living cells as well?
It has been well accepted by now that different cells of many species contain inorganic biomagnetic crystals. Joseph Kirschvink, at the California Institute of Technology, researched for many years various types of living creatures such as bacteria, insects, birds, fishes, etc. and has found that biomagnetites play an important role in their spatial orientation [4, 5]. He has also found that biomagnetic Fe3O4 iron-oxide crystals in the human brain are composed of 50–100 granules. According to his calculations, there are 5 million magnetic crystals in every gram of human brain, their size being on the order of 10–100 nm [6, 7]. Considering the mass of the whole human brain, we may assume the presence of a billion nanocrystals in our brain. According to Kirschvink’s experiments, there are functional connections between biomagnetites and organic molecules. Besides, the assumption that biomagnetites not only perceive magnetic fields but also can take part in the inheritance of magnetosome polarity was raised a long time ago [8, 9]. Here, we raise the possibility of having heredity in which information is not contained fully at the DNA level. In this connection, we note that the process of information storage occurs in biomagnetites and not in DNA, but this fixed information could be manifested at the DNA level.
Magnetobiology and associated problems
When we talk about magnetic effects on humans, two different magnetic field “types” are commonly distinguished: (1) a static magnetic field, which exists in the region of a large magnet; and (2) a magnetic field which is pulsed at frequencies higher than 10 Hz, often abbreviated as EMF (electromagnetic fields). The area of investigation of these special effects is termed “magnetobiology”, some sub-fields of which are extremely contentious, while others have already been established in medical applications. Nonetheless, there are some basic and altogether paradoxical problems in magnetobiology. For example, how can weak magnetic fields (e.g., Earth’s magnetic field) or ultra-weak electromagnetic expositions have effects on cellular processes? On the other hand, numerous scientists agree that static magnetic fields of up to 10 T have no obvious effects on long-term plant growth, mouse development, body temperature, or brain activity [10–12] to name a few biological phenomena of interest. Also, research indicates that humans are sensitive to small changes in magnetic field gradients, but not to the overall magnetic field [13].
There exists some strong interaction which results in the parallel alignment of all atomic magnetic moments inside the body. The magnitude of this spontaneous magnetic moment decreases if the sample temperature increases, because thermal fluctuations act against any order due to magnetic interactions [14]. Why do these random disturbances not destroy the magnetobiological effects? At the same time, how can living cells resist or compensate the strong effects of natural and artificial magnetic or electromagnetic environments?
According to [15], the mean noise potential energy without the presence of magnetic fields is 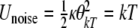 , where κ is a scalar coefficient, θkT is the angular displacement of a biomagnetite element which is placed in the cell and is free to rotate in that place and generate biological effects through that rotation, k is Boltzmann’s constant, and T is the absolute temperature. If this rotation affects biology, the magnetite system must be coupled to an element through some harness so that motion of the magnetite system changes the biologically sensitive conformation of that element. That transition must require an energy in excess of kT, if it were not to take place regularly through thermal agitation without any coupling to magnetic fields. Now we want to compare the energy of a magnetic field to thermal noise energy kT. Suppose that the magnetic field is in the form of B(t) = B0 cos ωt. We denote here μ for the magnetic moment of biomagnetite element. The total inserted torque on the biomagnetite element is obtained from a linear equation as below [15],
, where κ is a scalar coefficient, θkT is the angular displacement of a biomagnetite element which is placed in the cell and is free to rotate in that place and generate biological effects through that rotation, k is Boltzmann’s constant, and T is the absolute temperature. If this rotation affects biology, the magnetite system must be coupled to an element through some harness so that motion of the magnetite system changes the biologically sensitive conformation of that element. That transition must require an energy in excess of kT, if it were not to take place regularly through thermal agitation without any coupling to magnetic fields. Now we want to compare the energy of a magnetic field to thermal noise energy kT. Suppose that the magnetic field is in the form of B(t) = B0 cos ωt. We denote here μ for the magnetic moment of biomagnetite element. The total inserted torque on the biomagnetite element is obtained from a linear equation as below [15],
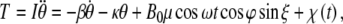 |
1 |
where β is a scalar coefficient and θ, 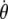 , and
, and 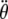 are angular displacement, angular velocity, and angular acceleration, respectively. ϕ is the angle between the direction of the applied torque and the final acceleration, ξ is the angle between the applied field B and the magnetic moment of biomagnetite μ, and χ(t) represents the effects of thermal agitation. Because of the linearity of Eq. 1 the magnetic and thermal noise amplitudes at every time instant can be written in the form
are angular displacement, angular velocity, and angular acceleration, respectively. ϕ is the angle between the direction of the applied torque and the final acceleration, ξ is the angle between the applied field B and the magnetic moment of biomagnetite μ, and χ(t) represents the effects of thermal agitation. Because of the linearity of Eq. 1 the magnetic and thermal noise amplitudes at every time instant can be written in the form 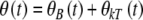 , where θB(t) is the solution without the presence of noise, thus χ(t) = 0, and θkT(t) is the solution without a magnetic field which means B(t) = 0. The general form of the solution for θB(t) is
, where θB(t) is the solution without the presence of noise, thus χ(t) = 0, and θkT(t) is the solution without a magnetic field which means B(t) = 0. The general form of the solution for θB(t) is 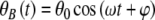 where θ0 is written in the form of Eq. 2,
where θ0 is written in the form of Eq. 2,
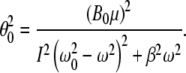 |
2 |
One can define two important energies here: 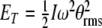 which describes the interaction energy between the magnetic field and the biomagnetite element, and
which describes the interaction energy between the magnetic field and the biomagnetite element, and 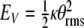 which represents potential energy of the biomagnetite. In the above energy relations, I is the moment of inertia of the magnetic element due to B(t), and
which represents potential energy of the biomagnetite. In the above energy relations, I is the moment of inertia of the magnetic element due to B(t), and 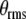 is obtained from
is obtained from 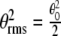 . If we let
. If we let 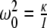 , then the ratio
, then the ratio 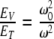 can be interpreted for different values of the variables. The energy of interaction, w, between the magnetic field, B, and the magnetic moment, μ, is
can be interpreted for different values of the variables. The energy of interaction, w, between the magnetic field, B, and the magnetic moment, μ, is 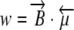 . For the Earth’s field, ω = 0 and
. For the Earth’s field, ω = 0 and 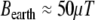 , we have
, we have 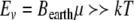 for biomagnetites with large net magnetic moments. We also have similar result for MRI magnetic field intensity in the 1–14 T range, although it is much stronger. Considering small values of ω, neglecting ω2 in the term
for biomagnetites with large net magnetic moments. We also have similar result for MRI magnetic field intensity in the 1–14 T range, although it is much stronger. Considering small values of ω, neglecting ω2 in the term 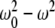 in Eq. 2 and bearing in mind the ratio
in Eq. 2 and bearing in mind the ratio 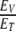 , we have
, we have 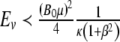 . Now, if we let
. Now, if we let 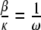 we have
we have 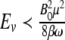 . For any magnetite, and requiring that the magnetic fields must not affect biology, Ev ≺ ≺ kT. By way of the required information in the above relationships, there is the possibility to investigate the effects of different magnetic fields on biomagnetites. The evoked human brain activity has ≤10 − 13 T intensity, and the magnetic fields due to spontaneous currents in the brain are about 10 − 12 T, while the Earth’s magnetic field is ≥10 − 5 T [14].
. For any magnetite, and requiring that the magnetic fields must not affect biology, Ev ≺ ≺ kT. By way of the required information in the above relationships, there is the possibility to investigate the effects of different magnetic fields on biomagnetites. The evoked human brain activity has ≤10 − 13 T intensity, and the magnetic fields due to spontaneous currents in the brain are about 10 − 12 T, while the Earth’s magnetic field is ≥10 − 5 T [14].
There are many models of weak magnetic effects on living cells, for example, with the help of biomagnetites, Eddy electric currents, classical and quantum oscillator models, cyclotron resonance, interference of quantum states of bound ions and electrons, coherent quantum excitations, parametric resonance, stochastic resonance, bifurcation, magnetosensitive free-radicals, etc. [16]. Adair [15] has shown that the energies transmitted to the magnetite elements by fields less than 5 × 10 − 6 T will be much less than thermal noise energies. Thus, the effects of such weak fields will be overwhelmed by thermal noise and cannot be expected to affect biology.
The basic question is “why is it that these models could not solve the basic problem of magnetobiology?” Perhaps, there are not real contradictions among the above-mentioned models, because living cells use several kinds of information processes simultaneously, but these processes are too complicated to find relations among them.
Electric, magnetic, electromagnetic, and acoustic signals produced by living cells
Living cells can generate and use electric, magnetic, and electromagnetic (both coherent and incoherent biophotons) waves and also acoustic waves as conformational changes in macromolecules called conformons (analogous to lattice vibrations of phonons in solid crystals) [17–23]. Electricity is a basic trait of cells, because all “biomolecules” are ions or biomolecules which are endowed with high electric dipole moments. When their charges move, an electromagnetic field is generated. Magnetic features can emerge from free radicals, organic molecules with metals or biomagnetites [24]. In addition, according to electron-spin resonance experiments, living cells or organisms can have paramagnetic features in their native states [25]. Here, we note that conformons originate from continuously moving molecules.
Different parts of cells (such as DNA, RNA, proteins) show piezoelectric and semiconductor properties [26–29]. The piezoelectric effect refers to that property of matter, which can convert electromagnetic oscillations to mechanical vibrations and vice versa, or electric oscillations to mechanical vibrations and vice versa. The piezoelectric property of cells can produce circular polarized light pulses which indicate that living molecules are not raceme mixtures of optically active molecules. Organic semiconductors have crystal-like structures and electrical conductivity as diodes. The electric fields of a wave can couple to the mobile carriers within a semiconductor structure and modify its electronic and elastic properties. Optical signals (biophotons) can be stored by the surface acoustic waves (conformons as mechanical vibrations in macromolecules) in the semiconductor in a photon-atom-bound way [6], and can be re-assembled into light after very long delay times and at a remote location of the sample.
The membrane lipid has a density of about 1011 pores m − 2, and it is not in a uniform non-conductive bulk phase [30]. It is a quasi-insulator, with conductive and non-conductive parts, and also there are semiconductor proteins in it. Finally, we note that the current–voltage relationship has a non-linear characteristic.
Cytoplasm is not simply an aqueous solution of macromolecules, but is a structurally and dynamically organized network (cytoskeleton) of interconnected (semiconductor) protein polymers in the ordered water/ion solution. Namely, living cells exhibit a liquid-crystal-like state [31, 32]. Liquid crystals show some of the orientational order of a solid, but the molecules are mobile. The regulated living structures can produce coherent or laser-like oscillations, which are called Fröhlich oscillations [33].
It seems that coherence—over ultra-short times—is a universal phenomenon and not limited to biochemical processes [34]. Many processes work on the ultra-short femtosecond time scale, which appears to avoid the effects of thermal noise or fluctuations and also can produce coherent biophotons.
Living systems show fractal features. Fractal systems, which are the best models of some fundamental aspects of the living and non-living world, have special features. Namely, fractals can resist strong forces; at the same time, they can use very weak ones [35–37]. Phase delay is a very important property of fractals, which can be generated by the noise of non-linear systems. Namely, properties of fractals can be related to the problem of useful noise [38].
Living cells and organisms can use non-linear creative white noise for signal amplification. In accordance with experiments, random noise can help neurons to react (as a non-linear resonance) to weak signals [39].
In brief, living cells can generate and use electric, magnetic, electromagnetic, and acoustic waves (conformons) and convert them from one form to another.
Ion gate model of biomagnetites
What are biomagnetites doing in various cells? In the case of bacteria, the answer may be simple. Magnetite crystals can take part in navigation processes and sense Earth’s or other magnetic fields, an effect called magnetotaxis. But in the case of birds, the answer is not simple at all. How can a migratory bird find its way home from 3,000 km away with the help of biomagnetites? Moreover, what are billions of magnetites doing in the human brain?
Kirschvink has proposed a model that biomagnetites can open or close ion gates in the cells while they perceive the Earth’s magnetic fields or different electromagnetic fields [40]. This idea has been met with many contradictory opinions, doubting that the Earth’s magnetic field would be strong enough to generate significant biological effects on biomagnetites.
According to Zeilinger, “Yet I am not convinced that living systems are just classical machines” [41]. However, living cells are quantum “devices” rather than simple mechanical and electrical machines. Many experiments show that ultra-weak forces work—fast and accurately—in cells as well as that information or regulation processes are much faster than molecular processes. If ultra-weak processes do not work accurately and very fast (at femtosecond time scales) in cells, different effects of natural and artificial environments can disintegrate these processes. Of course, various forms of natural and artificial radiation can have effects on the cellular processes but cells can compensate for this within a wide range. As we mentioned before, living cells can sense and use the Earth’s weak magnetic fields, which are 100 billion times stronger than the brain’s magnetic processes [42]. At the same time, during MRI experiments, we are exposed to very strong 1–14 T magnetic fields, which are much stronger than Earth’s magnetic field. If biomagnetites work as a regulator of ion gates, during MRI scans, magnetic radiation can have strong effects on biomagnetites which can be arranged in one direction and thus the brain’s processes can be collapsed. As a result, an ion gate model is hardly possible.
However, there exist additional biophysical possibilities as biomagnetites can take part in information processes in cells. We believe that it is insufficient for a migratory bird to find its way home by simply perceiving the Earth’s magnetic field. The migratory bird has to record the magnetic map or magnetic vector potential map of the entire journey. But how can a migratory bird find the exact way home, to its nest under the certain eaves of a certain house in a certain city from the distance of some thousand kilometers? Here we suggest that while biomagnetites are built up jointly with organic molecules and cellular electromagnetic fields in cells, these biomagnetites can record information of the Earth’s magnetic vector potential during the entire flight.
Biomagnetite crystal formation by biological control
In previous sections, we could see that living cells can create and use weak electric, magnetic, electromagnetic, and acoustic waves, and convert one of them into another. Electrons play a very important role in the information flow between organic and inorganic materials in various cells. Ge et al.—at Lawrence Berkeley National Laboratory—examined the behavior of electrons at interfaces [43]. A piece of inorganic silver was coated with organic paraffin and was illuminated with a tunable laser by a femtosecond pulse. The electrons came out from the silver surface and could bind to the lattice of organic paraffin as polarons. The polaron existed for 1,000 fs and came back via a tunnel effect to silver. As the authors emphasized, this phenomenon is very important in biochemical processes, namely a functional electric connection can exist between organic and inorganic materials. Since biomagnetites are in connection with surrounding organic protein molecules, these molecules can regulate the development of biomagnetites by electrical operating processes.
However, bioelectromagnetic forces can regulate the formation of biomagnetites in cells as well. Electromagnetic waves mostly affect the length of cell membranes which are in functional connection with biomagnetites [44]. In in vitro experiments, weak electromagnetic fields exert a direct influence on the kinetics of crystal formation [45]. Since living cells can produce coherent electromagnetic waves (biophotons) [46–49], these cellular electromagnetic forces can also regulate the formation of biogenic magnetites. Namely, a holographic lithography-like mechanism could work within cells (formation of biocrystals by interference of non-coplanar coherent biophotons) [50].
Ursula Liebl et al. proved evidence—with femtosecond spectroscopy—for driving of a reaction in a protein complex by coherent motions, and suggested the functional importance of coherent vibrations operating on a femtosecond timescale [51]. It seems that the ultra-short time coherence is a universal phenomenon in biochemical processes, thus femtosecond processes can produce coherent vibrations operating at an interface between organic molecules and inorganic crystals. At nano-levels, there are unusual magnetic and electric non-linear fluctuations, which make modeling relatively difficult. Because of quantum size effects, matter at the nanometer scale has very special properties, altered thermodynamics, and modified chemical reactivity.
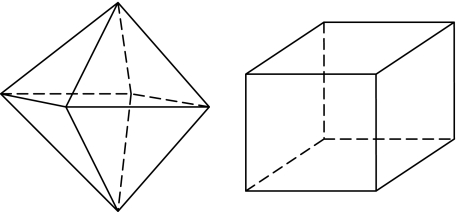
However, biomagnetic crystals are solitary and structurally well-ordered magnetic domains that are stably magnetized and the maximum magnetic moment per unit volume is required for magnetite [52, 53]. Biomagnetite crystal morphology is cubo-octahedral with the {111} direction (see Fig. 1), which yields unusual particle shapes in geological magnetite crystals, so the production of this biomineral must be under precise biological control. This biological control of biomagnetite formation can be achieved by surrounding organic protein molecules and coherent electromagnetic fields (biophotons) in cells.
Fig. 1.
Crystal morphology from left to right: octahedral {111} and cubic {100} forms
We should see whether the nanometer scale is the scale at which soft and hard materials sciences overlap.
Information storage in biomagnetites and the Aharonov–Bohm effect
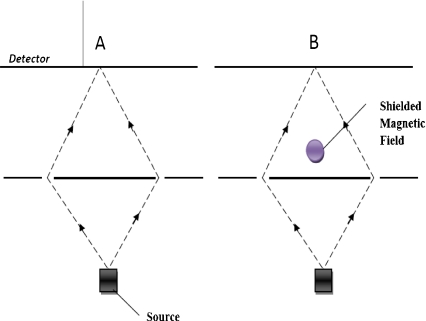
The quantum physical Aharonov–Bohm effect was proven via experiments on electron interference [54]. The essence of this effect is seen as follows. If we take a piece of static magnet, its static magnetic field is shielded but an effect still exists which can change the wave phase of electrons (see Fig. 2). This effect is more fundamental than a magnetic feature called the magnetic vector potential.
Fig. 2.
Aharonov–Bohm effect. In the interference experiment for electron particles, in part A there is no magnetic field around the set-up, but in part B there is a shielded magnetic field after the double slits which has no classical effect on the paths of electrons. The interference representations on detectors A and B are different. The magnetic field in part B has changed the wave phase of the electrons
Here we suggest that spin-modulated resistance could work in biomagnetites through the Aharonov–Bohm effect. In this effect, the phase of the electron wave depends on the magnetic vector potential, which causes a phase difference and interference between partial waves. Through the Aharonov–Bohm effect, weak geomagnetic fields can have effects on living cellular processes.
Biomagnetites can also be viewed as so-called ferromagnets. Manyala et al. argued that magnetoresistance which can rise from different mechanisms in certain ferromagnets is a quantum interference effect [55]. In addition, Tsukagoshi et al., in their experiments, reported that spin-polarized electrons can be injected into non-ferromagnetic materials (multi-walled carbon nanotubes) from a ferromagnet, finding direct evidence for the coherent transport of electron spins [56]. The abovementioned mechanisms can also work in cells. Namely, biomagnetites can take part in information storage and operating processes in cells through the Aharonov–Bohm effect.
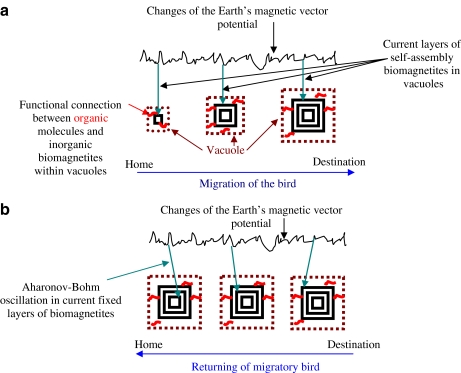
Layers in biomagnetites are shaped by a slow extraction which can be directed via electric and electromagnetic cellular processes. When the time of bird migration comes, migration intention might induce a genetic program in brain cells of migratory birds, which initiates and operates the development of biomagnetites. During migration, layers of biomagnetites and phases of non-conductive electrons in the layers are shaped by the current magnetic vector potential field of Earth (see Fig. 3a). These polarized states of non-conductive electrons—which are fixed in current layers in biomagnetites—might play the main role in magnetic information storage.
Fig. 3.
a Hypothetical model for role of biomagnetites in bird migration. b Hypothetical model for migratory return
During the return of a bird, previously fixed vector potentials of Earth’s magnetic fields could induce an Aharonov–Bohm type of oscillation in the fixed layer of biomagnetites (see Fig. 3b). Therefore, the electric resistance of biomagnetites could oscillate. Localized—previously fixed and spin-modulated—non-conductive electrons may act as scattering sites for the mobile electrons. Namely, oscillations of dephasing non-conductive (fixed) electrons could have an influence on conductive mobile electrons in biomagnetites. Then, there might be a coherent transport of mobile electrons’ spins into surrounding semiconductor protein molecules. Both the effects of electric resistance oscillations in biomagnetites and the transport of spins into proteins could induce conformational changes in organic proteins at the free rotations. Eventually, conformational changes in organic molecules might be amplified within cells, and then among cells, which could direct the movement of migratory birds. In this concept, biomagnetites might work as “devices” of spin-modulated information storage.
Biomagnetites can perceive the Earth’s magnetostatic or vector potential fields while the layers of them have taken shape, although the magnetostatic interaction between the magnetic nanoparticles is negligible in the 2D nanoscale limit [57]. Accordingly, biomagnetites’ magnetic state can be manipulated separately from the state of neighboring biomagnetites. Consequently, the vector potential of the Earth’s magnetic field (which cannot be shielded) plays the main role, and there is no need for a strong external magnetic field to have a mechanical effect (closing or opening ion gates) on biomagnetites.
Wernsdorfer and Sessoli have observed an Aharonov–Bohm type of oscillation in magnetic molecular clusters, analogous to the oscillations as a function of the external flux in a SQUID ring [58]. Their opinion is: “A great deal of information is contained in these oscillations both about the form of the molecular spin Hamiltonian and about the dephasing effect of the environment”. This spin “memory” concept for biomagnetites could guarantee a great amount of information and an operating system, which is needed for the migratory bird to find its way home from a distance of some thousand kilometers.
Signal amplification process of spin information
In reality, many biomagnetites and cells take part in these cooperative processes, but static magnetic forces of biomagnetites and environment have no important role in them, while magnetic vector potentials do. This concept does not need the existence of biomagnetites in most cells of the bird’s brain. Fixed vector potential information in biomagnetites could be converted into different electrical vibration signals in cells.
Aharonov–Bohm oscillations of electric resistance in biomagnetites and also transport of spins into semiconducting proteins can change conformations of organic molecules, which are in direct connection with biomagnetites. Then, conformational changes can oscillate and these oscillations which appear as conformons—in a polar biological system—generate cellular electromagnetic fields around themselves, which can mediate long-range interactions and also signal-amplifying processes [59, 60].
The living matrix is a structural and energetic continuum. Every cell contains a cytoskeleton that is connected, across the cell surface, with the extracellular connective tissue matrix. Structural components of this system include the connective tissue, cytoskeletal, musculoskeletal, and genetic networks. Thus, propagation of conformational changes could expand by this network in cells.
Coupled oscillations, resonant transfer, and electrodynamic coupling allow energy and information to flow through the network. Therefore, electromechanical, electrochemical, or electromagnetic signals could regulate the signal-amplifying processes within and between cells. How these hypothetical processes might interface with known processes and experimentally validated studies, however, remains to be seen.
Biomagnetites in the human brain
The Aharonov–Bohm effect could play important roles in biomolecular communication. This effect might be able to connect the different weak electric, magnetic, and electromagnetic signal processes in various cells. One might even speculate that magnetic informational processes could serve unconscious navigation of living creatures. It seems that humans also have an innate unconscious sense of direction by direction-sensitive cells [61]. However, biomagnetites may also have functional roles in human place cells, a hypothesis suggested by the existence of billions of biomagnetites in the brain, including in the hippocampus, where direction-sensitive place cells—which do not require vision for normal firing—are also found.
Summary
Living cells can generate and use electric, magnetic, electromagnetic, and acoustic waves (conformons), and convert from one form into another. The biological regulation of biomagnetite formation can be achieved by surrounding organic protein molecules and coherent cellular electromagnetic fields (biophotons).
In this paper, we have hypothesized that spin-modulated resistance can work in biomagnetites through the Aharonov–Bohm effect. Layers in biomagnetites are shaped by a slow extraction that is regulated by electric and electromagnetic cellular processes. When the time of a bird’s migration comes, it could induce a genetic program in brain cells of the migratory birds, which initiates and operates the development of biomagnetites. During migration, biomagnetic crystal layers and phases of non-conductive electrons within layers could be shaped by current magnetic vector potential field of the Earth. During the return of the migratory birds, earlier fixed vector potentials of Earth’s magnetic fields could induce an Aharonov–Bohm type of oscillation adequately and then fix the biomagnetites layer. Therefore, the electric resistance of biomagnetites might exhibit oscillations. Localized—previously fixed and spin-modulated—non-conductive electrons could act as scattering sites for the mobile electrons. Namely, oscillations of dephasing non-conductive (fixed) electrons could have an influence on conductive mobile electrons in biomagnetites. Then, there might be a coherent transport of mobile electrons’ spins into surrounding semiconductor protein molecules. Both effects, electric resistance oscillations in biomagnetites and transport of spin states into proteins, could induce conformational changes in the surrounding organic proteins in the free rotations. Eventually, conformation changes might be amplified by signal processes in cell, and then among cells, which could somehow direct the movement of a migratory bird. Within this concept, biomagnetites work as “devices” performing electron spin-modulated information storage. Here we also emphasize that biomagnetites might have functional roles in the human place cells.
References
- 1.Anderson, H.C.: Mechanism of pathologic calcification. Rheum. Dis. Clin. North Am. 2, 303–319 (1988) [PubMed]
- 2.Bókkon, I.: The world of nanobacteria. Biokémia, Q. Bull. Hung. Biochem. Soc. 2, 35–42 (2003)
- 3.Lang, C., Schüler, D., Faivre, D.: Synthesis of magnetite nanoparticles for bio- and nanotechnology: genetic engineering and biomimetics of bacterial magnetosomes. Macromol. Biosci. 7, 144–151 (2007) [DOI] [PubMed]
- 4.Kirschvink, J.L., Jones, D.S., MacFadden, B.J. (eds.): Magnetite Biomineralization and Magnetoreception in Organisms: A New Biomagnetism. Plenum, New York (1985)
- 5.Kirschvink, J.L.: South-seeking magnetic bacteria. J. Exp. Biol. 86, 345–347 (1980)
- 6.John, S., Burstein, E., Weisbuch, C. (eds.): Localization of light in disordered and periodic dielectrics, confined electrons and photons. Plenum, New York (1995)
- 7.Kirschvink, J.L., Kirschvink, A., Woodford, B.: Magnetite biomineralization in the human brain. PNAS 89, 7683–7687 (1992) [DOI] [PMC free article] [PubMed]
- 8.Hedges, R.W.: Inheritance of magnetosome polarity in magnetotropic bacteria. J. Theor. Biol. 112, 607–608 (1985) [DOI]
- 9.Nanney, D.L.: Heredity without Genes: Ciliate Explorations of Clonal Heredity, pp. 295–298. Elsevier, Amsterdam (1985)
- 10.Barnothy, J.M., Barnothy, M.F., Boszormenyi-Nagy, I.: Influence of magnetic field upon the leucocytes of the mouse. Nature 177, 577–578 (1956) [DOI] [PubMed]
- 11.Barnothy, J.M., Barnothy, M.F.: Biological effect of a magnetic field and the radiation syndrome. Nature 181, 1785–1786 (1958) [DOI] [PubMed]
- 12.Maret, G., Kiepenheuer, J., Boccara, N.: Biophysical Effects of Steady Magnetic Fields. Springer, Berlin (1986)
- 13.Rocard, Y.: Actions of a very weak magnetic gradient: the reflex of the dowser. In: Barnothy, M.F. (ed.) Biological Effects of Magnetic Fields, pp. 279–286. Plenum, New York (1964)
- 14.Andra, W., Novak, H.: Magnetism in Medicine. Wiley-VCH, Mannheim (2007)
- 15.Adair, R.K.: Constraints of thermal noise on the effects of weak 60-Hz magnetic fields acting on biological magnetite. PNAS 91, 2925–2929 (1994) [DOI] [PMC free article] [PubMed]
- 16.Binhi, V.N.: An analytical survey of theoretical studies in the area of magnetoreception. In: Repacholi, M.H., Rubtsova, N.B., Muc, A.M. (eds.) Electromagnetic Fields: Biological Effects and Hygienic Standardization, pp. 155–170. World Health Organization, Geneva (1999)
- 17.Bókkon, I.: Creative information. J. Biol. Syst. 11, 1–17 (2003) [DOI]
- 18.Clark, I.J.: The geometric curvature of microtubules may play a part in information processing. Bioelectrochemistry and Bioenergetics 41, 59–61 (1996) [DOI]
- 19.Fukada, E.: Electrical phenomena in biorheology. Biorheology 19, 15–27 (1982) [DOI] [PubMed]
- 20.Kobayashi, M., Takeda, M., Ito, K., Kato, H., Inaba, H.: Two-dimensional photon counting imaging and spatiotemporal characterization of ultraweak photon emission from a rat’s brain in vivo. J. Neurosci. Methods 93, 163–168 (1999) [DOI] [PubMed]
- 21.Nuccitelli, R.: Ionic currents and DC fields in multicellular animal tissues. Bioelectromagnetics 1, 147–157 (1992) [DOI] [PubMed]
- 22.Popp, F.A., Ruth, B., Bahr, W., Böhm, J., Grass, P., Grolig, G., Rattemeyer, M., Schmidt, H.G., Wulle, P.: Emission of visible and ultraviolet radiation by active biological systems. Collect. Phenom. 3, 187–214 (1981)
- 23.Popp, F.A., Yan, Y.: Delayed luminescence of biological systems in terms of coherent states. Phys. Lett. A 293, 93–97 (2002) [DOI]
- 24.Grundler, W., Kaiser, F., Keilmann, F., Walleczek, J.: Mechanisms of electromagnetic interaction with cellular systems. Naturwissenschaften 79, 551–559 (1992) [DOI] [PubMed]
- 25.Szent-Györgyi, A.: Electronic Biology and Cancer. Dekker, New York (1976)
- 26.Fink, H.W., Schönenberger, C.: Electrical conduction through DNA molecules. Nature 398, 407–410 (1999) [DOI] [PubMed]
- 27.Fukada, E.: Piezoelectricity of biopolymers. Biorheology 32, 593–609 (1995) [DOI] [PubMed]
- 28.Shamos, M.H., Lavine, L.S.: Piezoelectricity as a fundamental property of biological tissues. Nature 213, 267–269 (1967) [DOI] [PubMed]
- 29.Szent-Györgyi, A.: The Living State and Cancer. Magvető, Budapest (1983)
- 30.Bordi, F., Cametti, C., Natali, F.: Electrical conductivity and ion permeation in planar lipid membranes. Bioelectrochemistry and Bioenergetic 41, 197–200 (1996) [DOI]
- 31.Ho, M.W., Haffegee, J., Newton, R., Zhou, Y., Bolton, J.S., Ross, S.: Organisms as polyphasic crystals. Bioelectrochemistry and Bioenergetic 41, 81–91 (1996) [DOI]
- 32.Booth, C.H., Raynes, P.: Liquid-crystal displays. Physics World 10, 33–37 (1997)
- 33.Fröhlich, H.: Long-range coherence and energy storage in biological systems. Int. J. Quant. Chem. 2, 641–649 (1968) [DOI]
- 34.Vos, M.H., Rappaport, F., Lambry, J.C.H., Breton, J., Martin, J.L.: Visualization of coherent nuclear motion in a membrane protein by femtosecond spectroscopy. Nature 363, 320–325 (1993) [DOI]
- 35.Dewey, T.G.: Fractal aspects of protein structure and dynamics. Fractals 1, 179–189 (1993) [DOI]
- 36.Mandelbrot, B.: The Fractal Geometry of Nature. Freeman, San Francisco (1982)
- 37.West, B.J., Goldberger, A.L.: Physiology in fractal dimensions. Am. Sci. 75, 354–365 (1987)
- 38.McClintock, P.V.E.: Unsolved problems of noise. Nature 402, 23–24 (1999) [DOI] [PubMed]
- 39.Moss, F.: Noise is good for the brain. Physics World 2, 15–16 (1997)
- 40.Kirschvink, J.L., Kobayashi-Kirschvink, A., Diaz-Ricci, J.C., Kirschvink, S.J.: Magnetite in human tissues. Bioelectromagnetics 1, 101–113 (1992) [DOI] [PubMed]
- 41.Koenig, R.: Teleportation guru stakes out new ground. Science 288, 1327 (2000) [DOI]
- 42.Hahneiser, S., Kohlsmann, M., Hetscher, M., Kramer, K.D.: Development and application of a SQUID sensor array for the measurement of biomagnetic fields. Bioelectrochemistry and Bioenergetics 37, 51–53 (1995) [DOI]
- 43.Ge, N.-H., Wong, C.M., Lingle, R.L., McNeill, J.D., Gaffney, K.J., Harris, C.B.: Femtosecond dynamics of electron localization at interfaces. Science 279, 202–205 (1998) [DOI] [PubMed]
- 44.Pilla, A.A., Nasser, P.R., Kaufmann, J.J.: On the sensitivity of cells and tissues to therapeutic and environmental electromagnetic fields. Bioelectrochemistry and Bioenergetics 30, 161–169 (1993) [DOI]
- 45.Berton, R., Beruto, D., Bianco, B., Chiabrera, A., Giordani, M.: Effect of ELF electromagnetic exposure on precipitation of barium oxalate. Bioelectrochemistry and Bioenergetics 30, 13–25 (1993) [DOI]
- 46.Bajpai, R.P.: Quantum coherence of biophotons and living systems. Indian J. Exp. Biol. 41, 514–527 (2003) [PubMed]
- 47.Bendjaballah, C.H.: Introduction to Photon Communication. Springer, New York (1995)
- 48.Kobayashi, M., Takeda, M., Sato, T., Yamazaki, Y., Kaneko, K., Ito, K., Kato, H., Inaba, H.: In vivo imaging of spontaneous ultraweak photon emission from a rat’s brain correlated with cerebral energy metabolism and oxidative stress. Neurosci. Res. 34, 103–113 (1999) [DOI] [PubMed]
- 49.Popp, F.A.: Properties of biophotons and their theoretical implications. Indian J. Exp. Biol. 41, 391–402 (2003) [PubMed]
- 50.Campbell, M., Sharp, D.N., Harrison, M.T., Denning, R.G., Turbefield, A.: Fabrication of photonic crystals for the visible spectrum by holographic lithography. Nature 404, 53–56 (2000) [DOI] [PubMed]
- 51.Liebl, U., Lipowski, G., Négrerie, M., Lambry, J.C., Martin, J.L., Vos, M.H.: Coherent reaction dynamics in a bacteria cytochrome c oxidase. Nature 401, 181–184 (1999) [DOI] [PubMed]
- 52.Kirschvink, J.L.: Magnetite biomineralization and geomagnetic sensitivity in higher animals: an update and recommendation for future study. Bioelectromagnetics 10, 239–260 (1989) [DOI] [PubMed]
- 53.Mann, S., Frankel, R.B., Blakemore, R.P.: Structure, morphology and crystal growth of bacterial magnetite. Nature 310, 405–407 (1984) [DOI]
- 54.Imry, Y., Webb, R.A.: Quantum interference and the Aharonov–Bohm effect. Scientific American 260, 36–42 (1989)
- 55.Manyala, N., Sidis, Y., DiTusa, J.F., Aeppli, G., Young, D.P., Fisk, Z.: Magnetoresistance from quantum interference effects in ferromagnets. Nature 406, 581–584 (2000) [DOI] [PubMed]
- 56.Tsukagoshi, K., Alphenaar, B.W., Ago, H.: Coherent transport of electron spin in a ferromagnetically contacted carbon nanotube. Nature 401, 572–574 (2000)
- 57.Stamm, C., Marty, M., Vaterlaus, A., Weich, V., Egger, S., Maier, U., Ramsperger, U., Fuhrmann, H., Pescia, D.: Two-dimensional magnetic particles. Science 282, 449–451 (1998) [DOI] [PubMed]
- 58.Wernsdorfer, W., Sessoli, R.: Quantum phase interference and parity effects in magnetic molecular clusters. Science 284, 133–135 (1999) [DOI] [PubMed]
- 59.Fröhlich, H. (eds.): Biological coherence and response to external stimuli. Springer, Berlin (1988)
- 60.Fröhlich, H., Kremer, F. (eds.): Coherent excitations in biological systems. Springer, Berlin (1983)
- 61.Shapiro, M.: Plasticity, hippocampal place cells, and cognitive maps. Arch. Neurol. 58, 874–881 (2001) [DOI] [PubMed]
- 62.Dobson, J.: Investigation of age-related variations in biogenic magnetite levels in the human hippocampus. Exp. Brain Res. 144, 122–126 (2002) [DOI] [PubMed]
- 63.Dunn, J.R., Fuller, M., Zoeger, J., Dobson, J., Heller, F., Hammann, J., Caine, E., Moskowitz, B.M.: Magnetic material in the human hippocampus. Brain Res. Bull. 36, 149–153 (1995) [DOI] [PubMed]
- 64.Save, E., Cressant, A., Thinus-Blanc, C., Poucet, B.: Spatial firing of hippocampal place cells in blind rats. J. Neurosci. 18, 1818–1826 (1998) [DOI] [PMC free article] [PubMed]